Antimicrobial Effect of Copper Nanoparticles on Relevant Supragingival Oral Bacteria
Abstract
:1. Background
2. Methods
2.1. Nanoparticle Characterization
2.2. Bacterial Cultures
2.3. Antibacterial Activity of Nanoparticles
2.4. Viability Assays
2.5. Anti-Biofilm Activity of Nanoparticles
2.6. Anti-Biofilm Activity of Nanoparticles on Tooth Crowns
2.7. Scanning Electron Microscopy (SEM) Visualization
2.8. Statistical Analysis
3. Results
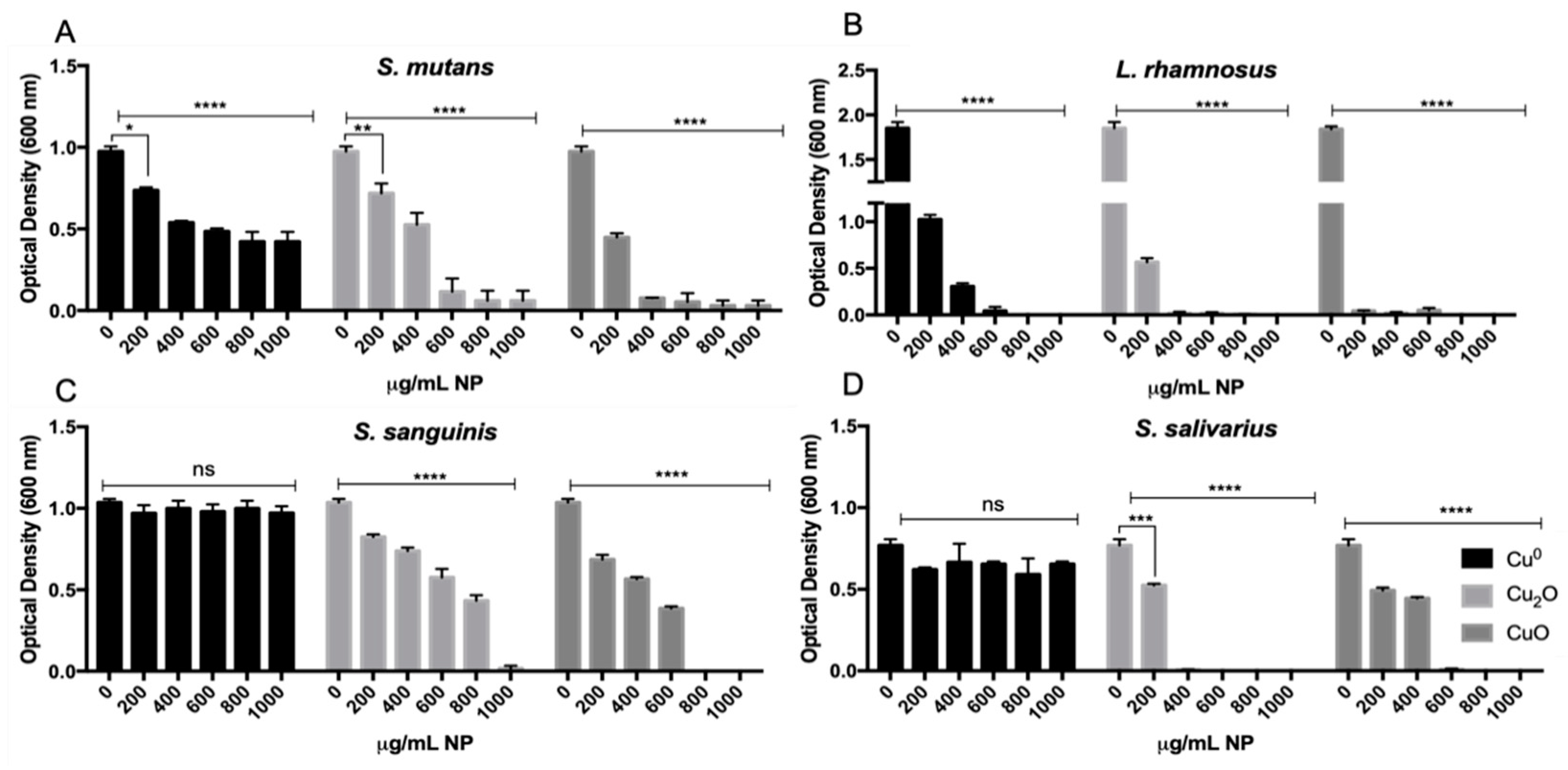
3.1. Effect of Cu NPs on the Growth of Pathogenic and Commensal Oral Bacteria
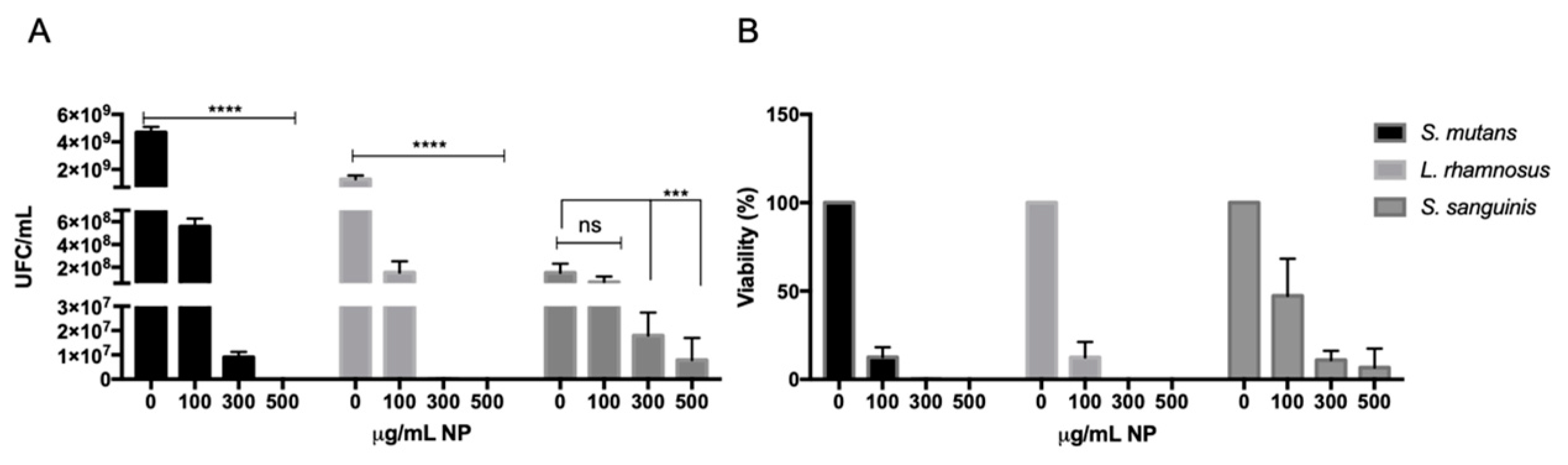
3.2. Effect of CuO NPs on the Viability of Caries-Associated Bacteria
3.3. Anti-Biofilm Activity of Cu NPs over Oral Bacteria
3.4. Anti-Biofilm Effect of Cu NPs against S. mutans on Tooth Crowns
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NPs | Nanoparticles |
| AOA | Anticavity oral agents |
| Cu NPs | Copper nanoparticles |
| CuxO | Copper oxide NPs |
| OD600 | Optical Density at 600 nm |
| OD570 | Optical Density at 570 nm |
| EPS | Extracellular polymeric substance |
| ROS | Reactive oxygen species |
| BHI | Brain Heart Infusion |
| CFU | Colony-Forming Unit |
| BFI | Biofilm Formation Index |
| SEM | Scanning electron microscopy |
| MIC | Minimal inhibitory concentration |
| MBC | Minimum bactericidal concentration |
| AIs | Autoinducers |
| QS | Quorum sensing |
| H2O2 | Hydrogen peroxide |
| CHX | Chlorhexidine |
References
- WHO. Global Oral Health Status Report; WHO: Geneva, Switzerland, 2022; Volume 57, ISBN 9789240061484. [Google Scholar]
- Deo, P.N.; Deshmukh, R. Oral Microbiome: Unveiling the Fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Belstrøm, D.; Constancias, F.; Liu, Y.; Yang, L.; Drautz-Moses, D.I.; Schuster, S.C.; Kohli, G.S.; Jakobsen, T.H.; Holmstrup, P.; Givskov, M. Metagenomic and Metatranscriptomic Analysis of Saliva Reveals Disease-Associated Microbiota in Patients with Periodontitis and Dental Caries. NPJ Biofilms Microbiomes 2017, 3, 23. [Google Scholar] [CrossRef]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Radaic, A.; Kapila, Y.L. The Oralome and Its Dysbiosis: New Insights into Oral Microbiome-Host Interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef]
- Marsh, P.D. Are Dental Diseases Examples of Ecological Catastrophes? Microbiology 2003, 149, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Nyvad, B. Ecological Hypothesis of Dentin and Root Caries. Caries Res. 2016, 50, 422–431. [Google Scholar] [CrossRef]
- Horiuchi, M.; Washio, J.; Mayanagi, H.; Takahashi, N. Transient Acid-Impairment of Growth Ability of Oral Streptococcus, Actinomyces, and Lactobacillus: A Possible Ecological Determinant in Dental Plaque. Oral Microbiol. Immunol. 2009, 24, 319–324. [Google Scholar] [CrossRef]
- Valdebenito, B.; Tullume-Vergara, P.O.; González, W.; Kreth, J.; Giacaman, R.A. In Silico Analysis of the Competition between Streptococcus Sanguinis and Streptococcus Mutans in the Dental Biofilm. Mol. Oral Microbiol. 2018, 33, 168–180. [Google Scholar] [CrossRef]
- Burton, J.P.; Wescombe, P.A.; Cadieux, P.A.; Tagg, J.R. Beneficial Microbes for the Oral Cavity: Time to Harness the Oral Streptococci? Benef Microbes 2011, 2, 93–101. [Google Scholar] [CrossRef]
- Burton, J.P.; Wescombe, P.A.; Macklaim, J.M.; Chai, M.H.C.; Macdonald, K.; Hale, J.D.F.; Tagg, J.; Reid, G.; Gloor, G.B.; Cadieux, P.A. Persistence of the Oral Probiotic Streptococcus Salivarius M18 Is Dose Dependent and Megaplasmid Transfer Can Augment Their Bacteriocin Production and Adhesion Characteristics. PLoS ONE 2013, 8, e65991. [Google Scholar] [CrossRef]
- Bescos, R.; Ashworth, A.; Cutler, C.; Brookes, Z.L.; Belfield, L.; Rodiles, A.; Casas-Agustench, P.; Farnham, G.; Liddle, L.; Burleigh, M.; et al. Effects of Chlorhexidine Mouthwash on the Oral Microbiome. Sci. Rep. 2020, 10, 5254. [Google Scholar] [CrossRef]
- Cheng, X.; He, F.; Si, M.; Sun, P.; Chen, Q. Effects of Antibiotic Use on Saliva Antibody Content and Oral Microbiota in Sprague Dawley Rats. Front. Cell. Infect. Microbiol. 2022, 12, 721691. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, G.M.; Tadakamadla, S.K.; Connelly, S.T.; Sforza, C.; Martín, C. Adverse Events Associated with Home Use of Mouthrinses: A Systematic Review. Ther. Adv. Drug Saf. 2019, 10, 2042098619854881. [Google Scholar] [CrossRef] [PubMed]
- Yun, Z.; Qin, D.; Wei, F.; Xiaobing, L. Application of Antibacterial Nanoparticles in Orthodontic Materials. Nanotechnol. Rev. 2022, 11, 2433–2450. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mukherjee, S.; Mishra, M. Nanoparticles Used in Dentistry: A Review. J. Oral Biol. Craniofac. Res. 2018, 8, 58–67. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Rab, S.; Suman, R. Applications of Nanotechnology in Medical Field: A Brief Review. Glob. Health J. 2023, 7, 70–77. [Google Scholar] [CrossRef]
- Pathakoti, K.; Manubolu, M.; Hwang, H.M. Nanotechnology Applications for Environmental Industry. In Handbook of Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 894–907. [Google Scholar] [CrossRef]
- Xu, V.W.; Nizami, M.Z.I.; Yin, I.X.; Yu, O.Y.; Lung, C.Y.K.; Chu, C.H. Application of Copper Nanoparticles in Dentistry. Nanomaterials 2022, 12, 805. [Google Scholar] [CrossRef] [PubMed]
- Lövestam, G.; Rauscher, H.; Roebben, G.; Klüttgen, B.S.; Gibson, N.; Putaud, J.-P.; Stamm, H. Considerations on a Definition of Nanomaterial for Regulatory Purposes. JCR Ref. Rep. 2010, 80, 41. [Google Scholar]
- Gawande, M.B.; Goswami, A.; Felpin, F.X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef]
- Santhoshkumar, J.; Agarwal, H.; Menon, S.; Rajeshkumar, S.; Venkat Kumar, S. A Biological Synthesis of Copper Nanoparticles and Its Potential Applications. In Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 199–221. [Google Scholar] [CrossRef]
- Ramos-Zúñiga, J.; Bruna, N.; Pérez-Donoso, J.M. Toxicity Mechanisms of Copper Nanoparticles and Copper Surfaces on Bacterial Cells and Viruses. Int. J. Mol. Sci. 2023, 24, 10503. [Google Scholar] [CrossRef]
- Begum, M.S.; Devi, R.K.; Professor, A. Wet Biochemical Synthesis of Copper Oxide Nanoparticles Coated on Titanium Dental Implants. Int. J. Adv. Res. Sci. Eng. Technol. 2016, 3, 1191–1194. [Google Scholar]
- Ma, X.; Zhou, S.; Xu, X.; Du, Q. Copper-Containing Nanoparticles: Mechanism of Antimicrobial Effect and Application in Dentistry—A Narrative Review. Front. Surg. 2022, 9, 905892. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of Antibacterial Activity of Copper Nanoparticles. Nanotechnology 2014, 25, 135101. [Google Scholar] [CrossRef] [PubMed]
- Raffi, M.; Mehrwan, S.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Yawar, W.; Ul Hasan, M.M. Investigations into the Antibacterial Behavior of Copper Nanoparticles against Escherichia coli. Ann. Microbiol. 2010, 60, 75–80. [Google Scholar] [CrossRef]
- Castro, M.; Fernandez, E.; Fluxá, P.P.; Gil, A.M.C.; Grez, P.V. Antimicrobial Effect against Streptococcus Mutans of an Adhesive System with Copper and Zinc Oxide Nanoparticles. Rev. Cuba. Investig. Biomed. 2020, 39, 1–14. [Google Scholar]
- Covarrubias, C.; Trepiana, D.; Corral, C. Synthesis of Hybrid Copper-Chitosan Nanoparticles with Antibacterial Activity against Cariogenic Streptococcus Mutans. Dent. Mater. J. 2018, 37, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Modaresi, F. The Use of Synergistically Antiplaque Nanoparticles in Treating Dental Caries. J. Dent. Health Oral Disord. Ther. 2017, 6, 144–149. [Google Scholar] [CrossRef]
- Jarpa, P. Footwear Molded in Plastic Material with Ventilation Holes of the Crocs Type Where Said Footwear Contains Copper-Based Bactericide, CL2013002853A1. 4 October 2013. Available online: https://patents.google.com/patent/CL2013002853A1/en?oq=CL2013002853A1 (accessed on 17 March 2024).
- Jarpa, P. Quick-Drying Disinfectant Composition of the Gel Type for Hands and Skin, That Does Not Contain Alcohol. WO2015035530A8. 11 September 2014. Available online: https://patents.google.com/patent/WO2015035530A8/en?oq=WO2015035530A8 (accessed on 17 March 2024).
- Lucero-Mejía, J.E.; Romero-Gómez, S.d.J.; Hernández-Iturriaga, M. A New Classification Criterion for the Biofilm Formation Index: A Study of the Biofilm Dynamics of Pathogenic Vibrio Species Isolated from Seafood and Food Contact Surfaces. J. Food Sci. 2020, 85, 2491–2497. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, F.; Kong, Q.; Lu, Y.; Zhang, B.; Wu, C.; Luo, R.; Wang, Y. Synergistic Chemical and Photodynamic Antimicrobial Therapy for Enhanced Wound Healing Mediated by Multifunctional Light-Responsive Nanoparticles. Biomacromolecules 2019, 20, 4581–4592. [Google Scholar] [CrossRef]
- Recalde, A.; van Wolferen, M.; Sivabalasarma, S.; Albers, S.V.; Navarro, C.A.; Jerez, C.A. The Role of Polyphosphate in Motility, Adhesion, and Biofilm Formation in Sulfolobales. Microorganisms 2021, 9, 193. [Google Scholar] [CrossRef]
- Ayoub, H.M.; Gregory, R.L.; Tang, Q.; Lippert, F. Influence of Salivary Conditioning and Sucrose Concentration on Biofilm-Mediated Enamel Demineralization. J. Appl. Oral Sci. 2020, 28, e20190501. [Google Scholar] [CrossRef]
- Thurnheer, T.; Bostanci, N.; Belibasakis, G.N. Microbial Dynamics during Conversion from Supragingival to Subgingival Biofilms in an in Vitro Model. Mol. Oral Microbiol. 2016, 31, 125–135. [Google Scholar] [CrossRef]
- Asahi, Y.; Miura, J.; Tsuda, T.; Kuwabata, S.; Tsunashima, K.; Noiri, Y.; Sakata, T.; Ebisu, S.; Hayashi, M. Simple Observation of Streptococcus Mutans Biofilm by Scanning Electron Microscopy Using Ionic Liquids. AMB Express 2015, 5, 6. [Google Scholar] [CrossRef]
- Woźniak-Budych, M.J.; Staszak, K.; Staszak, M. Copper and Copper-Based Nanoparticles in Medicine—Perspectives and Challenges. Molecules 2023, 28, 6687. [Google Scholar] [CrossRef]
- Harishchandra, B.D.; Pappuswamy, M.; PU, A.; Shama, G.; Pragatheesh, A.; Arumugam, V.A.; Periyaswamy, T.; Sundaram, R. Copper Nanoparticles: A Review on Synthesis, Characterization and Applications. Asian Pac. J. Cancer Biol. 2020, 5, 201–210. [Google Scholar] [CrossRef]
- Bakshi, M.; Kumar, A. Applications of Copper Nanoparticles in Plant Protection and Pollution Sensing: Toward Promoting Sustainable Agriculture. In Copper Nanostructures: Next-Generation of Agrochemicals for Sustainable Agroecosystems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 393–413. [Google Scholar] [CrossRef]
- Thomas, C.; Minty, M.; Vinel, A.; Canceill, T.; Loubières, P.; Burcelin, R.; Kaddech, M.; Blasco-Baque, V.; Laurencin-Dalicieux, S. Oral Microbiota: A Major Player in the Diagnosis of Systemic Diseases. Diagnostics 2021, 11, 1376. [Google Scholar] [CrossRef]
- Baker, J.L.; Edlund, A. Exploiting the Oral Microbiome to Prevent Tooth Decay: Has Evolution Already Provided the Best Tools? Front. Microbiol. 2019, 10, 437902. [Google Scholar] [CrossRef] [PubMed]
- Giannousi, K.; Sarafidis, G.; Mourdikoudis, S.; Pantazaki, A.; Dendrinou-Samara, C. Selective Synthesis of Cu2O and Cu/Cu2O NPs: Antifungal Activity to Yeast Saccharomyces Cerevisiae and DNA Interaction. Inorg. Chem. 2014, 53, 9657–9666. [Google Scholar] [CrossRef]
- Allaker, R.P. Critical Review in Oral Biology & Medicine: The Use of Nanoparticles to Control Oral Biofilm Formation. J. Dent. Res. 2010, 89, 1175–1186. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Cu and CuO Nanoparticles Immobilized by Silica Thin Films as Antibacterial Materials and Photocatalysts. Surf. Coat. Technol. 2010, 205, 219–223. [Google Scholar] [CrossRef]
- Hans, M.; Erbe, A.; Mathews, S.; Chen, Y.; Solioz, M.; Mücklich, F. Role of Copper Oxides in Contact Killing of Bacteria. Langmuir 2013, 29, 16160–16166. [Google Scholar] [CrossRef]
- Grytten, J.; Scheie, A.A.; Giertsen, E. Synergistic Antibacterial Effects of Copper and Hexetidine against Streptococcus Sobrinus and Streptococcus Sanguis. Acta Odontol. Scand. 1988, 46, 181–183. [Google Scholar] [CrossRef]
- Du, T.; Vijayakumar, A.; Desai, V. Effect of Hydrogen Peroxide on Oxidation of Copper in CMP Slurries Containing Glycine and Cu Ions. Electrochim. Acta 2004, 49, 4505–4512. [Google Scholar] [CrossRef]
- Beveridge, T.J.; Murray, R.G.E. Sites of Metal Deposition in the Cell Wall of Bacillus Subtilis. J. Bacteriol. 1980, 141, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, A.V.; Danilushkina, A.A.; Irisov, D.S.; Bruslik, N.L.; Fakhrullin, R.F.; Zakharov, Y.A.; Bukhmin, V.S.; Yarullina, D.R. Assessment of Resistance and Bioremediation Ability of Lactobacillus Strains to Lead and Cadmium. Int. J. Microbiol. 2017, 2017, 9869145. [Google Scholar] [CrossRef] [PubMed]
- Morales-Calderón, L.S.; Armenta-Ortiz, N.; Méndez-Trujillo, V.; Ruiz-Sanchez, E.; González-Mendoza, D.; Grimaldo-Juarez, O.; Cervantes-Diaz, L.; Aviles-Marin, M. Laura Selene Morales-Calderón Copper Induced Biofilm Formation and Changes on Photosynthetic Pigment in Euglena Gracilis. Afr. J. Microbiol. Res. 2012, 6, 1833–1836. [Google Scholar] [CrossRef]
- Singh, K. Characterization of a Copper Resistance and Transport System in Streptococcus mutans. ProQuest Dissertations and Thesis, University of Toronto, Toronto, ON, Canada, 2015; p. 148. [Google Scholar]
- Zhou, L.; Zhang, Y.; Ge, Y.; Zhu, X.; Pan, J. Regulatory Mechanisms and Promising Applications of Quorum Sensing-Inhibiting Agents in Control of Bacterial Biofilm Formation. Front. Microbiol. 2020, 11, 589640. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Hamza, H.K.; Mohammed, G.J. Anti-Quorum Sensing Effect of Streptococcus agalatiaceae by Zinc Oxide, Copper Oxide, and Titanium Oxide Nanoparticles. J. Phys. Conf. Ser. 2021, 1999, 012031. [Google Scholar] [CrossRef]
- Dong, L.; Tong, Z.; Linghu, D.; Lin, Y.; Tao, R.; Liu, J.; Tian, Y.; Ni, L. Effects of Sub-Minimum Inhibitory Concentrations of Antimicrobial Agents on Streptococcus Mutans Biofilm Formation. Int. J. Antimicrob. Agents 2012, 39, 390–395. [Google Scholar] [CrossRef]
- Qian, W.; Zhang, J.; Xiao, X. Research on Inhibition of Sodium Fluoride on Five Subgingival Bacteria in Vitro. Hua Xi Kou Qiang Yi Xue Za Zhi 1998.
- Li, X.; Wang, Y.; Jiang, X.; Zeng, Y.; Zhao, X.; Washio, J.; Takahashi, N.; Zhang, L. Investigation of Drug Resistance of Caries-Related Streptococci to Antimicrobial Peptide GH12. Front. Cell Infect. Microbiol. 2022, 12. [Google Scholar] [CrossRef]
- Doern, G.V.; Ferraro, M.J.; Brueggemann, A.B.; Ruoff, K.L. Emergence of High Rates of Antimicrobial Resistance among Viridans Group Streptococci in the United States. Antimicrob. Agents Chemother. 1996, 40, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Aliasghari, A.; Khorasgani, M.R.; Vaezifar, S.; Rahimi, F.; Younesi, H.; Khoroushi, M. Evaluation of Antibacterial Efficiency of Chitosan and Chitosan Nanoparticles on Cariogenic Streptococci: An in Vitro Study. Iran. J. Microbiol. 2016, 8, 93. [Google Scholar]



| Cu0 NPs | Cu2O NPs | CuO NPs | |
|---|---|---|---|
| CAS number | 7440-50-8 | 1317-38-0 | 1317-38-0 |
| Molecular weight | 63.5 g/mol | 143.9 g/mol | 79.6 g/mol |
| Color | Brown-red | green | black |
| Size | ~40–70 nm | ~40–60 nm | ~40–60 nm |
| Batch | 180314-RN | 230315-SP | 190726-MA |
| MIC Cu0 NPs (μg/mL) | MIC Cu2O NPs (μg/mL) | MIC CuO NPs (μg/mL) | |
|---|---|---|---|
| S. mutans | >1000 | 500 | 400 |
| L. rhamnosus | 800 | 300 | 200 |
| S. sanguinis | >1000 | 1000 | 800 |
| S. salivarius | >1000 | 400 | 600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oetiker, N.; Salinas, D.; Lucero-Mora, J.; Orellana, R.; Quiroz-Muñoz, M.; Bravo, D.; Pérez-Donoso, J.M. Antimicrobial Effect of Copper Nanoparticles on Relevant Supragingival Oral Bacteria. Microorganisms 2024, 12, 624. https://doi.org/10.3390/microorganisms12030624
Oetiker N, Salinas D, Lucero-Mora J, Orellana R, Quiroz-Muñoz M, Bravo D, Pérez-Donoso JM. Antimicrobial Effect of Copper Nanoparticles on Relevant Supragingival Oral Bacteria. Microorganisms. 2024; 12(3):624. https://doi.org/10.3390/microorganisms12030624
Chicago/Turabian StyleOetiker, Nia, Daniela Salinas, Joaquín Lucero-Mora, Rocío Orellana, Mariana Quiroz-Muñoz, Denisse Bravo, and José M. Pérez-Donoso. 2024. "Antimicrobial Effect of Copper Nanoparticles on Relevant Supragingival Oral Bacteria" Microorganisms 12, no. 3: 624. https://doi.org/10.3390/microorganisms12030624





