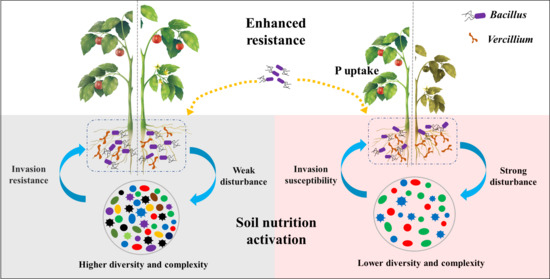
Difference in the Effect of Applying Bacillus to Control Tomato Verticillium Wilt in Black and Red Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Construction of the Bacillus Combinations
2.3. Assessment of Growth-Promoting Ability
2.4. Growth Curve Determination and Inhibitory Effect Analysis
2.5. Preparation of Bacillus Suspension and V. dahliae Spore Suspension
2.6. Pot Experiment
2.7. Determination of Nutrients in Tomato and Soil
2.8. Determination of Soil Enzyme Activity
2.9. Determination of Resistance-Related Enzymes in Tomato
2.10. Quantitative Analysis of Bacillus and V. dahliae
2.11. Microbial Community Analysis
2.12. Statistical Analysis
2.13. Data Availability
3. Results
3.1. Construction and Evaluation of Bacillus Combinations
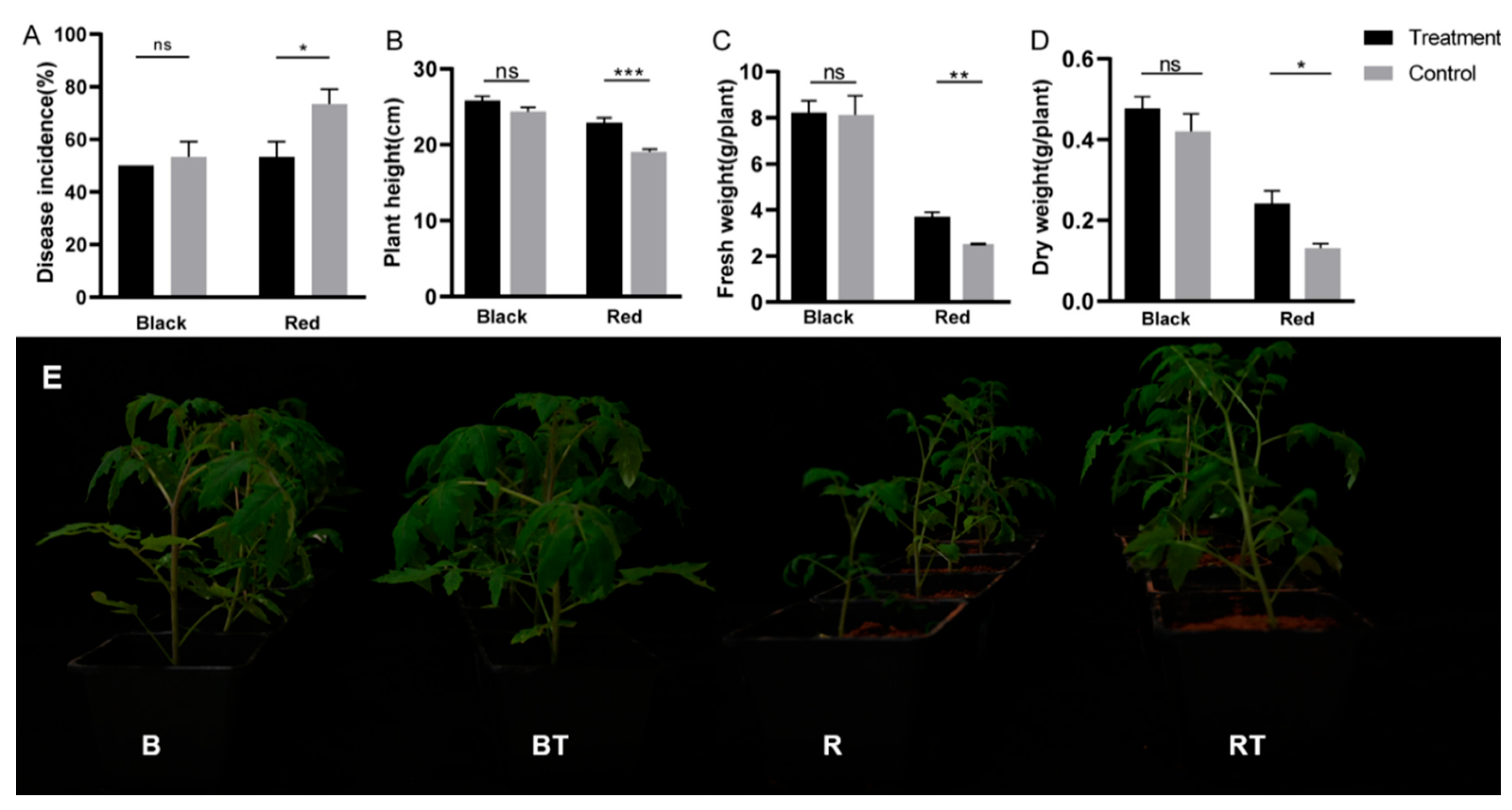
3.2. Effects of Bacillus Application on Tomato Biomass and Incidence of Verticillium Wilt
3.3. Effects of Bacillus Application on Tomato Nutrient Uptake in Two Soils
3.4. Effect of Bacillus Application on the Bacterial Community Diversity and Abundance of V. dahliae
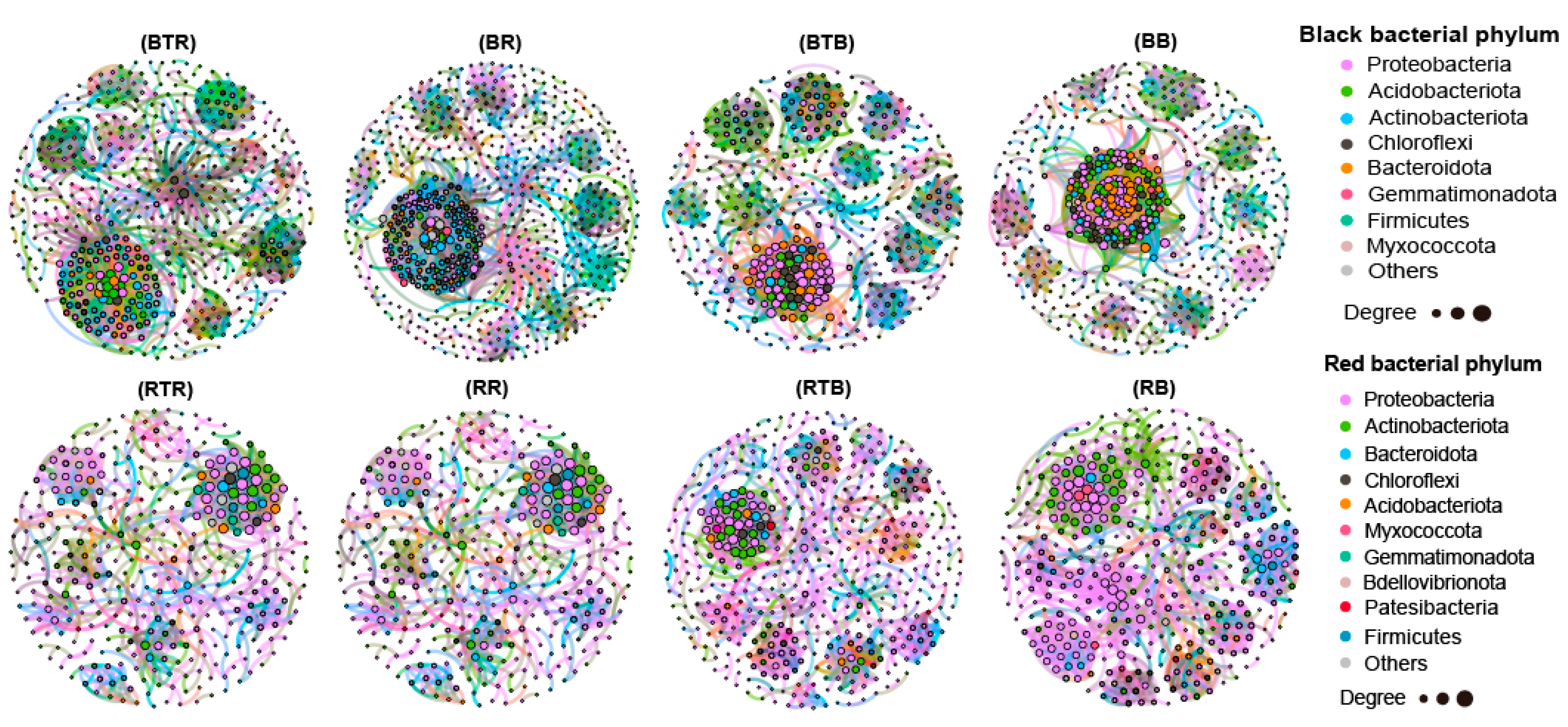
3.5. Effect of Bacillus Application on Microbial Networks
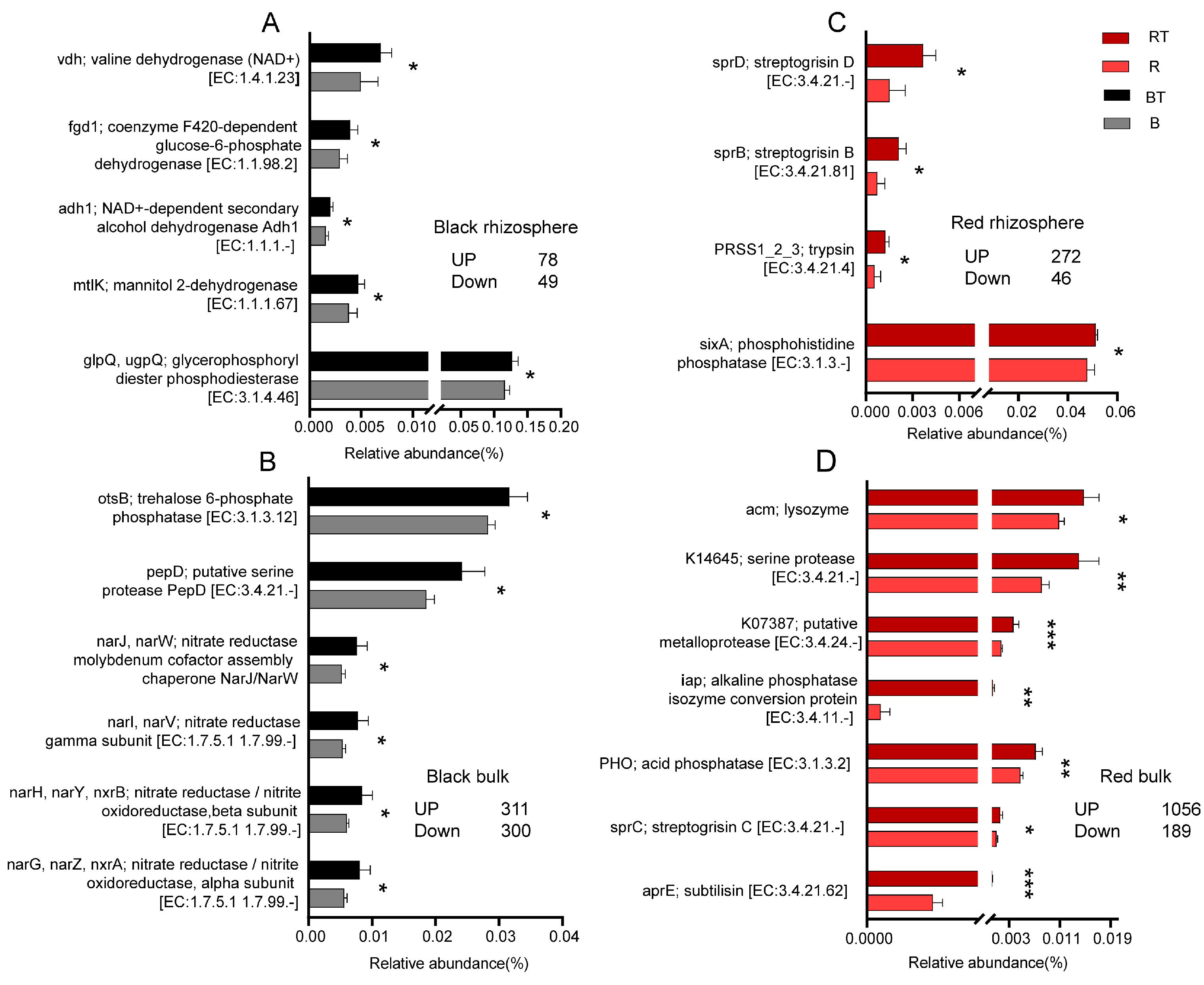
3.6. Regulation of Bacterial Community Function by the Application of Bacillus
4. Discussion
4.1. Application of Bacillus Can Significantly Inhibit the Occurrence of Tomato Verticillium Wilt in Red Soil
4.2. Application of Bacillus Significantly Promoted Phosphate Uptake in Red Soil
4.3. The Bacterial Community in Native Soil May Be the Key to Affect the Colonization of Bacillus and Then Affect the Disease-Preventing and Growth-Promoting Effect of Bacillus on Tomato
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Mutar, D.M.K.; Alzawar, N.S.A.; Noman, M.; Azizullah Li, D.; Song, F. Suppression of Fusarium Wilt in Watermelon by Bacillus amyloliquefaciens DHA55 through Extracellular Production of Antifungal Lipopeptides. J. Fungi 2023, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Yadav, R.; Ramakrishna, W. Bacillus subtilis impact on plant growth, soil health and environment: Dr. Jekyll and Mr. Hyde. J. Appl. Microbiol. 2022, 132, 3543–3562. [Google Scholar] [CrossRef] [PubMed]
- Aloo, B.N.; Makumba, B.A.; Mbega, E.R. The potential of Bacilli rhizobacteria for sustainable crop production and environ-mental sustainability. Microbiol. Res. 2019, 219, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The Significance of Bacillus spp. in Disease Suppression and Growth Pro-motion of Field and Vegetable Crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef] [PubMed]
- Schlemper, T.R.; Leite, M.F.A.; Lucheta, A.R.; Shimels, M.; Bouwmeester, H.J.; van Veen, J.A.; Kuramae, E.E. Rhizobacterial community structure differences among sorghum cultivars in different growth stages and soils. FEMS Microbiol. Ecol. 2017, 93, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Hu, Y.; Razavi, B.S.; Zhou, J.; Shen, J.; Nannipieri, P.; Wu, J.; Ge, T. Rare taxa of alkaline phosphomonoesterase-harboring microorganisms mediate soil phosphorus mineralization. Soil Biol. Biochem. 2019, 131, 62–70. [Google Scholar] [CrossRef]
- Su, X.; Lu, G.; Guo, H.; Zhang, K.; Li, X.; Cheng, H. The dynamic transcriptome and metabolomics profiling in Verticillium dahliae inoculated Arabidopsis thaliana. Sci. Rep. 2018, 8, 15404. [Google Scholar] [CrossRef] [PubMed]
- Ibiang, S.R.; Usami, T.; Sakamoto, K. Reduction of verticillium wilt in tomato by an arbuscular mycorrhizal fungus—Rhizophagus intraradices and an endophytic fungus—Penicillium pinophilum is cultivar dependent. Rhizosphere 2021, 20, 100440. [Google Scholar] [CrossRef]
- Tao, X.; Zhang, H.; Gao, M.; Li, M.; Zhao, T.; Guan, X. Pseudomonas species isolated via high-throughput screening significantly protect cotton plants against verticillium wilt. AMB Express 2020, 10, 193. [Google Scholar] [CrossRef]
- Fradin, E.F.; Zhang, Z.; Ayala, J.C.J.; Castroverde, C.D.M.; Nazar, R.N.; Robb, J.; Liu, C.-M.; Thomma, B.P.H.J. Genetic dissection of verticillium wilt resistance mediated by tomato Ve1. Plant Physiol. 2009, 150, 320–332. [Google Scholar] [CrossRef]
- Guo, Z. Study on the Difference in the Effect of Applying Bacillus to Control Tomato Verticillium wilt in Black and Red Soil. Ph.D. Thesis, Sun Yat-Sen University, Guangzhou, China, 2023. [Google Scholar]
- Wei, M.; Zhang, M.; Huang, G.; Yuan, Y.; Fu, C.; Yu, L. Coculture with two Bacillus velezensis strains enhances the growth of Anoectochilus plants via promoting nutrient assimilation and regulating rhizosphere microbial community. Ind. Crops Prod. 2020, 154, 112697. [Google Scholar] [CrossRef]
- Yuan, Y. Screening of Plant Growth-Promoting Rhizobacteria of Anoectochilus roxburghii (Wall.) LindI and Investigation of Promoting Effects. Ph.D. Thesis, Huazhong University of Science and Technology, Wuhan, China, 2022. [Google Scholar]
- Magallon-Servín, P.; Antoun, H.; Taktek, S.; Bashan, Y.; De-Bashan, L. The maize mycorrhizosphere as a source for isolation of arbuscular mycorrhizae-compatible phosphate rock-solubilizing bacteria. Plant Soil 2020, 451, 169–186. [Google Scholar] [CrossRef]
- Raturi, G.; Sharma, Y.; Rana, V.; Thakral, V.; Myaka, B.; Salvi, P.; Singh, M.; Dhar, H.; Deshmukh, R. Exploration of silicate solubilizing bacteria for sustainable agriculture and silicon biogeochemical cycle. Plant Physiol. Biochem. 2021, 166, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Michał, S.; Piotr, S.; Sławomir, O. A novel, simple, and sensitive colorimetric method to determine aromatic amino acid ami-notransferase activity using the Salkowski reagent. Folia Microbiol. 2012, 57, 1–4. [Google Scholar]
- Monteiro, D.R.; Takamiya, A.S.; Feresin, L.P.; Gorup, L.F.; de Camargo, E.R.; Delbem, A.C.B.; Henriques, M.; Barbosa, D.B. Susceptibility of Candida albicans and Candida glabrata biofilms to silver nanoparticles in intermediate and mature development phases. J. Prosthodont. Res. 2015, 59, 42–48. [Google Scholar] [CrossRef] [PubMed]
- NY/T 2419—2013; Determination of Total Nitrogen in Plant—Automatic Kjeldahl Apparatus Method. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2013.
- NY/T 2421-2013; Determination of Total Phosphorus in Plant—Vanadium Molybdate Blue Colorimetric Method. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2013.
- NY/T 2420-2013; Determination of Total Potassium in Plant—Flame Photometry Method. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2013.
- Fan, K.; Delgado-Baquerizo, M.; Guo, X.; Wang, D.; Wu, Y.; Zhu, M.; Yu, W.; Yao, H.; Zhu, Y.-G.; Chu, H. Suppressed N fixation and diazotrophs after four decades of fertilization. Microbiome 2019, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- HJ 632-2011; Determination of Total Phosphorus by Alkali Fusion—Mo-Sb Anti Spectrophotometric Method. Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2011.
- HJ 781-2016; Determination of 22 Metal Elements—Inductively Coupled Plasma Optical Emission Spectrometry. Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2016.
- NY/T 1121.7-2014; Soil Testing—Part 7: Method for Determination of Available Phosphorus in Soil. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2014.
- NY/T 889-2004; Determination of Exchangeable Potassium Andnon—Exchangeable Potassium Content in Soil. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2004.
- GB/T 32737-2016; Determination of Nitrate Nitrogen in Soil—Ultraviolet Spectrophotometry Method. General Administration of Quality Supervision; Inspection and Quarantine of the People’s Republic of China; Standardization Administration of the People’s Republic of China: Beijing, China, 2016.
- LY/T 1228-2015; Nitrogen Determination Methods of Forest Soils. The State Forestry Administration of the People’s Republic of China: Beijing, China, 2015.
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Ladd, J.; Butler, J. Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]
- Fradin, E.F.; Abd-El-Haliem, A.; Masini, L.; van den Berg, G.C.; Joosten, M.H.; Thomma, B.P. Interfamily transfer of tomato Ve1 mediates Verticillium resistance in Arabidopsis. Plant Physiol. 2011, 156, 2255–2265. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108 (Supp. S1), 4516–4522. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Jiang, Y.-H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, X.; Zhang, J.; Cai, Z.; Jiang, K.; Chang, Y. Deciphering the relative importance of soil and plant traits on the development of rhizosphere microbial communities. Soil Biol. Biochem. 2020, 148, 107909. [Google Scholar] [CrossRef]
- Eskandari, S.; Guppy, C.N.; Knox, O.G.; Backhouse, D.; Haling, R.E. Mycorrhizal Symbioses of Cotton Grown on Sodic Soils: A Review from an Australian Perspective. Pedosphere 2017, 27, 1015–1026. [Google Scholar] [CrossRef]
- Tao, C.; Li, R.; Xiong, W.; Shen, Z.; Liu, S.; Wang, B.; Ruan, Y.; Geisen, S.; Shen, Q.; Kowalchuk, G.A. Bio-organic fertilizers stimulate indigenous soil Pseudomonas populations to enhance plant disease suppression. Microbiome 2020, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Pishchik, V.N.; Vorobyev, N.I.; Moiseev, K.G.; Sviridova, O.V.; Surin, V.G. Influence of Bacillus subtilis on the physiological state of wheat and the microbial community of the soil under different rates of nitrogen fertilizers. Eurasian Soil Sci. 2015, 48, 77–84. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, D.; Guo, J.; Manter, D.K.; Reardon, K.F.; Vivanco, J.M. Bacillus spp. from rainforest soil promote plant growth under limited nitrogen conditions. J. Appl. Microbiol. 2015, 118, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Ahmad, M.; Raza, M.A.; Hilger, T.; Rasche, F. Phosphate-Solubilizing Bacillus sp. Modulate Soil Exoenzyme Activities and Improve Wheat Growth. Microb. Ecol. 2024, 87, 31. [Google Scholar] [CrossRef]
- Goswami, G.; Deka, P.; Das, P.; Bora, S.S.; Samanta, R.; Boro, R.C.; Barooah, M. Diversity and functional properties of acid-tolerant bacteria isolated from tea plantation soil of Assam. 3 Biotech 2017, 7, 229. [Google Scholar] [CrossRef]
- Chowdhury, N.; Hazarika, D.J.; Goswami, G.; Sarmah, U.; Borah, S.; Boro, R.C.; Barooah, M. Acid tolerant bacterium Bacillus amyloliquefaciens MBNC retains biocontrol efficiency against fungal phy-topathogens in low pH. Arch. Microbiol. 2022, 204, 124. [Google Scholar] [CrossRef] [PubMed]
- Egamberdiyeva, D. The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. Appl. Soil Ecol. 2007, 36, 184–189. [Google Scholar] [CrossRef]
- De Oliveira, A.L.M.; de Canuto, E.L.; Urquiaga, S.; Reis, V.M.; Baldani, J.I. Yield of micropropagated sugarcane varieties in different soil types following inoculation with diazotrophic bacteria. Plant Soil 2006, 284, 23–32. [Google Scholar] [CrossRef]
- Fallik, E.; Okon, Y. The response of maize (Zea mays) to Azospirillum inoculation in various types of soils in the field. World J. Microbiol. Biotechnol. 1996, 12, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Matse, D.T.; Huang, C.H.; Huang, Y.M.; Yen, M.Y. Effects of coinoculation of Rhizobium with plant growth promoting rhizobacteria on the nitrogen fixation and nutrient uptake of Trifolium repens in low phosphorus soil. J. Plant Nutr. 2019, 43, 739–752. [Google Scholar] [CrossRef]
- Liang, J.-L.; Liu, J.; Jia, P.; Yang, T.-T.; Zeng, Q.-W.; Zhang, S.-C.; Liao, B.; Shu, W.-S.; Li, J.-T. Novel phosphate-solubilizing bacteria enhance soil phosphorus cycling following ecological restoration of land degraded by mining. ISME J. 2020, 14, 1600–1613. [Google Scholar] [CrossRef] [PubMed]
- Raymond, N.S.; Gómez-Muñoz, B.; van der Bom, F.J.T.; Nybroe, O.; Jensen, L.S.; Müller-Stöver, D.S.; Oberson, A.; Richardson, A.E. Phosphate-solubilising microorganisms for improved crop productivity: A critical assessment. New Phytol. 2021, 229, 1268–1277. [Google Scholar] [CrossRef]
- Mawarda, P.C.; Mallon, C.A.; Le Roux, X.; Van Elsas, J.D.; Salles, J.F. Interactions between Bacterial Inoculants and Native Soil Bacterial Community: The Case of Spore-forming Bacillus spp. FEMS Microbiol. Ecol. 2022, 98, fiac127. [Google Scholar] [CrossRef]
- Mallon, C.A.; van Elsas, J.D.; Salles, J.F. Microbial Invasions: The Process, Patterns, and Mechanisms. Trends Microbiol. 2015, 23, 719–729. [Google Scholar] [CrossRef]
- Mallon, C.; Le Roux, X.; van Doorn, G.S.; Dini-Andreote, F.; Poly, F.; Salles, J.F. The impact of failure: Unsuccessful bacterial invasions steer the soil microbial community away from the invader’s niche. ISME J. 2018, 12, 728–741. [Google Scholar] [CrossRef]
- Ge, A.-H.; Liang, Z.-H.; Xiao, J.-L.; Zhang, Y.; Zeng, Q.; Xiong, C.; Han, L.-L.; Wang, J.-T.; Zhang, L.-M. Microbial assembly and association network in watermelon rhizosphere after soil fumigation for Fusarium wilt control. Agric. Ecosyst. Environ. 2021, 312, 107336. [Google Scholar] [CrossRef]
- Nikolaidou, C.; Monokrousos, N.; Kapagianni, P.D.; Orfanoudakis, M.; Dermitzoglou, T.; Papatheodorou, E.M. The Effect of Rhizophagus irregularis, Bacillus subtilis and Water Regime on the Plant–Microbial Soil System: The Case of Lactuca sativa. Agronomy 2021, 11, 2183. [Google Scholar] [CrossRef]
- Liu, Z.; Xiao, J.; Zhang, X.; Dou, S.; Gao, T.; Wang, D.; Zhang, D. Influence of Bacillus subtilis strain Z-14 on microbial communities of wheat rhizospheric soil infested with Gaeumannomyces graminis var. tritici. Front. Microbiol. 2022, 13, 923242. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Ma, G.; Chen, S.; Lin, C.; Zhao, X. Microbial Network and Soil Properties Are Changed in Bacterial Wilt-Susceptible Soil. Appl. Environ. Microbiol. 2019, 85, e00162-19. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Maiti, S.K. Different Soil Factors Influencing Dehydrogenase Activity in Mine Degraded Lands—State-of-Art Review. Water Air Soil Pollut. 2021, 232, 360. [Google Scholar] [CrossRef]
- Zhang, D.; Li, M.; Yang, Y.; Yu, H.; Xiao, F.; Mao, C.; Huang, J.; Yu, Y.; Wang, Y.; Wu, B.; et al. Nitrite and nitrate reduction drive sediment microbial nitrogen cycling in a eutrophic lake. Water Res. 2022, 220, 118637. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, R.; Liu, Z.; Yu, X.; Zheng, S.; Yuan, S. Nitrogen process in stormwater bioretention: The impact of alternate drying and rewetting on nitrogen migration and transformation. Environ. Sci. Pollut. Res. 2021, 28, 43803–43814. [Google Scholar] [CrossRef]
- Liu, C.; Song, Y.; Dong, X.; Wang, X.; Ma, X.; Zhao, G.; Zang, S. Soil Enzyme Activities and Their Relationships with Soil C, N, and P in Peatlands from Different Types of Permafrost Regions, Northeast China. Front. Environ. Sci. 2021, 9, 670769. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Lu, Z.; Liu, Z.; Zhou, W.; Yang, S.; Lv, J.; Wei, M. Difference in the Effect of Applying Bacillus to Control Tomato Verticillium Wilt in Black and Red Soil. Microorganisms 2024, 12, 797. https://doi.org/10.3390/microorganisms12040797
Guo Z, Lu Z, Liu Z, Zhou W, Yang S, Lv J, Wei M. Difference in the Effect of Applying Bacillus to Control Tomato Verticillium Wilt in Black and Red Soil. Microorganisms. 2024; 12(4):797. https://doi.org/10.3390/microorganisms12040797
Chicago/Turabian StyleGuo, Zhenhua, Ziyu Lu, Zhongwang Liu, Wei Zhou, Shuangyu Yang, Jiayan Lv, and Mi Wei. 2024. "Difference in the Effect of Applying Bacillus to Control Tomato Verticillium Wilt in Black and Red Soil" Microorganisms 12, no. 4: 797. https://doi.org/10.3390/microorganisms12040797
APA StyleGuo, Z., Lu, Z., Liu, Z., Zhou, W., Yang, S., Lv, J., & Wei, M. (2024). Difference in the Effect of Applying Bacillus to Control Tomato Verticillium Wilt in Black and Red Soil. Microorganisms, 12(4), 797. https://doi.org/10.3390/microorganisms12040797