The Changes in Fecal Bacterial Communities in Goats Offered Rumen-Protected Fat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Design, and Diets
2.2. Procedures and Sample Collection
2.3. Feed Sample Analysis
2.4. Fecal Short-Chain Fatty Acids Analysis
2.5. DNA Extraction, 16S rRNA Gene Amplification, and Sequencing
2.6. Statistical Analyses
3. Results
3.1. Growth Performance
3.2. Fecal Short-Chain Fatty Acids
3.3. Collective Sequencing Data Summary
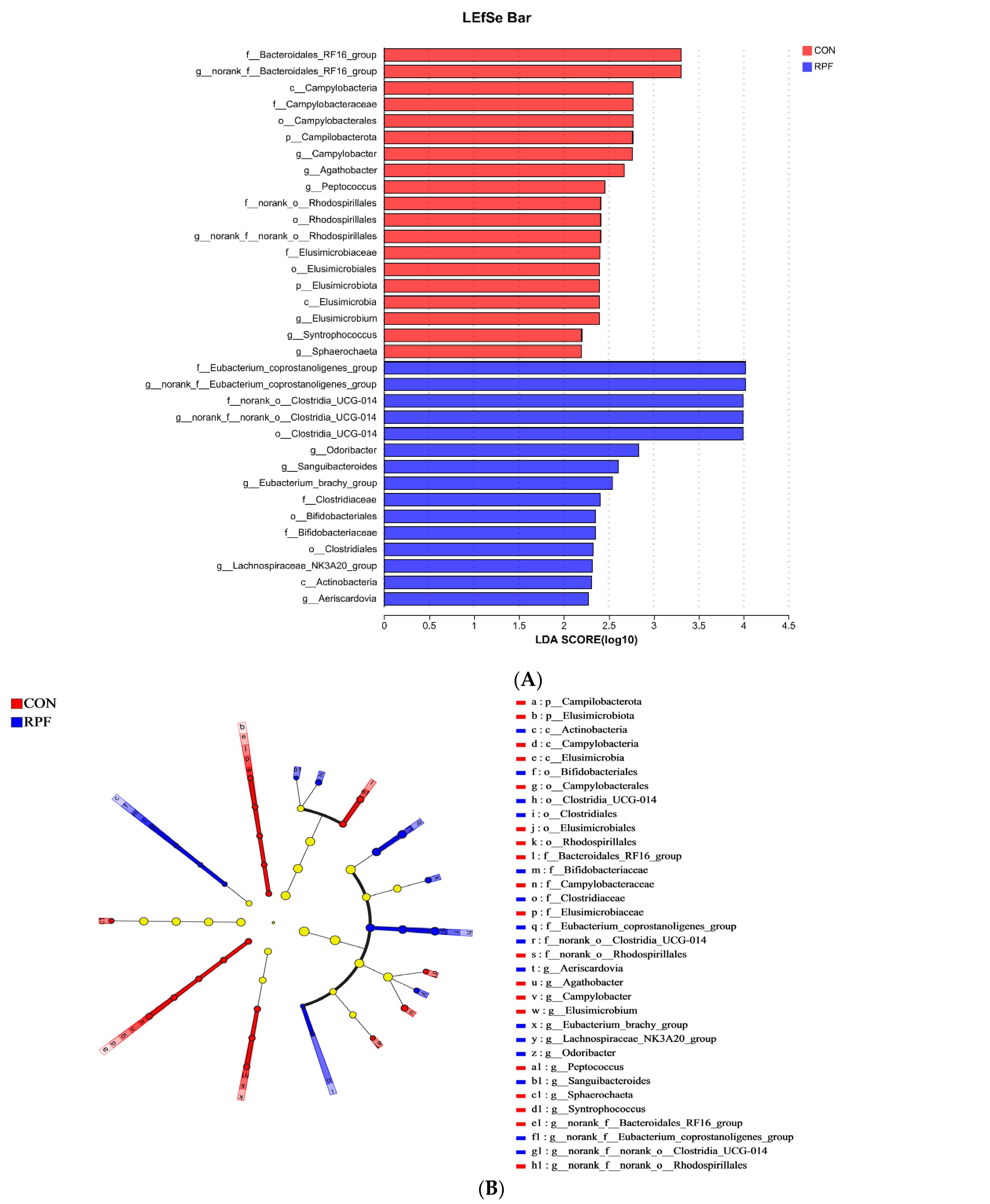
3.4. Microbial Community Composition in the Feces
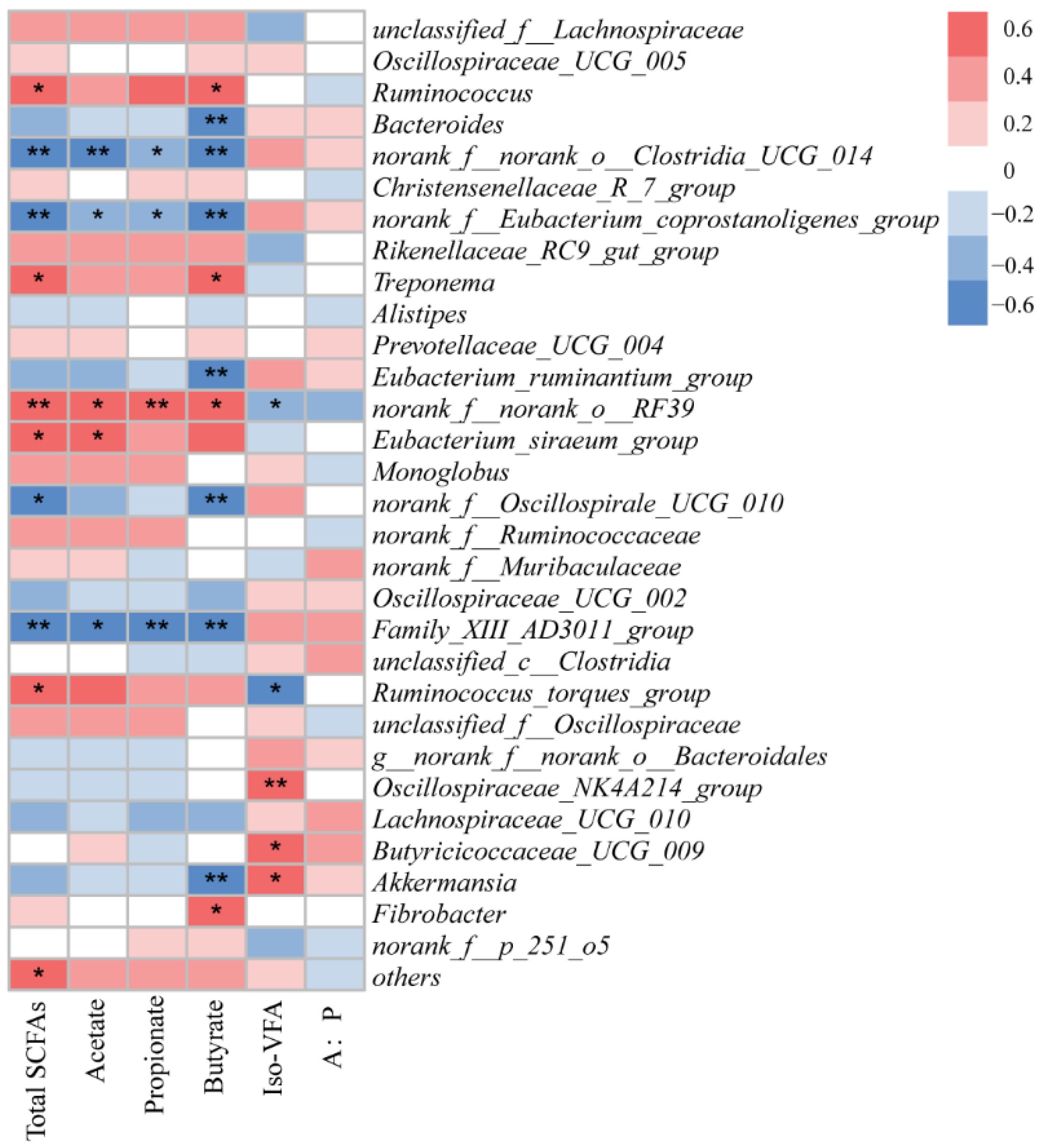
3.5. Correlations between Fecal Bacteria and Short-Chain Fatty Acids
4. Discussion
4.1. Effect of Dietary Rumen-Protected Fat on Growth Performance
4.2. Effects of Dietary Rumen-Protected Fat on Fecal Short-Chain Fatty Acid Concentrations
4.3. Microbial Community Composition in the Feces
4.4. Correlations between Fecal Bacteria and Short-Chain Fatty Acids
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- China National Commission of Animal Genetic Resources. Animal Genetic Resources in China: Sheep and Goats; China Agriculture Press: Beijing, China, 2011. [Google Scholar]
- Feng, H.; Shi, H.; Yang, F.; Yun, Y.; Wang, X. Impact of anthocyanins derived from Dioscorea alata L. on growth performance, carcass characteristics, antioxidant capacity, and immune function of Hainan black goats. Front. Vet. Sci. 2023, 10, 1283947. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, G.; Degen, A.; Ji, K.; Jiao, D.; Liang, Y.; Xiao, L.; Long, R.; Zhou, J. Effect of feed level and supplementary rumen protected lysine and methionine on growth performance, rumen fermentation, blood metabolites and nitrogen balance in growing Tan lambs fed low protein diets. Anim. Feed Sci. Technol. 2021, 279, 115024. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Liu, X.; Liu, R.; Wang, Y.; Huang, X.; Li, Y.; Liu, R.; Yang, X. Dietary folic acid addition reduces abdominal fat deposition mediated by alterations in gut microbiota and SCFA production in broilers. Anim. Nutr. 2023, 12, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Pormalekshahi, A.; Fatahnia, F.; Jafari, H.; Azarfar, A.; Varmaghany, S.; Taasoli, G. Interaction effect of ruminal undegradable protein level and rumen-protected conjugated linoleic acid (CLA) inclusion in the diet of growing goat kids on meat CLA content and quality traits. Br. J. Nutr. 2019, 122, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Manriquez, D.; Chen, L.; Melendez, P.; Pinedo, P. The effect of an organic rumen-protected fat supplement on performance, metabolic status, and health of dairy cows. BMC Vet. Res. 2019, 15, 450. [Google Scholar] [CrossRef] [PubMed]
- Bakker, C.E.; Blair, A.D.; Grubbs, J.K.; Taylor, A.R.; Brake, D.W.; Long, N.M.; Underwood, K.R. Effects of rumen-protected long-chain fatty acid supplementation during the finishing phase of beef steers on live performance, carcass characteristics, beef quality, and serum fatty acid profile. Transl. Anim. Sci. 2019, 3, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- de Lima, J.A.M.; Bezerra, L.R.; Feitosa, T.J.O.; Oliveira, J.R.; de Oliveira, D.L.V.; Mazzetto, S.E.; Cavalcanti, M.T.; Pereira Filho, J.M.; Oliveira, R.L.; de Oliveira, J.P.F.; et al. Production, characterization, and dietary supplementation effect of rumen-protected fat on ruminal function and blood parameters of sheep. Trop. Anim. Health Prod. 2023, 55, 142. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hao, L.Z.; Cao, X.L.; Yang, G.; Degen, A.A.; Xiao, L.; Liu, S.J.; Zhou, J.W. Effects of supplementary concentrate and/or rumen-protected lysine plus methionine on productive performance, milk composition, rumen fermentation, and bacterial population in grazing, lactating yaks (Bos grunniens), and average daily gain of their calves. Anim. Feed Sci. Technol. 2023, 297, 115591. [Google Scholar] [CrossRef]
- Behan, A.A.; Loh, T.C.; Fakurazi, S.; Kaka, U.; Kaka, A.; Samsudin, A.A. Effects of supplementation of rumen protected fats on rumen ecology and digestibility of nutrients in sheep. Animals 2019, 9, 400. [Google Scholar] [CrossRef]
- Liu, H.; Ran, T.; Zhang, C.F.; Yang, W.Z.; Wu, X.K.; Degen, A.; Long, R.J.; Shi, Z.J.; Zhou, J.W. Comparison of rumen bacterial communities between yaks (Bos grunniens) and Qaidam cattle (Bos taurus) fed a low protein diet with different energy levels. Front. Microbiol. 2022, 13, 982338. [Google Scholar] [CrossRef]
- Liu, H.Y.; Yan, C.R.; Hao, C.Y.; Wang, D.Q.; Liu, Y.Z.; Luo, Z.B.; Han, S.Z.; Wang, J.X.; Li, D.X.; Zhu, J.; et al. Dynamic changes in intestinal microbiota and metabolite composition of pre-weaned beef calves. Microb. Pathog. 2023, 175, 105991. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC: Arlington, VA, USA, 2006. [Google Scholar]
- Robertson, J.B. The Detergent System of Fiber Analysis. In Topics in Dietary Fiber Research; Spiller, G.A., Ed.; Springer: Boston, MA, USA, 1978; pp. 1–42. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Pappenheim, S.; Yener, S.; Nichols, K.; Dijkstra, J.; Hettinga, K.; van Valenberg, H.J.F. Feeding hydrogenated palm fatty acids and rumen-protected protein to lactating Holstein-Friesian dairy cows modifies milk fat triacylglycerol composition and structure, and solid fat content. J. Dairy Sci. 2022, 105, 2828–2839. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.; Simões, P.; Bexiga, R.; Silva, E.; Mateus, L.; Fernandes, T.; Alves, S.P.; Bessa, R.J.B.; Lopes-da-Costa, L. Effects of feeding rumen-protected linseed fat to postpartum dairy cows on plasma n-3 polyunsaturated fatty acid concentrations and metabolic and reproductive parameters. J. Dairy Sci. 2022, 105, 361–374. [Google Scholar] [CrossRef] [PubMed]
- da Rosa E Silva, P.I.J.L.; Zervoudakis, J.T.; da Silva Cabral, L.; Hatamoto-Zervoudakis, L.K.; da Freiria, L.B.; E Silva, Y.R.V.B.; Paulino, P.V.R.; Tsuneda, P.P.; Possamai, A.J. Effects of rumen-protected oil supplementation on finishing grazing beef cattle. Trop. Anim. Health Prod. 2020, 52, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Zhang, Z.Y.; Zhang, X.L.; Lu, C.M.; Yang, W.Z.; Xie, X.L.; Xin, H.S.; Lu, X.T.; Ni, M.B.; Yang, X.Y.; et al. Effects of dietary Clostridium butyricum and rumen protected fat on meat quality, oxidative stability, and chemical composition of finishing goats. J. Anim. Sci. Biotechnol. 2024, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Ngidi, M.E.; Loerch, S.C.; Fluharty, F.L.; Palmquist, D.L. Effects of calcium soaps of long-chain fatty acids on feedlot performance, carcass characteristics and ruminal metabolism of steers. J. Anim. Sci. 1990, 68, 2555–2565. [Google Scholar] [CrossRef]
- Bhatt, R.S.; Sahoo, A.; Shinde, A.K.; Karim, S.A. Change in body condition and carcass characteristics of cull ewes fed diets supplemented with rumen bypass fat. Livest. Sci. 2013, 157, 132–140. [Google Scholar] [CrossRef]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef]
- Li, Q.; Wang, M.; Zhang, Y.; Li, Y.; Zhang, X.; Li, H.; Peng, Y.; Zhu, C.; Zheng, P.; Yang, S.; et al. Pectin-derived oligogalacturonic acids ameliorate high-fat diet-induced obesity in mice by regulating gut microbiota and inflammation. J. Funct. Foods 2024, 112, 105928. [Google Scholar] [CrossRef]
- Jin, B.; Wang, R.; Hu, J.; Wang, Y.; Cheng, P.; Zhang, J.; Zhang, J.; Xue, G.; Zhu, Y.; Zhang, Y.; et al. Analysis of fecal microbiome and metabolome changes in goats with pregnant toxemia. BMC Vet. Res. 2024, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Tanca, A.; Fraumene, C.; Manghina, V.; Palomba, A.; Abbondio, M.; Deligios, M.; Pagnozzi, D.; Addis, M.F.; Uzzau, S. Diversity and functions of the sheep faecal microbiota: A multi-omic characterization. Microb. Biotechnol. 2017, 10, 541–554. [Google Scholar] [CrossRef]
- Huang, S.; Ji, S.; Wang, F.; Huang, J.; Alugongo, G.M.; Li, S. Dynamic changes of the fecal bacterial community in dairy cows during early lactation. AMB Express 2020, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Ran, T.; Jiao, P.; AlZahal, O.; Xie, X.; Beauchemin, K.A.; Niu, D.; Yang, W. Fecal bacterial community of finishing beef steers fed ruminally protected and non-protected active dried yeast. J. Anim. Sci. 2020, 98, skaa058. [Google Scholar] [CrossRef]
- Liu, J.; Pu, Y.Y.; Xie, Q.; Wang, J.K.; Liu, J.X. Pectin induces an in vitro rumen microbial population shift attributed to the pectinolytic Treponema group. Curr. Microbiol. 2015, 70, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, G.; Mikaelyan, A.; Fukui, C.; Matsuura, Y.; Watanabe, H.; Fujishima, M.; Brune, A. Fiber-associated spirochetes are major agents of hemicellulose degradation in the hindgut of wood-feeding higher termites. Proc. Natl. Acad. Sci. USA 2018, 115, E11996–E12004. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, M.; Brazel, D.M.; Swan, B.K.; Arnosti, C.; Chain, P.S.G.; Reitenga, K.G.; Xie, G.; Poulton, N.J.; Gomez, M.L.; Masland, D.E.D.; et al. Capturing single cell genomes of active polysaccharide degraders: An unexpected contribution of Verrucomicrobia. PLoS ONE 2012, 7, e35314. [Google Scholar] [CrossRef] [PubMed]
- Ransom-Jones, E.; Jones, D.L.; McCarthy, A.J.; McDonald, J.E. The Fibrobacteres: An important phylum of cellulose-degrading bacteria. Microb. Ecol. 2012, 63, 267–281. [Google Scholar] [CrossRef]
- Jiang, S.; Huo, D.; You, Z.; Peng, Q.; Ma, C.; Chang, H.; Lin, X.; Wang, L.; Zhang, J. The distal intestinal microbiome of hybrids of Hainan black goats and Saanen goats. PLoS ONE 2020, 15, e0228496. [Google Scholar] [CrossRef]
- Shabana, I.I.; Albakri, N.N.; Bouqellah, N.A. Metagenomic investigation of faecal microbiota in sheep and goats of the same ages. J. Taibah Univ. Sci. 2021, 15, 1–9. [Google Scholar] [CrossRef]
- Karri, S.; Vadela, M.B.; Gundi, V.A.K.B. Chapter 15—Fiber degradation strategies of bacteria in rumen ecosystem. In Recent Developments in Applied Microbiology and Biochemistry; Viswanath, B., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 153–159. [Google Scholar]
- Liu, H.; Li, Z.; Pei, C.; Degen, A.; Hao, L.; Cao, X.; Liu, H.; Zhou, J.; Long, R. A comparison between yaks and Qaidam cattle in in vitro rumen fermentation, methane emission, and bacterial community composition with poor quality substrate. Anim. Feed Sci. Technol. 2022, 291, 115395. [Google Scholar] [CrossRef]
- Christensen, L.; Sørensen, C.V.; Wøhlk, F.U.; Kjølbæk, L.; Astrup, A.; Sanz, Y.; Hjorth, M.F.; Benítez-Páez, A. Microbial enterotypes beyond genus level: Bacteroides species as a predictive biomarker for weight change upon controlled intervention with arabinoxylan oligosaccharides in overweight subjects. Gut Microbes 2020, 12, 1847627. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, M.F.; Blædel, T.; Bendtsen, L.Q.; Lorenzen, J.K.; Holm, J.B.; Kiilerich, P.; Roager, H.M.; Kristiansen, K.; Larsen, L.H.; Astrup, A. Prevotella-to-Bacteroides ratio predicts body weight and fat loss success on 24-week diets varying in macronutrient composition and dietary fiber: Results from a post-hoc analysis. Int. J. Obes. 2019, 43, 149–157. [Google Scholar] [CrossRef]
- Yi, S.; Dai, D.; Wu, H.; Chai, S.; Liu, S.; Meng, Q.; Zhou, Z. Dietary concentrate-to-forage ratio affects rumen bacterial community composition and metabolome of yaks. Front. Nutr. 2022, 9, 927206. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liang, T.; Zhang, Y.; Huang, K.; Yang, S.; Lv, H.; Chen, Y.; Zhang, C.; Guan, X. Vitexin alleviates high-fat diet induced brain oxidative stress and inflammation via anti-oxidant, anti-inflammatory and gut microbiota modulating properties. Free Radic. Biol. Med. 2021, 171, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Liang, N.; Zhang, X.; Han, C.; Nan, X. Functional differentiation related to decomposing complex carbohydrates of intestinal microbes between two wild zokor species based on 16SrRNA sequences. BMC Vet. Res. 2021, 17, 216. [Google Scholar] [CrossRef]
- Wan, F.; Wang, M.; Zhong, R.; Chen, L.; Han, H.; Liu, L.; Zhao, Y.; Lv, H.; Hou, F.; Yi, B.; et al. Supplementation with chinese medicinal plant extracts from lonicera hypoglauca and scutellaria baicalensis mitigates colonic inflammation by regulating oxidative stress and gut microbiota in a colitis mouse model. Front. Cell. Infect. Microbiol. 2021, 11, 798052. [Google Scholar] [CrossRef]


| Items | Content |
|---|---|
| Ingredients, % of DM | |
| Corn stalk | 50.0 |
| Corn grain (ground) | 15.0 |
| Soybean meal | 13.0 |
| Wheat bran | 5.00 |
| Barley grain | 8.00 |
| Distillers dried grains with solubles | 4.15 |
| Sodium bicarbonate | 1.00 |
| Limestone | 1.20 |
| Calcium hydrogen phosphate | 0.95 |
| Sodium chloride | 0.50 |
| Urea | 0.20 |
| Premix 1 | 1.00 |
| Chemical composition | |
| DM, % | 91.5 |
| CP 2, % | 13.5 |
| NDF, % | 41.0 |
| ADF, % | 23.5 |
| EE, % | 5.00 |
| Calcium, % | 0.76 |
| Phosphorus, % | 0.32 |
| Items | CON | RPF | SEM | p-Values |
|---|---|---|---|---|
| IBW, kg | 13.37 | 13.31 | 0.024 | 0.872 |
| FBW, kg | 16.52 | 16.73 | 0.091 | 0.027 |
| DMI, g/d | 906 | 920 | 20.4 | 0.733 |
| ADG, g/d | 75.1 | 87.5 | 3.78 | 0.012 |
| DMI:ADG | 12.1 | 10.5 | 0.30 | 0.041 |
| Items | CON | RPF | SEM | p-Values |
|---|---|---|---|---|
| Total SCFA, Mm | 14.9 | 13.6 | 0.33 | <0.01 |
| Acetate, Mm | 11.3 | 10.5 | 0.29 | 0.025 |
| Propionate, Mm | 1.84 | 1.65 | 0.078 | 0.036 |
| Butyrate, Mm | 1.25 | 0.83 | 0.087 | <0.01 |
| Iso-VFA, Mm | 0.57 | 0.65 | 0.044 | 0.117 |
| Acetate:Propionate | 6.21 | 6.50 | 0.423 | 0.511 |
| Items | CON | RPF | SEM | p-Values |
|---|---|---|---|---|
| Ace | 1518 | 1442 | 40.1 | 0.512 |
| Chao | 1480 | 1414 | 35.2 | 0.226 |
| Coverage | 0.996 | 0.996 | 0.0001 | 0.999 |
| Shannon | 5.31 | 5.27 | 0.05 | 0.519 |
| Simpson | 0.013 | 0.015 | 0.001 | 0.792 |
| Sobs | 1360 | 1280 | 38.13 | 0.851 |
| Items | CON | RPF | SEM | p-Values |
|---|---|---|---|---|
| Firmicutes | 69.0 | 72.1 | 0.52 | <0.001 |
| Bacteroidota | 22.9 | 22.6 | 0.26 | 0.610 |
| Spirochaetota | 5.80 | 3.32 | 0.411 | <0.001 |
| Verrucomicrobiota | 0.48 | 0.65 | 0.033 | 0.003 |
| Fibrobacterota | 0.57 | 0.41 | 0.031 | 0.003 |
| Others | 1.34 | 0.90 | 0.074 | <0.001 |
| Items | CON | RPF | SEM | p-Values |
|---|---|---|---|---|
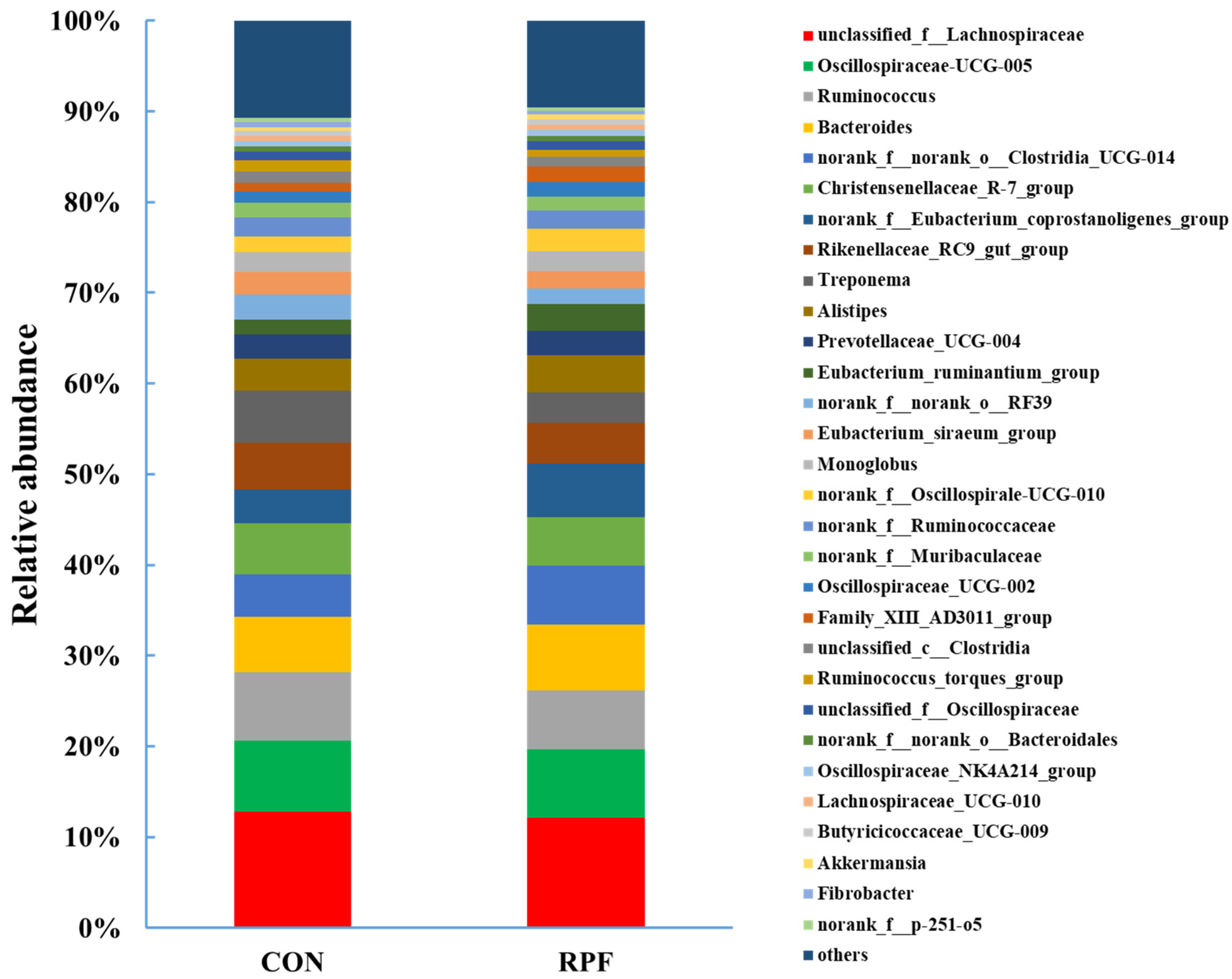
| unclassified_f__Lachnospiraceae | 12.8 | 12.1 | 0.243 | 0.173 |
| Oscillospiraceae-UCG-005 | 7.84 | 7.55 | 0.228 | 0.561 |
| Ruminococcus | 7.52 | 6.46 | 0.211 | <0.01 |
| Bacteroides | 6.12 | 7.27 | 0.240 | <0.01 |
| norank_f__norank_o__Clostridia_UCG-014 | 4.72 | 6.52 | 0.293 | <0.001 |
| Christensenellaceae_R-7_group | 5.55 | 5.33 | 0.100 | 0.301 |
| norank_f__Eubacterium_coprostanoligenes_group | 3.78 | 5.93 | 0.338 | <0.001 |
| Rikenellaceae_RC9_gut_group | 5.11 | 4.54 | 0.142 | 0.033 |
| Treponema | 5.77 | 3.30 | 0.393 | <0.001 |
| Alistipes | 3.56 | 4.09 | 0.215 | 0.233 |
| Prevotellaceae_UCG-004 | 2.66 | 2.67 | 0.099 | 0.956 |
| Eubacterium_ruminantium_group | 1.58 | 3.01 | 0.250 | <0.001 |
| norank_f__norank_o__RF39 | 2.83 | 1.72 | 0.201 | <0.01 |
| Eubacterium_siraeum_group | 2.45 | 1.89 | 0.110 | <0.01 |
| Monoglobus | 2.20 | 2.15 | 0.070 | 0.729 |
| norank_f__Oscillospirale-UCG-010 | 1.74 | 2.57 | 0.155 | 0.001 |
| norank_f__Ruminococcaceae | 2.06 | 1.92 | 0.122 | 0.606 |
| norank_f__Muribaculaceae | 1.66 | 1.57 | 0.129 | 0.758 |
| Oscillospiraceae_UCG-002 | 1.19 | 1.65 | 0.090 | <0.01 |
| Family_XIII_AD3011_group | 1.01 | 1.68 | 0.126 | <0.01 |
| unclassified_c__Clostridia | 1.18 | 1.10 | 0.084 | 0.648 |
| Ruminococcus_torques_group | 1.27 | 0.73 | 0.102 | <0.01 |
| unclassified_f__Oscillospiraceae | 0.97 | 0.94 | 0.030 | 0.618 |
| g__norank_f__norank_o__Bacteroidales | 0.61 | 0.61 | 0.048 | 0.923 |
| Oscillospiraceae_NK4A214_group | 0.58 | 0.62 | 0.052 | 0.662 |
| Lachnospiraceae_UCG-010 | 0.51 | 0.60 | 0.029 | 0.084 |
| Butyricicoccaceae_UCG-009 | 0.57 | 0.53 | 0.043 | 0.597 |
| Akkermansia | 0.42 | 0.56 | 0.051 | 0.199 |
| Fibrobacter | 0.57 | 0.41 | 0.054 | 0.163 |
| norank_f__p-251-o5 | 0.49 | 0.44 | 0.038 | 0.521 |
| others | 10.7 | 9.53 | 0.342 | 0.088 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Peng, W.; Mao, K.; Yang, Y.; Wu, Q.; Wang, K.; Zeng, M.; Han, X.; Han, J.; Zhou, H. The Changes in Fecal Bacterial Communities in Goats Offered Rumen-Protected Fat. Microorganisms 2024, 12, 822. https://doi.org/10.3390/microorganisms12040822
Liu H, Peng W, Mao K, Yang Y, Wu Q, Wang K, Zeng M, Han X, Han J, Zhou H. The Changes in Fecal Bacterial Communities in Goats Offered Rumen-Protected Fat. Microorganisms. 2024; 12(4):822. https://doi.org/10.3390/microorganisms12040822
Chicago/Turabian StyleLiu, Hu, Weishi Peng, Kaiyu Mao, Yuanting Yang, Qun Wu, Ke Wang, Meng Zeng, Xiaotao Han, Jiancheng Han, and Hanlin Zhou. 2024. "The Changes in Fecal Bacterial Communities in Goats Offered Rumen-Protected Fat" Microorganisms 12, no. 4: 822. https://doi.org/10.3390/microorganisms12040822





