Food Supplements to Mitigate Detrimental Effects of Pelvic Radiotherapy
Abstract
:1. Local Radiotherapy as Pelvic Cancer Treatment
2. Gastrointestinal Complications Associated with Pelvic Radiotherapy
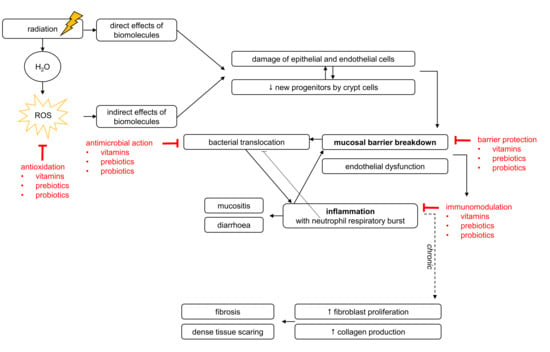
2.1. Mechanisms of Pelvic Radiotherapy-Induced Effects to the Healthy Intestine
2.1.1. Pelvic Radiotherapy-Induced Breakdown of Mucosal Homeostasis
2.1.2. Pelvic Radiotherapy-Induced Inflammation
2.2. Pelvic Radiotherapy-Induced Effects on Gut Microbiota
3. Nutritional Interventions for Pelvic Radiotherapy-Induced Side Effects
3.1. General Improvements in Gut Health
3.2. Preclinical Evidence for Reduction of Radiation-Induced GI Toxicity
3.3. Clinical Evidence for Reduction of Radiation-Induced GI Toxicity
3.4. Mechanisms of Radioprotection
3.4.1. Antimicrobial Capacities
3.4.2. Barrier-Enhancing Capacities
3.4.3. Immunomodulatory Capacities
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Haddock, M.G.; Sloan, J.A.; Bollinger, J.W.; Soori, G.; Steen, P.D.; Martenson, J.A. Patient assessment of bowel function during and after pelvic radiotherapy: Results of a prospective phase III North Central Cancer Treatment Group clinical trial. J. Clin. Oncol. 2007, 25, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Hogan, N.M.; Kerin, M.J.; Joyce, M.R. Gastrointestinal complications of pelvic radiotherapy: Medical and surgical management strategies. Curr. Probl. Surg. 2013, 50, 395–407. [Google Scholar] [CrossRef]
- Andreyev, H.J.N.; Wotherspoon, A.; Denham, J.W.; Hauer-Jensen, M. Defining pelvic-radiation disease for the survivorship era. Lancet Oncol. 2010, 11, 310–312. [Google Scholar] [CrossRef]
- Hauer-Jensen, M.; Denham, J.W.; Andreyev, H.J.N. Radiation enteropathy–pathogenesis, treatment and prevention. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Ahlberg, K.; Ekman, T.; Gaston-Johansson, F. The experience of fatigue, other symptoms and global quality of life during radiotherapy for uterine cancer. Int. J. Nurs. Stud. 2005, 42, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Husebye, E.; Hauer-Jensen, M.; Kjorstad, K.; Skar, V. Severe late radiation enteropathy is characterized by impaired motility of proximal small intestine. Dig. Dis. Sci. 1994, 39, 2341–2349. [Google Scholar] [CrossRef]
- Denham, J.W.; Hauer-Jensen, M. The radiotherapeutic injury—A complex “wound”. Radiother. Oncol. 2002, 63, 129–145. [Google Scholar] [CrossRef]
- Peters, L.J.; Ang, K.K.; Thames, H.D.J. Accelerated fractionation in the radiation treatment of head and neck cancer. A critical comparison of different strategies. Acta Oncol. 1988, 27, 185–194. [Google Scholar] [CrossRef]
- Bourne, R.G.; Kearsley, J.H.; Grove, W.D.; Roberts, S.J. The relationship between early and late gastrointestinal complications of radiation therapy for carcinoma of the cervix. Int. J. Radiat. Oncol. Biol. Phys. 1983, 9, 1445–1450. [Google Scholar] [CrossRef]
- Dörr, W.; Hendry, J.H. Consequential late effects in normal tissues. Radiother. Oncol. 2001, 61, 223–231. [Google Scholar] [CrossRef]
- François, A.; Milliat, F.; Guipaud, O.; Benderitter, M. Inflammation and immunity in radiation damage to the gut mucosa. BioMed Res. Int. 2013, 2013, 123241. [Google Scholar] [CrossRef] [PubMed]
- Simons, B.D.; Clevers, H. Stem cell self-renewal in intestinal crypt. Exp. Cell Res. 2011, 317, 2719–2724. [Google Scholar] [CrossRef] [PubMed]
- Darwich, A.S.; Aslam, U.; Ashcroft, D.M.; Rostami-Hodjegan, A. Meta-analysis of the turnover of intestinal epithelia in preclinical animal species and humans. Drug Metab. Dispos. 2014, 42, 2016–2022. [Google Scholar] [CrossRef]
- Umar, S. Intestinal stem cells. Curr. Gastroenterol. Rep. 2010, 12, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Bjerknes, M.; Cheng, H. The stem-cell zone of the small intestinal epithelium. I. Evidence from Paneth cells in the adult mouse. Am. J. Anat. 1981, 160, 51–63. [Google Scholar] [CrossRef]
- Banerjeea, S.; Aykin-Burnsa, N.; Kragera, K.J.; Shaha, S.K.; Melnykb, S.; Hauer-Jensena, M.; Pawar, S.A. Loss of C/EBPδ enhances IR-induced cell death by promoting oxidative stress and mitochondrial dysfunction. Free Radic. Biol. Med. 2016, 99, 296–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikkelsen, R.B.; Wardman, P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene 2003, 22, 5734–5754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desouky, O.; Ding, N.; Zhou, G. Targeted and non-targeted effects of ionizing radiation. J. Radiat. Res. Appl. Sci. 2015, 8, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.K.; Newman, V.L.; Romaine, P.L.; Hauer-Jensen, M.; Pollard, H.B. Use of biomarkers for assessing radiation injury and efficacy of countermeasures. Expert Rev. Mol. Diagn. 2016, 16, 65–81. [Google Scholar] [CrossRef]
- Yan, K.; Chia, L.; Li, X.; Ootani, A.; Su, J.; Lee, J.; Su, N.; Luo, Y.; Heilshorn, S.; Amieva, M.; et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc. Natl. Acad. Sci. USA 2012, 109, 466–471. [Google Scholar] [CrossRef]
- Schepers, A.G.; Vries, R.; Van Den Born, M.; Van De Wetering, M.; Clevers, H. Lgr5 intestinal stem cells have high telomerase activity and randomly segregate their chromosomes. EMBO J. 2011, 30, 1104–1109. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Yang, V.W.; Bialkowska, A.B. The Role of Intestinal Stem Cells in Epithelial Regeneration Following Radiation-Induced Gut Injury. Curr. Stem Cell Rep. 2017, 3, 320–332. [Google Scholar] [CrossRef] [Green Version]
- Barker, N. Adult intestinal stem cells: Critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 2013, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Booth, C.; Tudor, G.; Tudor, J.; Katz, B.P.; MacVittie, T.J. Acute gastrointestinal syndrome in high-dose irradiated mice. Health Phys. 2012, 103, 383–399. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.K.; Gangwar, R.; Manda, B.; Meena, A.S.; Yadav, N.; Szabo, E.; Balogh, A.; Lee, S.C.; Tigyi, G.; Rao, R. Rapid disruption of intestinal epithelial tight junction and barrier dysfunction by ionizing radiation in mouse colon in vivo: Protection by N-acetyl-l-cysteine. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G705–G715. [Google Scholar] [CrossRef]
- Wang, J.; Boerma, M.; Fu, Q.; Hauer-Jensen, M. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J. Gastroenterol. 2007, 13, 3047–3055. [Google Scholar] [CrossRef] [PubMed]
- Mollà, M.; Gironella, M.; Miquel, R.; Tovar, V.; Engel, P.; Biete, A.; Piqué, J.M.; Panés, J. Relative roles of ICAM-1 and VCAM-1 in the pathogenesis of experimental radiation-induced intestinal inflammation. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 264–273. [Google Scholar] [CrossRef]
- Mollà, M.; Panés, J. Radiation-induced intestinal inflammation. World J. Gastroenterol. 2007, 13, 3043–3046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, N.; Luster, A. The role of tissue resident cells in neutrophil recruitment. Trends Immunol. 2015, 36, 547–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Llorente, C.; Muñoz, S.; Gil, A. Role of Toll-like receptors in the development of immunotolerance mediated by probiotics. Proc. Nutr. Soc. 2010, 69, 381–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Wang, Z.D.; Chen, M.S.; Zhang, X.Q.; Shen, L.P.; Zhang, J.X.; Chen, Y. Activation of Toll-like receptors by intestinal microflora reduces radiation-induced DNA damage in mice. Mutat. Res. Genet. Toxicol. Environ. Mutagen 2014, 774, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Flament, C.; Rusakiewicz, S.; Routy, B.; Maria, P.; Duong, C.P.M.; Poirier-colame, V.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2016, 350, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Paul, W.E. Heterogeneity and plasticity of T helper cells. Cell Res. 2010, 20, 4–12. [Google Scholar] [CrossRef]
- Schaue, D.; McBride, W.H. T lymphocytes and normal tissue responses to radiation. Front. Oncol. 2012, 2, 119. [Google Scholar] [CrossRef]
- Zoetendal, E.G.; Akkermans, A.D.; De Vos, W.M. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl. Environ. Microbiol. 1998, 64, 3854–3859. [Google Scholar] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Harmsen, H.J.M.; Raangs, G.C.; He, T.; Degener, J.E.; Welling, G.W. Extensive set of 16S rRNA-based probes for detection of bacteria in human feces. Appl. Environ. Microbiol. 2002, 68, 2982–2990. [Google Scholar] [CrossRef]
- Vyas, U.; Ranganathan, N. Probiotics, prebiotics, and synbiotics: Gut and beyond. Gastroenterol. Res. Pract. 2012, 2012, 872716. [Google Scholar] [CrossRef]
- Heiss, C.N.; Olofsson, L.E. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J. Neuroendocrinol. 2019, e12684. [Google Scholar] [CrossRef]
- Mowat, A.M. To respond or not to respond—A personal perspective of intestinal tolerance. Nat. Rev. Immunol. 2018, 18, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Tordesillas, L.; Berin, M.C. Mechanisms of Oral Tolerance. Clin. Rev. Allergy Immunol. 2018, 55, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Bengmark, S. Ecological control of the gastrointestinal tract. The role of probiotic flora. Gut 1998, 42, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanderhoof, J.A.; Young, R.J. Use of probiotics in childhood gastrointestinal disorders. J. Pediatr. Gastroenterol. Nutr. 1998, 27, 323–332. [Google Scholar] [CrossRef]
- Holzapfel, W.H.; Haberer, P.; Snel, J.; Schillinger, U.; Huis in’t Veld, J.H. Overview of gut flora and probiotics. Int. J. Food Microbiol. 1998, 41, 85–101. [Google Scholar] [CrossRef]
- Vandenbergh, P. Lactic acid bacteria, their metabolic prodcuts and interference with microbial growth. FEMS Microbiol. Rev. 1993, 12, 221–238. [Google Scholar] [CrossRef]
- Davidson, J.N.; Hirsh, D.C. Bacterial competition as a means of preventing neonatal diarrhea in pigs. Infect. Immun. 1976, 13, 1773–1774. [Google Scholar] [PubMed]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current understanding of dysbiosis in disease in human and animal models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [Green Version]
- Ghavami, S.B.; Rostami, E.; Sephay, A.A.; Shahrokh, S.; Balaii, H.; Aghdaei, H.A.; Zali, M.R. Alterations of the human gut Methanobrevibacter smithii as a biomarker for inflammatory bowel diseases. Microb. Pathog. 2018, 117, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.M.; Pereira-Marques, J.; Pinto-Ribeiro, I.; Costa, J.L.; Carneiro, F.; Machado, J.C.; Figueiredo, C. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 2018, 67, 226–236. [Google Scholar] [CrossRef]
- Wang, A.; Ling, Z.; Yang, Z.; Kiela, P.R.; Wang, T.; Wang, C.; Cao, L.; Geng, F.; Shen, M.; Ran, X.; et al. Gut Microbial Dysbiosis May Predict Diarrhea and Fatigue in Patients Undergoing Pelvic Cancer Radiotherapy: A Pilot Study. PLoS ONE 2015, 10, e0126312. [Google Scholar] [CrossRef] [PubMed]
- Gerassy-Vainberg, S.; Blatt, A.; Danin-Poleg, Y.; Gershovich, K.; Sabo, E.; Nevelsky, A.; Daniel, S.; Dahan, A.; Ziv, O.; Dheer, R.; et al. Radiation induces proinflammatory dysbiosis: Transmission of inflammatory susceptibility by host cytokine induction. Gut 2018, 67, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Crawford, P.A.; Gordon, J.I. Microbial regulation of intestinal radiosensitivity. Proc. Natl. Acad. Sci. USA 2005, 102, 13254–13259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, B.R. Survival studies of whole-body X-irradiated germfree (axenic) mice. Radiat. Res. 1963, 20, 477–483. [Google Scholar] [CrossRef] [PubMed]
- McLauglin, M.M.; Dacquisto, M.P.; Jacobus, D.P.; Horowitz, R.E. Effects of the germfree state on responses of mice to whole-body irradiation. Radiat. Res. 1964, 23, 333–349. [Google Scholar] [CrossRef]
- Jervis, H.R.; McLaughlin, M.M.; Johnson, M.C. Effect of neutron-gamma radiation on the morphology of the mucosa of the small intestine of germfree and conventional mice. Radiat. Res. 1971, 45, 613–628. [Google Scholar] [CrossRef]
- Manichanh, C.; Varela, E.; Martinez, C.; Antolin, M.; Llopis, M.; Dore, J.; Giralt, J.; Guarner, F.; Malagelada, J.-R. The gut microbiota predispose to the pathophysiology of acute postradiotherapy diarrhea. Am. J. Gastroenterol. 2008, 103, 1754–1761. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.; Park, S.-J. High-throughput 16S rRNA gene sequencing reveals alterations of mouse intestinal microbiota after radiotherapy. Anaerobe 2015, 33, 1–7. [Google Scholar] [CrossRef]
- Nam, Y.-D.; Kim, H.J.; Seo, J.-G.; Kang, S.W.; Bae, J.-W. Impact of Pelvic Radiotherapy on Gut Microbiota of Gynecological Cancer Patients Revealed by Massive Pyrosequencing. PLoS ONE 2013, 8, e82659. [Google Scholar] [CrossRef] [PubMed]
- Touchefeu, Y.; Montassier, E.; Nieman, K.; Gastinne, T.; Potel, G.; Bruley Des Varannes, S.; Le Vacon, F.; De La Cochetière, M.F. Systematic review: The role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis—Current evidence and potential clinical applications. Aliment. Pharmacol. Ther. 2014, 40, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.; Unnikrishnan, M.K.; Nagappa, A.N. Phytochemicals as radioprotective agents—A Review. Indian J. Nat. Prod. Resour. 2011, 2, 137–150. [Google Scholar]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef]
- Bailey, D.M.; Davies, B. Acute mountain sickness; prophylactic benefits of antioxidant vitamin supplementation at high altitude. High Alt. Med. Biol. 2001, 2, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.M.; Bartsch, P.; Knauth, M.; Baumgartner, R.W. Emerging concepts in acute mountain sickness and high-altitude cerebral edema: From the molecular to the morphological. Cell Mol. Life Sci. 2009, 66, 3583–3594. [Google Scholar] [CrossRef]
- Xu, C.; Sun, R.; Qiao, X.; Xu, C.; Shang, X.; Niu, W.; Chao, Y. Effect of Vitamin E Supplementation on Intestinal Barrier Function in Rats Exposed to High Altitude Hypoxia Environment. Korean J. Physiol. Pharmacol. 2014, 18, 313. [Google Scholar] [CrossRef] [PubMed]
- Czarnewski, P.; Das, S.; Parigi, S.M.; Villablanca, E.J. Retinoic acid and its role in modulating intestinal innate immunity. Nutrients 2017, 9, 68. [Google Scholar] [CrossRef]
- Yousefi, B.; Azizzadeh, F. The histopathalogical effects of retinoic acid on the tissues. Pakistan J. Biol. Sci. 2010, 13, 927–936. [Google Scholar] [CrossRef]
- De Medeiros, P.H.Q.S.; Pinto, D.V.; de Almeida, J.Z.; Rêgo, J.M.C.; Rodrigues, F.A.P.; Lima, A.Â.M.; Bolick, D.T.; Guerrant, R.L.; Oriá, R.B. Modulation of intestinal immune and barrier functions by vitamin A: Implications for current understanding of malnutrition and enteric infections in children. Nutrients 2018, 10, 1128. [Google Scholar] [CrossRef] [PubMed]
- Tielsch, J.M.; Rahmathullah, L.; Thulasiraj, R.D.; Katz, J.; Coles, C.; Sheeladevi, S.; John, R.; Prakash, K. Newborn vitamin A dosing reduces the case fatality but not incidence of common childhood morbidities in South India. J. Nutr. 2007, 137, 2470–2474. [Google Scholar] [CrossRef]
- Lester Packer, J.F. Vitamin C in Health and Disease; CRC Press: New York, NY, USA, 1997; pp. 28–30. [Google Scholar]
- Fischer, N.; Seo, E.J.; Efferth, T. Prevention from radiation damage by natural products. Phytomedicine 2018, 47, 192–200. [Google Scholar] [CrossRef]
- Mohammed, B.M.; Fisher, B.J.; Kraskauskas, D.; Farkas, D.; Brophy, D.F.; Fowler, A.A.; Natarajan, R. Vitamin C: A novel regulator of neutrophil extracellular trap formation. Nutrients 2013, 5, 3131–3151. [Google Scholar] [CrossRef]
- Danielski, L.G.; Walczewski, E.; de Jesus, C.R.; Florentino, D.; Giustina ADella Goldim, M.P.; Kanis, L.A.; Pereira, G.W.; Pereira, V.D.; Felisberto, F.; et al. Preoperative vitamin C supplementation improves colorectal anastomotic healing and biochemical parameters in malnourished rats. Int. J. Colorectal. Dis. 2016, 31, 1759–1766. [Google Scholar] [CrossRef]
- Rall, L.C.; Meydani, S.N. Vitamin B6 and immune competence. Nutr. Rev. 1993, 51, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Al-Numair, K.S.; Waly, M.I.; Ali, A.; Essa, M.M.; Farhat, M.F.; Alsaif, M.A. Dietary folate protects against azoxymethane-induced aberrant crypt foci development and oxidative stress in rat colon. Exp. Biol. Med. 2011, 236, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, A.J.; Behan, N.A.; Matias, F.M.G.; Green, J.; Caldwell, D.; Brooks, S.P.J. Dietary folate does not significantly affect the intestinal microbiome, inflammation or tumorigenesis in azoxymethane-dextran sodium sulphate-treated mice. Br. J. Nutr. 2013, 109, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Gaïa, N.; Johansson, M.; Ståhlman, M.; Backhed, F.; Delzenne, N.M.; Schrenzel, J.; François, P.; Cani, P.D. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J. 2014, 8, 2116–2130. [Google Scholar] [CrossRef]
- Paul, H.A.; Bomhof, M.R.; Vogel, H.J.; Reimer, R.A. Diet-induced changes in maternal gut microbiota and metabolomic profiles influence programming of offspring obesity risk in rats. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dewulf, E.M.; Cani, P.D.; Claus, S.P.; Fuentes, S.; Puylaert, P.G.B.; Neyrinck, A.M.; Bindels, L.B.; De Vos, W.M.; Gibson, G.R.; Thissen, J.P.; et al. Insight into the prebiotic concept: Lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 2013, 62, 1112–1121. [Google Scholar] [CrossRef]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Wang, J.; Sailer, M.; Theis, S.; Verbeke, K.; Raes, J. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut 2017, 66, 1968–1974. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Lu, X.; Yang, X. Stachyose-enriched alpha-galacto-oligosaccharides regulate gut microbiota and relieve constipation in mice. J. Agric. Food Chem. 2013, 61, 11825–11831. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lu, X.; Yang, X. Evaluation of clinical safety and beneficial effects of stachyose-enriched α-galacto-oligosaccharides on gut microbiota and bowel function in humans. Food Funct. 2017, 8, 262–269. [Google Scholar] [CrossRef]
- CJagetia, G. Radioprotective Potential of Plants and Herbs against the Effects of Ionizing Radiation. J. Clin. Biochem. Nutr. 2007, 40, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Selma, M.V.; Espin, J.C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef]
- Anhe, F.F.; Varin, T.V.; Le Barz, M.; Desjardins, Y.; Levy, E.; Roy, D.; Marette, A. Gut Microbiota Dysbiosis in Obesity-Linked Metabolic Diseases and Prebiotic Potential of Polyphenol-Rich Extracts. Curr. Obes. Rep. 2015, 4, 389–400. [Google Scholar] [CrossRef]
- Selma, M.V.; Espin, J.C.; Tomas-Barberan, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P.E. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, X.; Cao, S.; Wang, L.; Wang, D.; Yang, H.; Feng, Y.; Wang, S.; Li, L. Caffeic acid ameliorates colitis in association with increased Akkermansia population in the gut microbiota of mice. Oncotarget 2016, 7, 31790–31799. [Google Scholar] [CrossRef]
- Song, H.; Chu, Q.; Yan, F.; Yang, Y.; Han, W.; Zheng, X. Red pitaya betacyanins protects from diet-induced obesity, liver steatosis and insulin resistance in association with modulation of gut microbiota in mice. J. Gastroenterol. Hepatol. 2016, 31, 1462–1469. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Sanchez-Alcoholado, L.; Perez-Martinez, P.; Andres-Lacueva, C.; Cardona, F.; Tinahones, F.; Queipo-Ortuno, M.I. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct. 2016, 7, 1775–1787. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Kaila, M.; Isolauri, E.; Soppi, E.; Virtanen, E.; Laine, S.; Arvilommi, H. Enhancement of the circulating antibody secreting cell response in human diarrhea by a human Lactobacillus strain. Pediatr. Res. 1992, 32, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Kaila, M.; Isolauri, E.; Saxelin, M.; Arvilommi, H.; Vesikari, T. Viable versus inactivated Lactobacillus strain GG in acute rotavirus diarrhoea. Arch. Dis. Child. 1995, 72, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Guarino, A.; Guandalini, S.; Lo Vecchio, A. Probiotics for Prevention and Treatment of Diarrhea. J. Clin. Gastroenterol. 2015, 49 (Suppl. 1), S37–S45. [Google Scholar] [CrossRef]
- Conway, P.L.; Gorbach, S.L.; Goldin, B.R. Survival of lactic acid bacteria in the human stomach and adhesion to intestinal cells. J. Dairy Sci. 1987, 70, 1–12. [Google Scholar] [CrossRef]
- Yan, F. Probiotic Bacterium Prevents Cytokine-induced Apoptosis in Intestinal Epithelial Cells. J. Biol. Chem. 2002, 277, 50959–50965. [Google Scholar] [CrossRef]
- Johnson-Henry, K.C.; Donato, K.A.; Shen-Tu, G.; Gordanpour, M.; Sherman, P.M. Lactobacillus rhamnosus Strain GG Prevents Enterohemorrhagic Escherichia coli O157:H7-Induced Changes in Epithelial Barrier Function. Infect. Immun. 2008, 76, 1340–1348. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, C.; Dong, Y.; Zhang, M.; Wang, Y.; Li, F.; Li, X.; McClain, C.; Yang, S.; Feng, W. Inhibition of miR122a by Lactobacillus rhamnosus GG culture supernatant increases intestinal occludin expression and protects mice from alcoholic liver disease. Toxicol. Lett. 2015, 234, 194–200. [Google Scholar] [CrossRef]
- Orlando, A.; Linsalata, M.; Notarnicola, M.; Tutino, V.; Russo, F. Lactobacillus GG restoration of the gliadin induced epithelial barrier disruption: The role of cellular polyamines. BMC Microbiol. 2014, 14, 19. [Google Scholar] [CrossRef]
- Kumar, A.; Wu, H.; Collier-Hyams, L.S.; Hansen, J.M.; Li, T.; Yamoah, K.; Pan, Z.-Q.; Jones, D.P.; Neish, A.S. Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. EMBO J. 2007, 26, 4457–4466. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.W.; Myers, L.E.S.; Ray, L.; Song, S.-C.; Nasr, T.R.; Berardinelli, A.J.; Kundu, K.; Murthy, N.; Hansen, J.M.; Neish, A.S. Lactobacillus rhamnosus blocks inflammatory signaling in vivo via reactive oxygen species generation. Free Radic. Biol. Med. 2009, 47, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Vong, L.; Lorentz, R.J.; Assa, A.; Glogauer, M.; Sherman, P.M. Probiotic Lactobacillus rhamnosus inhibits the formation of neutrophil extracellular traps. J. Immunol. 2014, 192, 1870–1877. [Google Scholar] [CrossRef]
- Pieścik-Lech, M.; Urbańska, M.; Szajewska, H. Lactobacillus GG (LGG) and smectite versus LGG alone for acute gastroenteritis: A double-blind, randomized controlled trial. Eur. J. Pediatr. 2013, 172, 247–253. [Google Scholar] [CrossRef]
- Pant, A.R.; Graham, S.M.; Allen, S.J.; Harikul, S.; Sabchareon, A.; Cuevas, L.; Hart, C.A. Lactobacillus GG and acute diarrhoea in young children in the tropics. J. Trop. Pediatr. 1996, 42, 162–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raza, S.; Graham, S.M.; Allen, S.J.; Sultana, S.; Cuevas, L.; Hart, C.A. Lactobacillus GG promotes recovery from acute nonbloody diarrhea in Pakistan. Pediatr. Infect. Dis. J. 1995, 14, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Shornikova, A.V.; Isolauri, E.; Burkanova, L.; Lukovnikova, S.; Vesikari, T. A trial in the Karelian Republic of oral rehydration and Lactobacillus GG for treatment of acute diarrhoea. Acta Paediatr. 1997, 86, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Makizaki, Y.; Oikawa, Y.; Tanaka, Y.; Maeda, A.; Shimakawa, M.; Komoto, S.; Moriguchi, K.; Ohno, H.; Taniguchi, K. Oral administration of Bifidobacterium bifidum G9-1 alleviates rotavirus gastroenteritis through regulation of intestinal homeostasis by inducing mucosal protective factors. PLoS ONE 2017, 12, e0173979. [Google Scholar] [CrossRef]
- Karczewski, J.; Troost, F.J.; Konings, I.; Dekker, J.; Kleerebezem, M.; Brummer, R.-J.M.; Wells, J.M. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G851–G859. [Google Scholar] [CrossRef]
- Yang, F.; Wang, A.; Zeng, X.; Hou, C.; Liu, H.; Qiao, S. Lactobacillus reuteri I5007 modulates tight junction protein expression in IPEC-J2 cells with LPS stimulation and in newborn piglets under normal conditions. BMC Microbiol. 2015, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Tien, M.-T.; Girardin, S.E.; Regnault, B.; Le Bourhis, L.; Dillies, M.-A.; Coppee, J.-Y.; Bourdet-Sicard, R.; Sansonetti, P.J.; Pedron, T. Anti-inflammatory effect of Lactobacillus casei on Shigella-infected human intestinal epithelial cells. J. Immunol. 2006, 176, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Eun, C.S.; Kim, Y.S.; Han, D.S.; Choi, J.H.; Lee, A.R.; Park, Y.K. Lactobacillus casei prevents impaired barrier function in intestinal epithelial cells. APMIS 2011, 119, 49–56. [Google Scholar] [CrossRef]
- Toumi, R.; Abdelouhab, K.; Rafa, H.; Soufli, I.; Raissi-Kerboua, D.; Djeraba, Z.; Touil-Boukoffa, C. Beneficial role of the probiotic mixture Ultrabiotique on maintaining the integrity of intestinal mucosal barrier in DSS-induced experimental colitis. Immunopharmacol. Immunotoxicol. 2013, 35, 403–409. [Google Scholar] [CrossRef]
- Park, J.-S.; Choi, J.; Jhun, J.; Kwon, J.Y.; Lee, B.-I.; Yang, C.W.; Park, S.-H.; Cho, M.-L. Lactobacillus acidophilus Improves Intestinal Inflammation in an Acute Colitis Mouse Model by Regulation of Th17 and Treg Cell Balance and Fibrosis Development. J. Med. Food 2018, 21, 215–224. [Google Scholar] [CrossRef]
- Pearce, J.L.; Hamilton, J.R. Controlled trial of orally administered Lactobacilli in acute infantile diarrhea. J. Pediatr. 1974, 84, 261–262. [Google Scholar] [CrossRef]
- Haton, C.; Francois, A.; Vandamme, M.; Wysocki, J.; Griffiths, N.M.; Benderitter, M. Imbalance of the antioxidant network of mouse small intestinal mucosa after radiation exposure. Radiat. Res. 2007, 167, 445–453. [Google Scholar] [CrossRef]
- Felemovicius, I.; Bonsack, M.E.; Baptista, M.L.; Delaney, J.P. Intestinal radioprotection by vitamin E (alpha-tocopherol). Ann. Surg. 1995, 222, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Srinivasan, V.; Toles, R.; Jobe, L.; Seed, T.M. Nutritional approaches to radioprotection: Vitamin E. Mil. Med. 2002, 167 (Suppl. 2), 57–59. [Google Scholar] [PubMed]
- Singh, P.K.; Wise, S.Y.; Ducey, E.J.; Fatanmi, O.O.; Elliott, T.B.; Singh, V.K. alpha-Tocopherol succinate protects mice against radiation-induced gastrointestinal injury. Radiat. Res. 2012, 177, 133–145. [Google Scholar] [CrossRef]
- Brown, S.L.; Kolozsvary, A.; Liu, J.; Jenrow, K.A.; Ryu, S.; Kim, J.H. Antioxidant diet supplementation starting 24 hours after exposure reduces radiation lethality. Radiat. Res. 2010, 173, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Thotala, D.; Chetyrkin, S.; Hudson, B.; Hallahan, D.; Voziyan, P.; Yazlovitskaya, E. Pyridoxamine protects intestinal epithelium from ionizing radiation-induced apoptosis. Free Radic. Biol. Med. 2009, 47, 779–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roche, M.; Neti, P.V.S.V.; Kemp, F.W.; Agrawal, A.; Attanasio, A.; Douard, V.; Muduli, A.; Azzam, E.I.; Norkus, E.; Brimacombe, M.; et al. Radiation-induced reductions in transporter mRNA levels parallel reductions in intestinal sugar transport. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R173–R182. [Google Scholar] [CrossRef]
- Roche, M.; Kemp, F.W.; Agrawal, A.; Attanasio, A.; Neti, P.V.S.V.; Howell, R.W.; Ferraris, R.P. Marked changes in endogenous antioxidant expression precede vitamin A, C and E-protectable, radiation-induced reductions in small intestinal nutrient transport. Free Radic. Biol. Med. 2011, 50, 55–65. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kinoshita, M.; Shinomiya, N.; Hiroi, S.; Sugasawa, H.; Matsushita, Y.; Majima, T.; Saitoh, D.; Seki, S. Pretreatment with Ascorbic Acid Prevents Lethal Gastrointestinal Syndrome in Mice Receiving a Massive Amount of Radiation. J. Radiat. Res. 2010, 51, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Kinoshita, M.; Yamamoto, T.; Ito, M.; Nishida, T.; Takeuchi, M.; Saitoh, D.; Seki, S.; Mukai, Y. Treatment of irradiated mice with high-dose ascorbic acid reduced lethality. PLoS ONE 2015, 10, e0117020. [Google Scholar] [CrossRef] [PubMed]
- Adaramoye, O.; Ogungbenro, B.; Anyaegbu, O.; Fafunso, M. Protective effects of extracts of Vernonia amygdalina, Hibiscus sabdariffa and vitamin C against radiation-induced liver damage in rats. J. Radiat. Res. 2008, 49, 123–131. [Google Scholar] [CrossRef]
- Shimoi, K.; Masuda, S.; Furugori, M.; Esaki, S.; Kinae, N. Radioprotective effect of antioxidative flavonoids in gamma-ray irradiated mice. Carcinogenesis 1994, 15, 2669–2672. [Google Scholar] [CrossRef] [PubMed]
- Shimoi, K.; Masuda, S.; Shen, B.; Furugori, M.; Kinae, N. Radioprotective effects of antioxidative plant flavonoids in mice. Mutat. Res. 1996, 350, 153–161. [Google Scholar] [CrossRef]
- Benkovic, V.; Horvat Knezevic, A.; Dikic, D.; Lisicic, D.; Orsolic, N.; Basic, I.; Kosalec, I.; Kopjar, N. Radioprotective effects of propolis and quercetin in γ-irradiated mice evaluated by the alkaline comet assay. Phytomedicine 2008, 15, 851–858. [Google Scholar] [CrossRef]
- Orsolić, N.; Benković, V.; Horvat-Knezević, A.; Kopjar, N.; Kosalec, I.; Bakmaz, M.; Mihaljević, Z.; Bendelja, K.; Basić, I. Assessment by survival analysis of the radioprotective properties of propolis and its polyphenolic compounds. Biol. Pharm. Bull. 2007, 30, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Van Hijum, S.A.F.T.; van Geel-Schutten, G.H.; Rahaoui, H.; van der Maarel, M.J.E.C.; Dijkhuizen, L. Characterization of a novel fructosyltransferase from Lactobacillus reuteri that synthesizes high-molecular-weight inulin and inulin oligosaccharides. Appl. Environ. Microbiol. 2002, 68, 4390–4398. [Google Scholar] [CrossRef]
- Haak, B.W.; Littmann, E.R.; Chaubard, J.-L.; Pickard, A.J.; Fontana, E.; Adhi, F.; Gyaltshen, Y.; Ling, L.; Morjaria, S.M.; Peled, J.U.; et al. Impact of gut colonization with butyrate-producing microbiota on respiratory viral infection following allo-HCT. Blood 2018, 131, 2978–2986. [Google Scholar] [CrossRef] [PubMed]
- Qishen, P.; Guo, B.J.; Kolman, A. Radioprotective effect of extract from Spirulina platensis in mouse bone marrow cells studied by using the micronucleus test. Toxicol. Lett. 1989, 48, 165–169. [Google Scholar] [CrossRef]
- Sanders, M.E.; Benson, A.; Lebeer, S.; Merenstein, D.J.; Klaenhammer, T.R. Shared mechanisms among probiotic taxa: Implications for general probiotic claims. Curr. Opin. Biotechnol. 2018, 49, 207–216. [Google Scholar] [CrossRef]
- Seal, M.; Naito, Y.; Barreto, R.; Lorenzetti, A.; Safran, P.; Marotta, F. Experimental radiotherapy-induced enteritis: A probiotic interventional study. J. Dig. Dis. 2007, 8, 143–147. [Google Scholar] [CrossRef]
- Ki, Y.; Kim, W.; Cho, H.; Ahn, K.; Choi, Y.; Kim, D. The effect of probiotics for preventing radiation-induced morphological changes in intestinal mucosa of rats. J. Korean Med. Sci. 2014, 29, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Ciorba, M.A.; Riehl, T.E.; Rao, M.S.; Moon, C.; Ee, X.; Nava, G.M.; Walker, M.R.; Marinshaw, J.M.; Stappenbeck, T.S.; Stenson, W.F. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut 2012, 61, 829–838. [Google Scholar] [CrossRef]
- Riehl, T.E.; Alvarado, D.; Ee, X.; Zuckerman, A.; Foster, L.; Kapoor, V.; Thotala, D.; Ciorba, M.A.; Stenson, W. Lactobacillus rhamnosus GG protects the intestinal epithelium from radiation injury through release of lipoteichoic acid, macrophage activation and the migration of mesenchymal stem cells. Gut 2018. [Google Scholar] [CrossRef]
- Demirer, S.; Aydintug, S.; Aslim, B.; Kepenekci, I.; Sengül, N.; Evirgen, O.; Gerceker, D.; Andrieu, M.N.; Ulusoy, C.; Karahüseyinoglu, S. Effects of probiotics on radiation-induced intestinal injury in rats. Nutrition 2006, 22, 179–186. [Google Scholar] [CrossRef]
- Demirer, S.; Ulusu, N.N.; Aslim, B.; Kepenekci, I.; Ulusoy, C.; Andrieu, M.N.; Erkek, B.; Aydintug, S. Protective effects of Lactobacillus delbrueckii subsp bulgaricus B3 on intestinal enzyme activities after abdominal irradiation in rats. Nutr. Res. 2007, 27, 300–305. [Google Scholar] [CrossRef]
- Liu, Q.; Nobaek, S.; Adawi, D.; Mao, Y.; Wang, M.; Molin, G.; Ekelund, M.; Jeppsson, B. Administration of Lactobacillus plantarum 299v reduces side-effects of external radiation on colon anastomotic healing in an experimental model. Colorectal Dis. 2001, 3, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.; Bruninga, K.; Mutlu, E.A.; Losurdo, J.; Choudhary, S.; Keshavarzian, A. Successful and sustained treatment of chronic radiation proctitis with antioxidant vitamins E and C. Am. J. Gastroenterol. 2001, 96, 1080. [Google Scholar] [CrossRef]
- García-Peris, P.; Velasco, C.; Lozano, M.A.; Moreno, Y.; Paron, L.; de la Cuerda, C.; Bretón, I.; Camblor, M.; García-Hernández, J.; Guarner, F.; et al. Effect of a mixture of inulin and fructo-oligosaccharide on Lactobacillus and Bifidobacterium intestinal microbiota of patients receiving radiotherapy: A randomised, double-blind, placebo-controlled trial. Nutr. Hosp. 2012, 27, 1908–1915. [Google Scholar]
- Liu, Z.; Zhang, Z.; Qiu, L.; Zhang, F.; Xu, X.; Wei, H.; Tao, X. Characterization and bioactivities of the exopolysaccharide from a probiotic strain of Lactobacillus plantarum WLPL04. J. Dairy Sci 2017, 100, 6895–6905. [Google Scholar] [CrossRef]
- Fuccio, L.; Frazzoni, L.; Guido, A. Prevention of pelvic radiation disease. World J. Gastrointest. Pharmacol. Ther. 2015, 6, 1–9. [Google Scholar] [CrossRef]
- Delia, P.; Sansotta, G.; Donato, V.; Messina, G.; Frosina, P.; Pergolizzi, S.; De Renzis, C. Prophylaxis of diarrhoea in patients submitted to radiotherapeutic treatment on pelvic district: Personal experience. Dig. Liver Dis. 2002, 34 (Suppl. 2), S84–S86. [Google Scholar] [CrossRef]
- Delia, P.; Sansotta, G.; Donato, V.; Frosina, P.; Messina, G.; De Renzis, C.; Famularo, G. Use of probiotics for prevention of radiation-induced diarrhea. World J. Gastroenterol. 2007, 13, 912–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salminen, E.; Elomaa, I.; Minkkinen, J.; Vapaatalo, H.; Salminen, S. Preservation of intestinal integrity during radiotherapy using live Lactobacillus acidophilus cultures. Clin. Radiol. 1988, 39, 435–437. [Google Scholar] [CrossRef]
- Urbancsek, H.; Kazar, T.; Mezes, I.; Neumann, K. Results of a double-blind, randomized study to evaluate the efficacy and safety of Antibiophilus in patients with radiation-induced diarrhoea. Eur. J. Gastroenterol. Hepatol. 2001, 13, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Chitapanarux, I.; Chitapanarux, T.; Traisathit, P.; Kudumpee, S.; Tharavichitkul, E. Randomized controlled trial of live lactobacillus acidophilus plus bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients. Radiat. Oncol. 2010, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Österlund, P.; Ruotsalainen, T.; Korpela, R.; Saxelin, M.; Ollus, A.; Valta, P.; Kouri, M.; Elomaa, I.; Joensuu, H. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: A randomised study. Br. J. Cancer 2007, 97, 1028. [Google Scholar] [CrossRef]
- Germain, I.; Desjardins, J.; Demers, M.; Dagnault, A. Phase III Study: Impact of Probiotics on Diarrhea in Patients Treated with Pelvic Radiation. Int. J. Radiat. Oncol. 2011, 81, S667–S668. [Google Scholar] [CrossRef]
- Demers, M.; Dagnault, A.; Desjardins, J. A randomized double-blind controlled trial: Impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin. Nutr. 2014, 33, 761–767. [Google Scholar] [CrossRef]
- Giralt, J.; Regadera, J.P.; Verges, R.; Romero, J.; de la Fuente, I.; Biete, A.; Villoria, J.; Cobo, J.M.; Guarner, F. Effects of probiotic Lactobacillus casei DN-114 001 in prevention of radiation-induced diarrhea: Results from multicenter, randomized, placebo-controlled nutritional trial. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 1213–1219. [Google Scholar] [CrossRef]
- Timko, J. Probiotics as prevention of radiation-induced diarrhoea. J. Radiother. Pract. 2010, 9, 201–208. [Google Scholar] [CrossRef]
- Doron, S.; Snydman, D.R. Risk and safety of probiotics. Clin. Infect. Dis. 2015, 60 (Suppl. 2), S129–S134. [Google Scholar] [CrossRef]
- Oggioni, M.R.; Pozzi, G.; Valensin, P.E.; Galieni, P.; Bigazzi, C. Recurrent septicemia in an immunocompromised patient due to probiotic strains of Bacillus subtilis. J. Clin. Microbiol. 1998, 36, 325–326. [Google Scholar]
- Besselink, M.G.; van Santvoort, H.C.; Buskens, E.; Boermeester, M.A.; van Goor, H.; Timmerman, H.M.; Nieuwenhuijs, V.B.; Bollen, T.L.; van Ramshorst, B.; Witteman, B.J.; et al. Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet 2008, 371, 651–659. [Google Scholar] [CrossRef]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C.J. Genes and Molecules of Lactobacilli Supporting Probiotic Action. Microbiol. Mol. Biol. Rev. 2008, 72, 728–764. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ko, G. New perspectives regarding the antiviral effect of vitamin A on norovirus using modulation of gut microbiota. Gut Microbes 2017, 8, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ko, G.P. Antiviral effect of Vitamin A on norovirus infection via modulation of the gut microbiome. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Miki, T.; Goto, R.; Fujimoto, M.; Okada, N.; Hardt, W.D. The Bactericidal Lectin RegIIIβ Prolongs Gut Colonization and Enteropathy in the Streptomycin Mouse Model for Salmonella Diarrhea. Cell Host Microbe 2017, 21, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Kanjan, P.; Hongpattarakere, T. Prebiotic efficacy and mechanism of inulin combined with inulin-degrading Lactobacillus paracasei I321 in competition with Salmonella. Carbohydr. Polym. 2017, 169, 236–244. [Google Scholar] [CrossRef]
- Hino, S.; Takemura, N.; Sonoyama, K.; Morita, A.; Kawagishi, H.; Aoe, S.; Morita, T. Small Intestinal Goblet Cell Proliferation Induced by Ingestion of Soluble and Insoluble Dietary Fiber Is Characterized by An Increase in Sialylated Mucins in Rats. J. Nutr. 2012, 142, 1429–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, K.; Kono, H.; Hosomura, N.; Tsuchiya, M.; Ohgiku, M.; Tanaka, N.; Fujii, H. Medium-chain triglycerides enhance mucous secretion and cell proliferation in the rat. J. Gastroenterol. 2009, 44, 204–211. [Google Scholar] [CrossRef]
- Moran, E.T. Nutrients central to maintaining intestinal absorptive efficiency and barrier integrity with fowl. Poult. Sci. 2017, 96, 1348–1363. [Google Scholar] [CrossRef]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.-J. Review article: The role of butyrate on colonic function. Aliment Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar]
- Huang, C.; Song, P.; Fan, P.; Hou, C.; Thacker, P.; Ma, X. Dietary Sodium Butyrate Decreases Postweaning Diarrhea by Modulating Intestinal Permeability and Changing the Bacterial Communities in Weaned Piglets. J. Nutr. 2015, 145, 2774–2780. [Google Scholar] [CrossRef] [Green Version]
- Vernia, P.; Fracasso, P.L.; Casale, V.; Villotti, G.; Marcheggiano, A.; Stigliano, V.; Pinnaro, P.; Bagnardi, V.; Caprilli, R. Topical butyrate for acute radiation proctitis: Randomised, crossover trial. Lancet 2000, 356, 1232–1235. [Google Scholar] [CrossRef]
- Maggio, A.; Magli, A.; Rancati, T.; Fiorino, C.; Valvo, F.; Fellin, G.; Ricardi, U.; Munoz, F.; Cosentino, D.; Cazzaniga, L.F.; et al. Daily Sodium Butyrate Enema for the Prevention of Radiation Proctitis in Prostate Cancer Patients Undergoing Radical Radiation Therapy: Results of a Multicenter Randomized Placebo-Controlled Dose-Finding Phase 2 Study. Int. J. Radiat. Oncol. Biol. Phys. 2018, 89, 518–524. [Google Scholar] [CrossRef]
- Buffie, C.G.; Pamer, E.G. Microbiota-mediated colonization resistance against intestinal pathogens. Nat. Rev. Immunol. 2013, 13, 790–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Keersmaecker, S.C.J.; Verhoeven, T.L.A.; Desair, J.; Marchal, K.; Vanderleyden, J.; Nagy, I. Strong antimicrobial activity of Lactobacillus rhamnosus GG against Salmonella typhimurium is due to accumulation of lactic acid. FEMS Microbiol. Lett. 2006, 259, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Durant, J.A.; Corrier, D.E.; Ricke, S.C. Short-chain volatile fatty acids modulate the expression of the hilA and invF genes of Salmonella typhimurium. J. Food Prot. 2000, 63, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Alakomi, H.; Skyttä, E.; Saarela, M.; Helander, I.M. Lactic Acid Permeabilizes Gram-Negative Bacteria by Disrupting the Outer Membrane Lactic Acid Permeabilizes Gram-Negative Bacteria by Disrupting the Outer Membrane. Appl. Environ. Microbiol. 2005, 66, 2000–2005. [Google Scholar]
- Presser, K.A.; Ratkowsky, D.A.; Ross, T. Modelling the growth rate of Escherichia coli as a function of pH and lactic acid concentration. Appl. Environ. Microbiol. 1997, 63, 2355–2360. [Google Scholar] [PubMed]
- Doron, S.; Gorbach, S.L. Probiotics: Their role in the treatment and prevention of disease. Expert Rev. Anti Infect. Ther. 2006, 4, 261–275. [Google Scholar] [CrossRef]
- Eijsink, V.G.H.; Axelsson, L.; Diep, D.B.; Havarstein, L.S.; Holo, H.; Nes, I.F. Production of class II bacteriocins by lactic acid bacteria; an example of biological warfare and communication. Antonie van Leeuwenhoek 2002, 81, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Olmos, M.I.; Oberhelman, R.A. Probiotic agents and infectious diseases: A modern perspective on a traditional therapy. Clin. Infect. Dis. 2001, 32, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, R.; Li, J. Lactobacillus reuteri ATCC 55730 and L22 display probiotic potential in vitro and protect against Salmonella-induced pullorum disease in a chick model of infection. Res. Vet. Sci. 2012, 93, 366–373. [Google Scholar] [CrossRef]
- Cazorla, S.I.; Maldonado-Galdeano, C.; Weill, R.; De Paula, J.; Perdigon, G.D.V. Oral Administration of Probiotics Increases Paneth Cells and Intestinal Antimicrobial Activity. Front. Microbiol. 2018, 9, 736. [Google Scholar] [CrossRef]
- Gorbunov, N.V.; Garrison, B.R.; Kiang, J.G. Response of crypt paneth cells in the small intestine following total-body gamma-irradiation. Int. J. Immunopathol. Pharmacol. 2010, 23, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Hudault, S.; Lievin, V.; Bernet-Camard, M.F.; Servin, A.L. Antagonistic activity exerted in vitro and in vivo by Lactobacillus casei (strain GG) against Salmonella typhimurium C5 infection. Appl. Environ. Microbiol. 1997, 63, 513–518. [Google Scholar]
- Bernet-Camard, M.F.; Lievin, V.; Brassart, D.; Neeser, J.R.; Servin, A.L.; Hudault, S. The human Lactobacillus acidophilus strain LA1 secretes a nonbacteriocin antibacterial substance(s) active in vitro and in vivo. Appl. Environ. Microbiol. 1997, 63, 2747–2753. [Google Scholar] [PubMed]
- Le Bouguenec, C. Adhesins and invasins of pathogenic Escherichia coli. Int. J. Med. Microbiol. 2005, 295, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Schachtsiek, M.; Hammes, W.P.; Hertel, C. Characterization of Lactobacillus coryniformis DSM 20001 T Surface Protein Cpf Mediating Coaggregation with and Aggregation among Pathogens Characterization of Lactobacillus coryniformis DSM 20001 T Surface Protein Cpf Mediating Coaggregatio. Appl. Environ. Microbiol. 2004, 70, 7078–7085. [Google Scholar] [CrossRef]
- Bergonzelli, G.E.; Granato, D.; Pridmore, R.D.; Marvin-Guy, L.F.; Donnicola, D. GroEL of Lactobacillus johnsoni La1 (NCC533) Is cell surface Associated: Potential Role in Interactions with the Host and the Gastric Pathogen Helicobacter pylori. Infect. Immun. 2006, 74, 425–434. [Google Scholar] [CrossRef]
- Vollbracht, C.; Schneider, B.; Leendert, V.; Weiss, G.; Auerbach, L.; Beuth, J. Intravenous Vitamin C Administration Improves Quality of Life in Breast Cancer Patients during Chemo-/Radiotherapy and Aftercare: Results of a Retrospective, Multicentre, Epidemiological Cohort Study in Germany. In Vivo 2011, 25, 983–990. [Google Scholar]
- Padmanabhan, S.; Waly, M.I.; Taranikanti, V.; Guizani, N.; Ali, A.; Rahman, M.S.; Al-Attabi, Z.; Al-Malky, R.N.; Al-Maskari, S.N.M.; Al-Ruqaishi, B.R.S.; et al. Folate/Vitamin B12 Supplementation Combats Oxidative Stress-Associated Carcinogenesis in a Rat Model of Colon Cancer. Nutr. Cancer 2018. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mumm, J.B.; Herbst, R.; Kolbeck, R.; Wang, Y. IL-22 Increases Permeability of Intestinal Epithelial Tight Junctions by Enhancing Claudin-2 Expression. J. Immunol. 2017, 199, 3316–3325. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Pearson, K.; Kim, J.-H.; Kamdar, K.; DePaolo, R.W. Retinoic acid can exacerbate T cell intrinsic TLR2 activation to promote tolerance. PLoS ONE 2015, 10, e0118875. [Google Scholar] [CrossRef] [PubMed]
- Reifen, R.; Levy, E.; Berkovich, Z.; Tirosh, O. Vitamin A exerts its antiinflammatory activities in colitis through preservation of mitochondrial activity. Nutrition 2015, 31, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.-R.; Chang, S.-Y.; Chang, J.-H.; Kim, J.-O.; Yang, J.-Y.; Kim, C.-H.; Kweon, M.-N. Downregulation of Th17 Cells in the Small Intestine by Disruption of Gut Flora in the Absence of Retinoic Acid. J. Immunol. 2010, 184, 6799–6806. [Google Scholar] [CrossRef] [Green Version]
- Roche, M.; Neti, P.V.S.V.; Kemp, F.W.; Azzam, E.I.; Ferraris, R.P.; Howell, R.W. High Levels of Dietary Supplement Vitamins A, C and E are Absorbed in the Small Intestine and Protect Nutrient Transport Against Chronic Gamma Irradiation. Radiat. Res. 2015, 184, 470–481. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.L.; Wang, C.H.; Kuo, Y.W.; Tsai, C.H. Antioxidative and hepatoprotective effects of fructo-oligosaccharide in d-galactose-treated Balb/cJ mice. Br. J. Nutr. 2011, 105, 805–809. [Google Scholar] [CrossRef]
- Yen, C.H.; Kuo, Y.W.; Tseng, Y.H.; Lee, M.C.; Chen, H.L. Beneficial effects of fructo-oligosaccharides supplementation on fecal bifidobacteria and index of peroxidation status in constipated nursing-home residents-A placebo-controlled, diet-controlled trial. Nutrition 2011, 27, 323–328. [Google Scholar] [CrossRef]
- Lee, M.T.; Lin, W.C.; Yu, B.; Lee, T.T. Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals—A review. Asian-Australas. J. Anim. Sci. 2017, 30, 299–308. [Google Scholar] [CrossRef]
- Wood, S.M.; Mastaloudis, A.F.; Hester, S.N.; Gray, R.; Kern, D.; Namkoong, J.; Draelos, Z.D. Protective effects of a novel nutritional and phytonutrient blend on ultraviolet radiation-induced skin damage and inflammatory response through aging defense mechanisms. J. Cosmet. Dermatol. 2017, 16, 491–499. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Han, Q.; Guo, Y.; Zhang, B.; D’Inca, R. Dietary live yeast and mannan-oligosaccharide supplementation attenuate intestinal inflammation and barrier dysfunction induced by Escherichia coli in broilers. Br. J. Nutr. 2016, 116, 1878–1888. [Google Scholar] [CrossRef] [PubMed]
- Akbari, P.; Braber, S.; Alizadeh, A.; Verheijden, K.A.; Schoterman, M.H.; Kraneveld, A.D.; Garssen, J.; Fink-Gremmels, J. Galacto-oligosaccharides Protect the Intestinal Barrier by Maintaining the Tight Junction Network and Modulating the Inflammatory Responses after a Challenge with the Mycotoxin Deoxynivalenol in Human Caco-2 Cell Monolayers and B6C3F1 Mice. J. Nutr. 2015, 145, 1604–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, E.G.; Licht, T.R.; Leser, T.D.; Bahl, M.I. Dietary Xylo-oligosaccharide stimulates intestinal bifidobacteria and lactobacilli but has limited effect on intestinal integrity in rats. BMC Res. Notes 2014, 7, 1–14. [Google Scholar] [CrossRef]
- Amasheh, M.; Schlichter, S.; Amasheh, S.; Mankertz, J.; Zeitz, M.; Fromm, M.; Schulzke, J.D. Quercetin enhances epithelial barrier function and increases claudin-4 expression in Caco-2 cells. J. Nutr. 2008, 138, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hara, H. Quercetin enhances intestinal barrier function through the assembly of zonula occludens-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells. J. Nutr. 2009, 139, 965–974. [Google Scholar] [CrossRef]
- Lin, M.; Yen, C. Antioxidative ability of pactic acid bacteria. J. Agric. Food Chem. 1999, 47, 1460–1466. [Google Scholar] [CrossRef]
- Lee, J.; Hwang, K.-T.; Heo, M.-S.; Lee, J.-H.; Park, K.-Y. Resistance of Lactobacillus plantarum KCTC 3099 from Kimchi to oxidative stress. J. Med. Food 2005, 8, 299–304. [Google Scholar] [CrossRef]
- Li, S.; Huang, R.; Shah, N.P.; Tao, X.; Xiong, Y.; Wei, H. Antioxidant and antibacterial activities of exopolysaccharides from Bifidobacterium bifidum WBIN03 and Lactobacillus plantarum R315. J. Dairy Sci. 2014, 97, 7334–7343. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, W.; Rui, X.; Chen, X.; Jiang, M.; Dong, M. Characterization of a novel exopolysaccharide with antitumor activity from Lactobacillus plantarum 70810. Int. J. Biol. Macromol. 2014, 63, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shah, N.P. Antioxidant and antibacterial activities of sulphated polysaccharides from Pleurotus eryngii and Streptococcus thermophilus ASCC 1275. Food Chem. 2014, 165, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ji, J.; Rui, X.; Yu, J.; Tang, W.; Chen, X.; Jiang, M.; Dong, M. Production of exopolysaccharides by Lactobacillus helveticus MB2-1 and its functional characteristics in vitro. LWT Food Sci. Technol. 2014, 59, 732–739. [Google Scholar] [CrossRef]
- London, L.E.E.; Kumar, A.H.S.; Wall, R.; Casey, P.G.; Sullivan, O.O.Õ.; Shanahan, F.; Hill, C.; Cotter, P.D.; Fitzgerald, G.F.; Ross, R.P.; et al. Exopolysaccharide-Producing Probiotic Lactobacilli Reduce Serum Cholesterol and Modify Enteric Microbiota in ApoE deficient mice. J. Nutr. 2014, 144, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Pan, T.M.; Wu, Y.J.; Chang, S.J.; Chang, M.S.; Hu, C.Y. Exopolysaccharide activities from probiotic Bifidobacterium: Immunomodulatory effects (on J774A.1 macrophages) and antimicrobial properties. Int. J. Food Microbiol. 2010, 144, 104–110. [Google Scholar] [CrossRef]
- Ballal, S.A.; Veiga, P.; Fenn, K.; Michaud, M.; Kim, J.H.; Gallini, C.A.; Glickman, J.N.; Quéré, G.; Garault, P.; Béal, C.; et al. Host lysozyme-mediated lysis of Lactococcus lactis facilitates delivery of colitis-attenuating superoxide dismutase to inflamed colons. Proc. Natl. Acad. Sci. USA 2015, 112, 7803–7808. [Google Scholar] [CrossRef]
- Fanning, S.; Hall, L.J.; Cronin, M.; Zomer, A.; MacSharry, J.; Goulding, D.; Motherway, M.O.; Shanahan, F.; Nally, K.; Dougan, G.; et al. Bifidobacterial surface-exopolysaccharide facilitates commensal-host interaction through immune modulation and pathogen protection. Proc. Natl. Acad. Sci. USA 2012, 109, 2108–2113. [Google Scholar] [CrossRef] [Green Version]
- Dertli, E.; Colquhoun, I.J.; Gunning, A.P.; Bongaerts, R.J.; Le Gall, G.; Bonev, B.B.; Mayer, M.J.; Narbad, A. Structure and biosynthesis of two exopolysaccharides produced by Lactobacillus johnsonii FI9785. J. Biol. Chem. 2013, 288, 31938–31951. [Google Scholar] [CrossRef]
- Lee, I.-C.; Caggianiello, G.; van Swam, I.I.; Taverne, N.; Meijerink, M.; Bron, P.A.; Spano, G.; Kleerebezem, M. Strain-Specific Features of Extracellular Polysaccharides and Their Impact on Lactobacillus plantarum-Host Interactions. Appl. Environ. Microbiol. 2016, 82, 3959–3970. [Google Scholar] [CrossRef] [PubMed]
- Le, M.H.A.; Galle, S.; Yang, Y.; Landero, J.L.; Beltranena, E.; Gänzle, M.G.; Zijlstra, R.T. Effects of feeding fermented wheat with Lactobacillus reuteri on gut morphology, intestinal fermentation, nutrient digestibility, and growth performance in weaned pigs. J. Anim. Sci. 2016, 94, 4677–4687. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Jiang, Y.; Zhao, W.; Guo, T.; Cao, Y.; Teng, J.; Hao, X.; Zhao, J.; Yang, Z. Antioxidant status and gut microbiota change in an aging mouse model as influenced by exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibetan kefir. J. Dairy Sci. 2017, 100, 6025–6041. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; del Carmen, S.; Miyoshi, A.; Azevedo, V.; Sesma, F.; Langella, P.; Bermudez-Humaran, L.G.; Watterlot, L.; Perdigon, G.; de Moreno de LeBlanc, A. Use of superoxide dismutase and catalase producing lactic acid bacteria in TNBS induced Crohn’s disease in mice. J. Biotechnol. 2011, 151, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulos, B.G.; Misiakos, E.P.; Fotiadis, C.; Stoidis, C.N. Antioxidant properties of probiotics and their protective effects in the pathogenesis of radiation-induced enteritis and colitis. Dig. Dis. Sci. 2011, 56, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Wuyts, S.; Wittouck, S.; De Boeck, I.; Allonsius, C.N.; Pasolli, E.; Segata, N.; Lebeer, S. Large-Scale Phylogenomics of the Lactobacillus casei Group Highlights Taxonomic Inconsistencies and Reveals Novel Clade-Associated Features. mSystems 2017, 2, e00061-17. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.F.; Wang, S.X.; Zhang, D.Y.; Liu, H.; Shan, D.C.; Wang, Y.M. Lactobacillus plantarum ZLP001: In vitro assessment of antioxidant capacity and effect on growth performance and antioxidant status in weaning piglets. Asian-Australas. J. Anim. Sci. 2012, 25, 1153–1158. [Google Scholar] [CrossRef]
- Dowarah, R.; Verma, A.K.; Agarwal, N.; Patel, B.H.M.; Singh, P. Effect of swine based probiotic on performance, diarrhoea scores, intestinal microbiota and gut health of grower-finisher crossbred pigs. Livest. Sci. 2017, 195, 74–79. [Google Scholar] [CrossRef]
- Aluwong, T.; Kawu, M.; Raji, M.; Dzenda, T.; Govwang, F.; Sinkalu, V.; Ayo, J. Effect of Yeast Probiotic on Growth, Antioxidant Enzyme Activities and Malondialdehyde Concentration of Broiler Chickens. Antioxidants 2013, 2, 326–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klayraung, S.; Okonogi, S. Antibacterial and Antioxidant Activities of Acid and Bile Resistant Strains of Lactobacillus fermentum Isolated from Miang. Braz. J. Microbiol. 2009, 40, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Uskova, M.A.; Kravchenko, L.V. Antioxidant properties of lactic acid bacteria—Probiotic and yogurt strains. Vopr. Pitan. 2009, 78, 18–23. [Google Scholar]
- Capcarova, M.; Weiss, J.; Hrncar, C.; Kolesarova, A.; Pal, G. Effect of Lactobacillus fermentum and Enterococcus faecium strains on internal milieu, antioxidant status and body weight of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2010, 94, e215–e224. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 2012, 28, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Buffinton, G.D.; Doe, W.F. Depleted mucosal antioxidant defences in inflammatory bowel disease. Free Radic. Biol. Med. 1995, 19, 911–918. [Google Scholar] [CrossRef]
- Loguercio, C.; D’Argenio, G.; Delle Cave, M.; Cosenza, V.; Della Valle, N.; Mazzacca, G.; Del Vecchio Blanco, C. Glutathione supplementation improves oxidative damage in experimental colitis. Dig. Liver Dis. 2003, 35, 635–641. [Google Scholar] [CrossRef]
- Lutgendorff, F.; Nijmeijer, R.M.; Sandstrom, P.A.; Trulsson, L.M.; Magnusson, K.-E.; Timmerman, H.M.; van Minnen, L.P.; Rijkers, G.T.; Gooszen, H.G.; Akkermans, L.M.A.; et al. Probiotics prevent intestinal barrier dysfunction in acute pancreatitis in rats via induction of ileal mucosal glutathione biosynthesis. PLoS ONE 2009, 4, e4512. [Google Scholar] [CrossRef] [PubMed]
- Witschi, A.; Reddy, S.; Stofer, B.; Lauterburg, B.H. The systemic availability of oral glutathione. Eur. J. Clin. Pharmacol. 1992, 43, 667–669. [Google Scholar] [CrossRef]
- Mansour, H.H.; Hafez, H.F.; Fahmy, N.M.; Hanafi, N. Protective effect of N-acetylcysteine against radiation induced DNA damage and hepatic toxicity in rats. Biochem. Pharmacol. 2008, 75, 773–780. [Google Scholar] [CrossRef]
- Wu, W.; Abraham, L.; Ogony, J.; Matthews, R.; Goldstein, G.; Ercal, N. Effects of N-acetylcysteine amide (NACA), a thiol antioxidant on radiation-induced cytotoxicity in Chinese hamster ovary cells. Life Sci. 2008, 82, 1122–1130. [Google Scholar] [CrossRef]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome, and immune system: Envisioning the future. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Wu, Y.; Xie, F.; Du, K.; Wang, Y.; Shi, L.; Ji, L.; Liu, T.; Ma, X. Dimethyl fumarate reduces the risk of mycotoxins via improving intestinal barrier and microbiota. Oncotarget 2017, 8, 44625–44638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pompei, A.; Cordisco, L.; Amaretti, A.; Zanoni, S.; Raimondi, S.; Matteuzzi, D.; Rossi, M. Administration of folate-producing Bifidobacteria enhances folate status in Wistar rats. J. Nutr. 2007, 137, 2742–2746. [Google Scholar] [CrossRef] [PubMed]
- Strozzi, G.P.; Mogna, L. Quantification of folic acid in human feces after administration of Bifidobacterium probiotic strains. J. Clin. Gastroenterol. 2008, 42 (Suppl. 3), S179–S184. [Google Scholar] [CrossRef]
- Garg, S.; Zheng, J.; Wang, J.; Authier, S.; Pouliot, M.; Hauer-Jensen, M. Segmental Differences in Radiation-Induced Alterations of Tight Junction-Related Proteins in Non-Human Primate Jejunum, Ileum and Colon. Radiat. Res. 2016, 185, 50–59. [Google Scholar] [CrossRef]
- Kiang, J.G.; Fukumoto, R.; Gorbunov, N.V. Lipid Peroxidation After Ionizing Irradiation Leads to Apoptosis and Autophagy. In Lipid Peroxidation; InTech: Rijeka, Croatia, 2012; pp. 261–278. [Google Scholar]
- Farquhar, M.G.; Palade, G.E. Junctional complexes in various epithelia. J. Cell Biol. 1963, 17, 375–412. [Google Scholar] [CrossRef] [PubMed]
- De Santis, S.; Cavalcanti, E.; Mastronardi, M.; Jirillo, E.; Chieppa, M. Nutritional Keys for Intestinal Barrier Modulation. Front. Immunol. 2015, 6, 612. [Google Scholar] [CrossRef]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of Tight Junction Permeability by Intestinal Bacteria and Dietary Components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef] [Green Version]
- La Fata, G.; Weber, P.; Mohajeri, M.H. Probiotics and the Gut Immune System: Indirect Regulation. Probiotics Antimicrob. Proteins 2018, 10, 11–21. [Google Scholar] [CrossRef]
- Anderson, R.C.; Cookson, A.L.; McNabb, W.C.; Park, Z.; McCann, M.J.; Kelly, W.J.; Roy, N.C. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C.; Cookson, A.L.; McNabb, W.C.; Kelly, W.J.; Roy, N.C. Lactobacillus plantarum DSM 2648 is a potential probiotic that enhances intestinal barrier function. FEMS Microbiol. Lett. 2010, 309, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhang, Z.; Hang, X.; Jiang, Y.L. plantarum prevents Enteroinvasive Escherichia coli-induced tight junction proteins changes in intestinal epithelial cells. BMC Microbiol. 2009, 9, 63. [Google Scholar] [CrossRef]
- Trivedi, K.; Barrett, K.E.; Silvia, C.R.-L. Probiotic inhibition of the entry of enteroinvasive E. coli into, human intestinal epithelial cells involves both Rho-dependent and -independent pathways. Gastroenterology 2003, 124, A106. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, X.; Zhao, J.; Zhang, H.; Zhai, Q.; Narbad, A.; Chen, W. A mixture of Lactobacillus species isolated from traditional fermented foods promote recovery from antibiotic-induced intestinal disruption in mice. J. Appl. Microbiol. 2018, 124, 842–854. [Google Scholar] [CrossRef]
- Cornick, S.; Tawiah, A.; Chadee, K. Roles and regulation of the mucus barrier in the gut. Tissue barriers. 2015, 3, e982426. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.; Park, S.; Lee, J.; Myung, J.K.; Jang, W.S.; Lee, S.J.; Myung, H.; Lee, C.; Kim, H.; Lee, S.S.; et al. Rebamipide alleviates radiation-induced colitis through improvement of goblet cell differentiation in mice. J. Gastroenterol. Hepatol. 2018, 33, 878–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celebioglu, H.; Svensson, B. Dietary Nutrients, Proteomes, and Adhesion of Probiotic Lactobacilli to Mucin and Host Epithelial Cells. Microorganisms 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Sharma, P.; Kalia, N.; Singh, J.; Kaur, S. Anti-biofilm Properties of the Fecal Probiotic Lactobacilli Against Vibrio spp. Front. Cell Infect. Microbiol. 2018, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Mack, D.R.; Michail, S.; Wei, S.; McDougall, L.; Hollingsworth, M.A. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. 1999, 276, G941–G950. [Google Scholar]
- Hafez, M.M. Upregulation of Intestinal Mucin Expression by the Probiotic Bacterium E. coli Nissle 1917. Probiotics Antimicrob. Proteins 2012, 4, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Mattar, A.F.; Teitelbaum, D.H.; Drongowski, R.A.; Yongyi, F.; Harmon, C.M.; Coran, A.G. Probiotics up-regulate MUC-2 mucin gene expression in a Caco-2 cell-culture model. Pediatr. Surg. Int. 2002, 18, 586–590. [Google Scholar] [PubMed]
- Caballero-Franco, C.; Keller, K.; De Simone, C.; Chadee, K. The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G315–G322. [Google Scholar]
- Tareb, R.; Bernardeau, M.; Gueguen, M.; Vernoux, J.P. In vitro characterization of aggregation and adhesion properties of viable and heat-killed forms of two probiotic Lactobacillus strains and interaction with foodborne zoonotic bacteria, especially Campylobacter jejuni. J. Med. Microbiol. 2013, 62, 637–649. [Google Scholar] [CrossRef]
- Jiang, H.; Przybyszewski, J.; Mitra, D.; Becker, C.; Brehm-Stecher, B.; Tentinger, A.; MacDonald, R.S. Soy Protein Diet, but Not Lactobacillus rhamnosus GG, Decreases Mucin-1, Trefoil Factor-3, and Tumor Necrosis Factor- in Colon of Dextran Sodium Sulfate-Treated C57BL/6 Mice. J. Nutr. 2011, 141, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Ghosh, S.P.; Ha, C.T.; Fu, D.; Elliott, T.B.; Bolduc, D.L.; Villa, V.; Whitnall, M.H.; Landauer, M.R.; Xiao, M. Delta-tocotrienol protects mice from radiation-induced gastrointestinal injury. Radiat. Res. 2013, 180, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Kominsky, D.J.; Keely, S.; Macmanus, C.F.; Glover, L.E.; Scully, M.; Collins, C.B.; Bowers, B.E.; Campbell, E.L.; Colgan, P. An Endogenously Anti-Inflammatory Role for Methylation in Mucosal Inflammation Identified through Metabolite Profiling. J. Immunol. 2011, 186, 6505–6514. [Google Scholar] [CrossRef] [Green Version]
- Sanguri, S.; Gupta, D. Mannan oligosaccharide requires functional ETC and TLR for biological radiation protection to normal cells. BMC Cell Biol. 2018, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.O.; Sheikh, H.I.; Ha, S.D.; Martins, A.; Reid, G. G-CSF-mediated inhibition of JNK is a key mechanism for Lactobacillus rhamnosus-induced suppression of TNF production in macrophages. Cell Microbiol. 2006, 8, 1958–1971. [Google Scholar] [CrossRef]
- Roessler, A.; Friedrich, U.; Vogelsang, H.; Bauer, A.; Kaatz, M.; Hipler, U.C.; Schmidt, I.; Jahreis, G. The immune system in healthy adults and patients with atopic dermatitis seems to be affected differently by a probiotic intervention. Clin. Exp. Allergy 2008, 38, 93–102. [Google Scholar] [PubMed]
- Haller, D.; Serrant, P.; Granato, D.; Schiffrin, E.J.; Blum, S. Activation of human NK cells by staphylococci and lactobacilli requires cell contact-dependent costimulation by autologous monocytes. Clin. Diagn. Lab. Immunol. 2002, 9, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Smits, H.H.; Engering, A.; Van Der Kleij, D.; De Jong, E.C.; Schipper, K.; Van Capel, T.M.M.; Zaat, B.A.J.; Yazdanbakhsh, M.; Wierenga, E.A.; Van Kooyk, Y.; et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J. Allergy Clin. Immunol. 2005, 115, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.G.; Kayama, H.; Ueda, Y.; Takahashi, T.; Asahara, T.; Tsuji, H.; Tsuji, N.M.; Kiyono, H.; Ma, J.S.; Kusu, T.; et al. Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathog. 2012, 8, 1–15. [Google Scholar] [CrossRef]
| Probiotic | Gut Improving Effect | Type of Study | References |
|---|---|---|---|
| Individual L. acidophilus strains ADH or N2, or L. bulgaricus (strain not mentioned), or Streptococcus thermophiles (strain not mentioned) | Survival in human stomach and adhesion to IECs † | In vitro In vivo (healthy pigs and humans) | [98] |
| L. rhamnosus GG ATCC 53103 | Prevention of cytokine-induced apoptosis in IECs † | In vitro | [99] |
| Prevention of E. coli-induced changes in epithelial barrier function | In vitro | [100] | |
| Restoration of intestinal integrity of murine ileum through occludin expression | In vivo (mice with alcoholic liver disease) | [101] | |
| Prevention of increased intestinal paracellular permeability in Caco-2 cells; Restoration of tight junction proteins such as ZO-1 †, claudin-1, and occludin | In vitro | [102] | |
| Induction of inflammatory tolerance of the intestinal mucosa | In vitro In vivo (healthy mice) | [103,104] | |
| Local dampening of innate immune responses with desensitization towards luminal antigens | In vitro | [105] | |
| Effective treatment of acute gastroenteritis | In vivo (children with acute gastroenteritis) | [106] | |
| Faster recovery of acute non-bloody diarrhea | In vivo (children with acute diarrhea) | [107,108,109] | |
| B. bifidum G9-1 | Induction of mucosal protective factors including MUC2-4 †, TGFβ1 † and TFF3 †; Alleviation of diarrhea partly through protection against human rotavirus induced lesions | In vivo (mice pups with rotavirus gastroenteritis) | [110] |
| L. plantarum WCFS1 | Restoration of tight junction proteins ZO-1 † and occludin | In vitro In vivo (healthy humans) | [111] |
| L. reuteri I5007 | Restoration of tight junction proteins claudin-1, occluding, and ZO-1 † | In vitro In vivo (healthy piglets) | [112] |
| L. casei DN-114 001 | Prevention of transcription of numerous pro-inflammatory genes encoding cytokines, chemokines and adherence molecules | In vitro | [113] |
| Restoration of tight junction protein ZO-1 † in Caco-2 cells | In vitro | [114] | |
| Ultrabiotique® (L. acidophilus + B. lactis + L. plantarum + B. breve) | Improvement of clinical symptoms and histological alterations; Down regulation of nitric oxide production by peritoneal macrophages; Enhancement of mucus production with modification of microflora | In vivo (mice with colitis) | [115] |
| L. acidophilus (strain not mentioned) | Overall attenuation of the severity of DSS †-induced colitis, specifically by suppressing pro-inflammatory cytokines | In vitro In vivo (mice with colitis) | [116] |
| Mixture of Streptococcus thermophilus + L. acidophilus + L. bulgaricus (strains not mentioned) | No improvement of acute diarrhea | In vivo (children with acute diarrhea) | [117] |
| Probiotic | Radiation Dose | Method of Supplementation | Duration of Supplementation | Results of Supplementation | References |
|---|---|---|---|---|---|
| Microflorana®-F (L. acidophilus + L. helveticus + Bifidobacterium spp) | Abdominal X irradiation with 1 × 20 Gy | Oral gavage of 1 mL of probiotic solution three times daily | Started seven days before the irradiation procedure and maintained until 14 days thereafter | Improved overall survival; Improved endotoxin levels; Reduced incidence of bacterial contamination | [137] |
| L. acidophilus (strain not mentioned) | Abdominal-pelvic irradiation with either 1 × 10, 15 or 20 Gy | Oral gavage of 2 mL of probiotic solution (108 CFU †) | Started six days before irradiation and maintained until three days thereafter | Improved morphology of the small intestine after 10 or 15 Gy; No improvements of jejunal villi height when irradiated with 15 Gy; No improvements when irradiated with 20 Gy | [138] |
| L. rhamnosus GG ATCC 53103 | Whole body γ irradiation with 1 × 12 Gy | Oral gavage of probiotic solution (5 × 10⁷ CFU †), daily | Three consecutive days before irradiation | Reduced epithelial apoptosis particularly at crypt bases; Improved crypt survival; No detectable shifts in bacterial compositions | [139] |
| Oral gavage or intraperitoneal injection of lipoteichoic acid (5 mg/kg), a radioprotective agent in L. rhamnosus GG, daily | Three consecutive days before irradiation | Improved small intestinal crypt survival | [140] | ||
| Total abdominal X irradiation with 7 or 8 × 4 Gy | Intraperitoneal injection of lipoteichoic acid (5 mg/kg), a radioprotective agent in L. rhamnosus GG, daily | One hour before each fractionated radiation dose | Improved post-radiation weight recovery and survival | ||
| L. delbrueckii subsp. Bulgaricus B3 strain | Abdominal-pelvic γ irradiation with 1 × 11 Gy | Oral gavage of 2 mL of probiotic solution (1010 CFU †/mL), daily | Seven consecutive days after irradiation | Reduced scores for inflammation and vascularity; Accelerated healing; Decreased bacterial translocation; Reduced diarrhea | [141,142] |
| L. plantarum 299v | Lower abdominal X irradiation with 2 × 10 Gy | Oral gavage of probiotic solution (2 × 109 CFU †), twice daily | Started one day after irradiation and was continued throughout the experiment for a maximum of 15 days, except for the operation day | Increased collagen content; Decreased mucosal myeloperoxidase activity | [143] |
| Probiotic | Summary of Study | Method of Supplementation | Duration of Supplementation | Results | References |
|---|---|---|---|---|---|
| L. acidophilus NCDO 1748 | Patients (n = 24) with gynecological tumors, 80 Gy | A formulated drink (≥2 × 109 CFU †) and 6.5% lactulose as bacterial substrate, once daily | Started five days prior to radiotherapy, daily throughout the radiotherapy period including the interval, and continued for 10 days thereafter | Reduced diarrhea | [150] |
| L. casei DN-114 001 | Patients (n = 85) with gynecological tumors, 45–50 Gy with weekly cisplatin treatment (40 mg/m2) | A formulated drink (108 CFU †/g), three times daily | Started one week prior to radiotherapy | Improved stool consistency; No significant differences in need for rescue anti-diarrheal therapy, neither diarrhea severity | [156] |
| Infloran® (L. acidophilus and B. bifidum) | Patients (n = 63) with locally advanced cervical cancer receiving 50 Gy with additional brachytherapy of four times 7 Gy with weekly cisplatin during radiotherapy procedure | Two oral capsules (2 × 109 CFU †/g of each bacteria), twice daily | Started 7 days prior to radiotherapy and maintained during radiotherapy | Reduced severity of diarrhea;Reduced need of rescue anti-diarrheal therapy; Improved stool consistency | [152] |
| VSL#3® (Streptococcus thermophilus BT01, B. breve BB02, B. longum BL03, B. infantis BI04, L. acidophilus BA05, L. plantarum BP06, L. paracasei BP07, L. delbrueckii subsp. bulgaricus BD08) | Patients (n = 190) with pelvic tumors, 60–70 Gy | A formulation (450 × 109 CFU †/g), three times daily | Started on the first day of radiotherapy, for 6 to 7 consecutive weeks of therapy | Reduced number of patients suffering from radiation induced toxicity; Reduced severity of toxicity | [148,149] |
| Patients (n = 239) with postoperative cervical, sigmoid or rectal tumors, 60–70 Gy | Improved number of bowel movements; Delayed need for additional anti-diarrheal therapy | ||||
| Bifilact® (L. acidophilus LAC-361 and B. longum BB-536) | Patients (n = 246) with rectal, cervical, endometrial or prostatic cancers that had radiotherapy with or without surgery or chemotherapy | Oral capsules (1.3 × 1012 CFU †), twice daily or three times daily | Started on the first day of radiotherapy and maintained up until the last day of radiotherapy | Reduced severity of diarrhea with standard dosing | [154,155] |
| “5” Strain Dophilus® (L. rhamnosus HA-111, B. Breve HA-129, L. acidophilus HA-122, B. longum HA-135, L. Casei HA-108) | Patients (n = 42) with abdominal-pelvic cancers who received post-operative radiotherapy or radiotherapy with chemotherapy, 50–67 Gy | Oral capsules (6 × 1012 CFU †), twice daily | Started on the first day of radiotherapy and maintained up until the last day of radiotherapy | Reduced incidence and severity of diarrhea | [157] |
| Antibiophilus® (L. rhamnosus LCR 35) | Patients (n = 206) with several lower abdominal and pelvic tumors, 50 Gy | Oral capsules (1.5 × 109 CFU †), plus lactulose as bacterial substrate, three times daily | Started in case of diarrhea and maintained up to one week, depending on the response of the diarrhea | Improved number of bowel movements; Improved fecal consistency; | [151] |
| Gefiluss® (L. rhamnosus GG ATCC 53103) | Patients (n = 39) with colorectal cancers receiving 45–50.4 Gy and 24 weeks of 5-FU chemotherapy | Oral capsules (1–2 × 1010 CFU †), twice daily | Started at the start of adjuvant 5-FU chemotherapy and maintained for 24 weeks | Reduced severity of diarrhea; Less abdominal discomfort reported; Lower need for hospital care; Reduced need for chemotherapy dose adjustments due to bowel toxicity | [153] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segers, C.; Verslegers, M.; Baatout, S.; Leys, N.; Lebeer, S.; Mastroleo, F. Food Supplements to Mitigate Detrimental Effects of Pelvic Radiotherapy. Microorganisms 2019, 7, 97. https://doi.org/10.3390/microorganisms7040097
Segers C, Verslegers M, Baatout S, Leys N, Lebeer S, Mastroleo F. Food Supplements to Mitigate Detrimental Effects of Pelvic Radiotherapy. Microorganisms. 2019; 7(4):97. https://doi.org/10.3390/microorganisms7040097
Chicago/Turabian StyleSegers, Charlotte, Mieke Verslegers, Sarah Baatout, Natalie Leys, Sarah Lebeer, and Felice Mastroleo. 2019. "Food Supplements to Mitigate Detrimental Effects of Pelvic Radiotherapy" Microorganisms 7, no. 4: 97. https://doi.org/10.3390/microorganisms7040097