Plant Tissue Localization and Morphological Conversion of Azospirillum brasilense upon Initial Interaction with Allium cepa L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Media
2.2. Introduction of Reporter Genes into A. brasilense
2.3. Plasmid Stability
2.4. Evaluation of Plant Growth Promotion by A. brasilense
2.5. Plant Tissue Localization and Morphological Conversion of A. brasilense in the Onion Seedlings
2.6. Morphological Observation of A. brasilense by Scanning Electron Microscopy (SEM)
2.7. Detection of Intracellular PHB Granules by Nile Blue Staining
2.8. Biochemical Analyses of A. brasilense under Nitrogen-restricted Conditions
2.9. Statistical Analysis
3. Results
3.1. Effect of A. brasilense on the Growth of Onion Seedlings
3.2. Stability of Plasmids pHRGFPGUS and pBBR1MCS-2::mCherry in A. brasilense
3.3. Plant Tissue Localization of A. brasilense in the Onion Seedlings
3.4. Morphological Conversion of A. brasilense Cells Localized in the Onion Seedlings
3.5. Biochemical Characteristics of Morphologically Converted A. brasilense Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Herschkovitz, Y.; Lerner, A.; Davidov, Y.; Rothballer, M.; Hartmann, A.; Okon, Y.; Jurkevitch, E. Inoculation with the plant-growth-promoting rhizobacterium Azospirillum brasilense causes little disturbance in the rhizosphere and rhizoplane of maize (Zea mays). Microb. Ecol. 2005, 50, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Steenhoudt, O.; Vanderleyden, J. Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: genetic, biochemical and ecological aspects. FEMS Microbiol. Rev. 2000, 24, 487–506. [Google Scholar] [CrossRef] [PubMed]
- Spaepen, S.; Dobbelaere, S.; Croonenborghs, A.; Vanderleyden, J. Effects of Azospirillum brasilense indole-3-acetic acid production on inoculated wheat plants. Plant Soil 2008, 312, 15–23. [Google Scholar] [CrossRef]
- Hall, P.G.; Krieg, N.R. Swarming of Azospirillum brasilense on solid media. Can. J. Microbiol. 1983, 29, 1592–1594. [Google Scholar] [CrossRef]
- Okon, Y.; Cakmakci, L.; Nur, I.; Chet, I. Aerotaxis and chemotaxis of Azospirillum brasilense: a note. Microb. Ecol. 1980, 6, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, G.; Greer, S.E.; Zhulin, I.B. Energy taxis is the dominant behavior in Azospirillum brasilense. J. Bacteriol. 2000, 182, 6042–6048. [Google Scholar] [CrossRef] [PubMed]
- Okon, Y.; Itzigsohn, R. Poly-β-hydroxybutyrate metabolism in Azospirillum brasilense and the ecological role of PHB in the rhizosphere. FEMS Microbiol. Lett. 1992, 103, 131–139. [Google Scholar] [CrossRef]
- Pereg Gerk, L.; Gilchrist, K.; Kennedy, I.R. Mutants with enhanced nitrogenase activity in hydroponic Azospirillum brasilense -wheat associations. Appl. Environ. Microbiol. 2000, 66, 2175–2184. [Google Scholar] [CrossRef] [PubMed]
- Katupitiya, S.; Millet, J.; Vesk, M.; Viccars, L.; Zeman, A.; Lidong, Z.; Elmerich, C.; Kennedy, I.R. A mutant of Azospirillum brasilense Sp7 impaired in flocculation with a modified colonization pattern and superior nitrogen fixation in association with wheat. Appl. Environ. Microbiol. 1995, 61, 1987–1995. [Google Scholar]
- Sadasivan, L.; Neyra, C.A. Flocculation in Azospirillum brasilense and Azospirillum lipoferum: exopolysaccharides and cyst formation. J. Bacteriol. 1985, 163, 716–723. [Google Scholar]
- Burdman, S.; Jurkevitch, E.; Schwartsburd, B.; Hampel, M.; Okon, Y. Aggregation in Azospirillum brasilense: effects of chemical and physical factors and involvement of extracellular components. Microbiology 1998, 144, 1989–1999. [Google Scholar] [CrossRef] [PubMed]
- Burdman, S.; Okon, Y.; Jurkevitch, E. Surface characteristics of Azospirillum brasilense in relation to cell aggregation and attachment to plant roots. Crit. Rev. Microbiol. 2000, 26, 91–110. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo-and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Bible, A.N.; Khalsa-Moyers, G.K.; Mukherjee, T.; Green, C.S.; Mishra, P.; Purcell, A.; Aksenova, A.; Hurst, G.B.; Alexandre, G. Metabolic adaptations of Azospirillum brasilense to oxygen stress by cell-to-cell clumping and flocculation. Appl. Environ. Microbiol. 2015, 81, 8346–8357. [Google Scholar] [CrossRef] [PubMed]
- Nur, I.; Okon, Y.; Henis, Y. Effect of dissolved oxygen tension on production of carotenoids, poly-β-hydroxybutyrate, succinate oxidase and superoxide dismutase by Azospirillum brasilense Cd grown in continuous culture. J. Gen. Microbiol. 1982, 128, 2937–2943. [Google Scholar] [CrossRef]
- Palacios, O.A.; Gomez-Anduro, G.; Bashan, Y.; de-Bashan, L.E. Tryptophan, thiamine and indole-3-acetic acid exchange between Chlorella sorokiniana and the plant growth-promoting bacterium Azospirillum brasilense. FEMS Microbiol. Ecol. 2016, 92, fiw077. [Google Scholar] [CrossRef] [PubMed]
- Kamilova, F.; Kravchenko, L.V.; Shaposhnikov, A.I.; Azarova, T.; Makarova, N.; Lugtenberg, B. Organics acids, sugars, and L-tryptophane in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria. Mol. Plant Microbe Interact. 2006, 19, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Rascio, N.; Rocca, N.L. Biological nitrogen fixation. In Encyclopedia of Ecology; Fath, B.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 2, pp. 264–279. ISBN 978-0-444-64130-4. [Google Scholar]
- Oke, V.; Long, S.R. Bacteroid formation in the Rhizobium–legume symbiosis. Curr. Opin. Biotechnol. 1999, 2, 641–646. [Google Scholar] [CrossRef]
- Wagner, S.C. Biological nitrogen fixation. Nat. Educ. Knowl. 2011, 3, 15. [Google Scholar]
- Pereg Gerk, L.; Paquelin, A.; Gounon, P.; Kennedy, I.R.; Elmerich, C. A transcriptional regulator of the LuxR-UhpA family, FlcA, controls flocculation and wheat root surface colonization by Azospirillum brasilense Sp7. Mol. Plant Microbe Interact. 1998, 11, 177–187. [Google Scholar] [CrossRef]
- Santos, A.R.S.; Etto, R.M.; Furmam, R.W.; Freitas, D.L.; Santos, K.F.D.N.; Souza, E.M.; Pedrosa, F.O.; Ayub, R.A.; Steffens, M.B.R.; Galvão, C.W. Labeled Azospirillum brasilense wild type and excretion-ammonium strains in association with barley roots. Plant Physiol. Biochemi. 2017, 118, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Tajepkema, J.D.; Yocum, C.S. Measurement of oxygen partial pressure within soybean nodules by oxygen microelectrodes. Planta. 1974, 119, 351–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okon, Y.; Kapulnik, Y. Development and function of Azospirillum- inoculated roots. Plant Soil 1986, 90, 3–16. [Google Scholar] [CrossRef]
- Ministry of Agriculture, Forestry and Fisheries. Available online: http://www.maff.go.jp/hokkaido/toukei/index.html (accessed on 1 March 2019).
- Vanstockem, M.; Michiels, K.; Vanderleyden, J.; Van Gool, A.P. Transposon mutagenesis of Azospirillum brasilense and Azospirillum lipoferum: physical analysis of Tn5 and Tn5-mob insertion mutants. Appl. Environ. Microbiol. 1987, 53, 410–415. [Google Scholar] [PubMed]
- Kovach, M.E.; Elzer, P.H.; Hill, D.S.; Robertson, G.T.; Farris, M.A.; Roop, R.M., II; Peterson, K.M. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 1995, 166, 175–176. [Google Scholar] [CrossRef]
- Ramos, H.J.O.; Roncato-Maccari, L.D.; Souza, E.M.; Soares-Ramos, J.R.; Hungria, M.; Pedrosa, F.O. Monitoring Azospirillum-wheat interactions using the gfp and gusA genes constitutively expressed from a new broad-host range vector. J. Biotechnol. 2002, 97, 243–252. [Google Scholar] [CrossRef]
- Ditta, G.; Stanfield, S.; Corbin, D.; Helinski, D. Broad host range DNA cloning system for Gram-negative bacteria: Construction of a gene bank of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 1980, 77, 7347–7351. [Google Scholar] [CrossRef]
- Bashan, Y.; Mitiku, G.; Whitmoyer, R.E.; Levanony, H. Evidence that fibrillar anchoring is essential for Azospirillum brasilense Cd attachment to sand. Plant Soil 1991, 132, 73–83. [Google Scholar] [CrossRef]
- Gage, D.J.; Bobo, T.; Long, S.R. Use of green fluorescent protein to visualize the early events of symbiosis between Rhizobium meliloti and alfalfa (Medicago sativa). J. Bacteriol. 1996, 178, 7159–7166. [Google Scholar] [CrossRef]
- Ostle, A.G.; Holt, J.G. Nile Blue A as a fluorescent stain for poly-3- hydroxybutyrate. Appl. Environ. Microbiol. 1982, 44, 238–241. [Google Scholar]
- Karr, D.B.; Waters, J.K.; Emerich, D.W. Analysis of poly-3-hydroxybutyrate in Rhizobium japonicum bacteroids by ion-exclusion high-pressure liquid chromatography and UV detection. Appl. Environ. Microbiol. 1983, 46, 1339–1344. [Google Scholar] [PubMed]
- Perrig, D.; Boiero, M.L.; Masciarelli, O.A.; Penna, C.; Ruiz, O.A.; Cassán, F.D.; Luna, M.V. Plant-growth-promoting compounds produced by two agronomically important strains of Azospirillum brasilense, and implications for inoculant formulation. Appl. Microbiol. Biotechnol. 2007, 75, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Fu, H.A.; Burris, R.H. Influence of amino acids on nitrogen fixation ability and growth of Azospirillum spp. Appl. Environ. Microbiol. 1988, 54, 87–93. [Google Scholar] [PubMed]
- Kaushik, R.; Saxena, A.K.; Tilak, K.V.B.R. Selection of Tn5::lacZ mutants isogenic to wild type Azospirillum brasilense strains capable of growing at sub-optimal temperature. World J. Microbiol. Biotechnol. 2000, 16, 567–570. [Google Scholar] [CrossRef]
- Sridevi, S.; Ramakrishnan, K. Effects of combined inoculation of AM Fungi and Azospirillum on the growth and yield of onion (Allium cepa L.). J. Phytol. 2010, 2, 88–90. [Google Scholar]
- Hou, X.; McMillan, M.; Coumans, J.V.F.; Poljak, A.; Raftery, M.J.; Pereg, L. Cellular responses during morphological transformation in Azospirillum brasilense and its flcA knockout mutant. PLoS One 2014, 9, e114435. [Google Scholar] [CrossRef] [PubMed]
- Naher, K.; Miwa, H.; Okazaki, S.; Yasuda, M. Effects of different sources of nitrogen on endophytic colonization of rice plants by Azospirillum sp. B510. Microb. Environ. 2018, 33, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Berg, R.H.; Tyler, M.E.; Novick, N.J.; Vasil, V.; Vasil, I.K. Biology of Azospirillum-sugarcane association: enhancement of nitrogenase activity. Appl. Environ. Microbiol. 1980, 39, 642–649. [Google Scholar]
- Ramirez-Mata, A.; Pacheco, M.R.; Moreno, S.J.; Xiqui-Vazquez, M.L.; Baca, B.E. Versatile use of Azospirillum brasilense strains tagged with egfp and mCherry genes for the visualization of biofilms associated with wheat roots. Microbiol. Res. 2018, 215, 155–163. [Google Scholar] [CrossRef]
- Wang, C.; Sheng, X.; Equi, R.C.; Trainer, M.A.; Charles, T.C.; Sobral, B.W. Influence of the poly-3-hydroxybutyrate (PHB) granule-associated proteins (PhaP1 and PhaP2) on PHB accumulation and symbiotic nitrogen fixation in Sinorhizobium meliloti Rm1021. J. Bacteriol. 2007, 189, 9050–9056. [Google Scholar] [CrossRef]
- Thies, J.E.; Grossman, J.M. The soil habitat and soil ecology. In Biological strategies for sustainable soil systems; Uphoff, M., Ball, A.S., Fernandes, E., Herren, H., Husson, O., Laing, M., Palm, C., Pretty, J., Sanchez, P., Sanginga, N., et al., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 59–78. ISBN 978-1-5744-4583-1. [Google Scholar]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef] [Green Version]
- Idris, E.E.; Iglesias, D.J.; Talon, M.; Borriss, R. Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol. Plant Microbe Interact. 2007, 20, 619–626. [Google Scholar] [CrossRef]
- Malinich, E.A.; Bauer, C.E. Transcriptome analysis of Azospirillum brasilense vegetative and cyst states reveals large-scale alterations in metabolic and replicative gene expression. Microb. Genom. 2018, 4, 8. [Google Scholar] [CrossRef]
 , not inoculated; ■, inoculated.
, not inoculated; ■, inoculated.
 , not inoculated; ■, inoculated.
, not inoculated; ■, inoculated.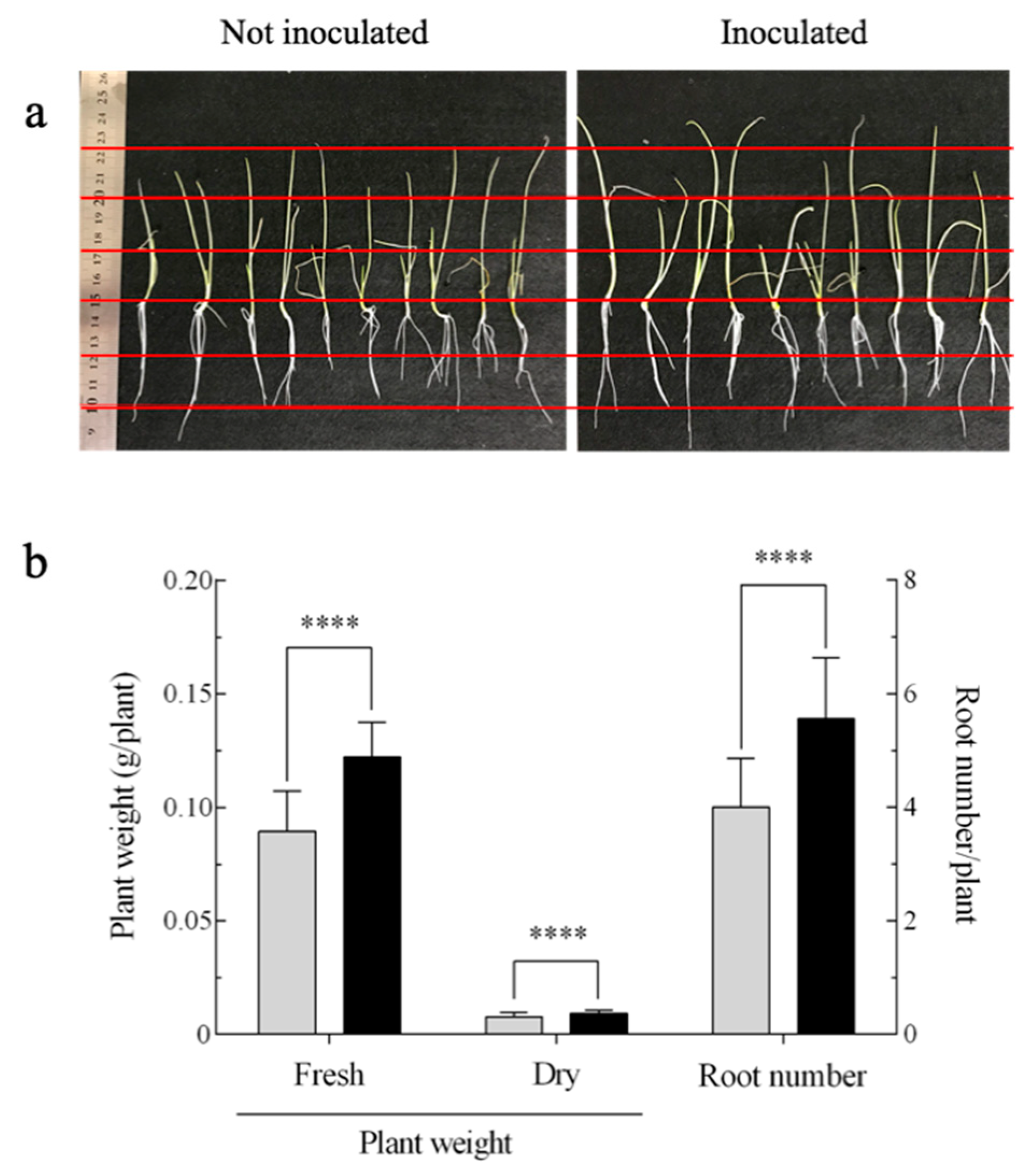





 ) or nitrogen-restricted MMAB medium (■), and the levels of poly-β-hydroxybutyrate (PHB), indole-3-acetic acid (IAA), and acetylene reduction activity (ARA) in the cells were analyzed as described in Materials and Methods. Values are expressed as the means ± standard deviation (SD) of three replicates. Means in the same parameter (PHB, IAA, ARA) with different letters (a, b, c) above each bar are significantly different from each other at p < 0.05 by Tukey’s HSD test. N.D., not detected.
) or nitrogen-restricted MMAB medium (■), and the levels of poly-β-hydroxybutyrate (PHB), indole-3-acetic acid (IAA), and acetylene reduction activity (ARA) in the cells were analyzed as described in Materials and Methods. Values are expressed as the means ± standard deviation (SD) of three replicates. Means in the same parameter (PHB, IAA, ARA) with different letters (a, b, c) above each bar are significantly different from each other at p < 0.05 by Tukey’s HSD test. N.D., not detected.
 ) or nitrogen-restricted MMAB medium (■), and the levels of poly-β-hydroxybutyrate (PHB), indole-3-acetic acid (IAA), and acetylene reduction activity (ARA) in the cells were analyzed as described in Materials and Methods. Values are expressed as the means ± standard deviation (SD) of three replicates. Means in the same parameter (PHB, IAA, ARA) with different letters (a, b, c) above each bar are significantly different from each other at p < 0.05 by Tukey’s HSD test. N.D., not detected.
) or nitrogen-restricted MMAB medium (■), and the levels of poly-β-hydroxybutyrate (PHB), indole-3-acetic acid (IAA), and acetylene reduction activity (ARA) in the cells were analyzed as described in Materials and Methods. Values are expressed as the means ± standard deviation (SD) of three replicates. Means in the same parameter (PHB, IAA, ARA) with different letters (a, b, c) above each bar are significantly different from each other at p < 0.05 by Tukey’s HSD test. N.D., not detected.
| Plant Tissue | Days after Inoculation | ||
|---|---|---|---|
| 0 | 3 | 7 | |
| Bulb | 1.73 ± 0.40 ab | 2.17 ± 0.52 bc | 4.67 ± 1.14 f |
| Roots | |||
| Base | 1.75 ± 0.34 ab | 2.75 ± 0.43 d | 4.24 ± 1.09 f |
| Middle | 1.87 ± 0.37 ab | 2.38 ± 0.47 cd | 6.61 ± 1.57 g |
| Tip | 1.66 ± 0.31 a | 3.40 ± 0.53 e | 4.55 ± 1.20 f |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, L.; Orikasa, Y.; Sakamoto, H.; Ohwada, T. Plant Tissue Localization and Morphological Conversion of Azospirillum brasilense upon Initial Interaction with Allium cepa L. Microorganisms 2019, 7, 275. https://doi.org/10.3390/microorganisms7090275
Hong L, Orikasa Y, Sakamoto H, Ohwada T. Plant Tissue Localization and Morphological Conversion of Azospirillum brasilense upon Initial Interaction with Allium cepa L. Microorganisms. 2019; 7(9):275. https://doi.org/10.3390/microorganisms7090275
Chicago/Turabian StyleHong, Leidong, Yoshitake Orikasa, Hisayo Sakamoto, and Takuji Ohwada. 2019. "Plant Tissue Localization and Morphological Conversion of Azospirillum brasilense upon Initial Interaction with Allium cepa L." Microorganisms 7, no. 9: 275. https://doi.org/10.3390/microorganisms7090275
APA StyleHong, L., Orikasa, Y., Sakamoto, H., & Ohwada, T. (2019). Plant Tissue Localization and Morphological Conversion of Azospirillum brasilense upon Initial Interaction with Allium cepa L. Microorganisms, 7(9), 275. https://doi.org/10.3390/microorganisms7090275




