Recovery of Metals from Acid Mine Drainage by Bioelectrochemical System Inoculated with a Novel Exoelectrogen, Pseudomonas sp. E8
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Pseudomonas sp. E8
2.2. Configuration of MFC Reactors and MEC Reactors
2.3. Startup and Operation of the MFC Reactor and MEC Reactor
2.4. Measurement and Calculation
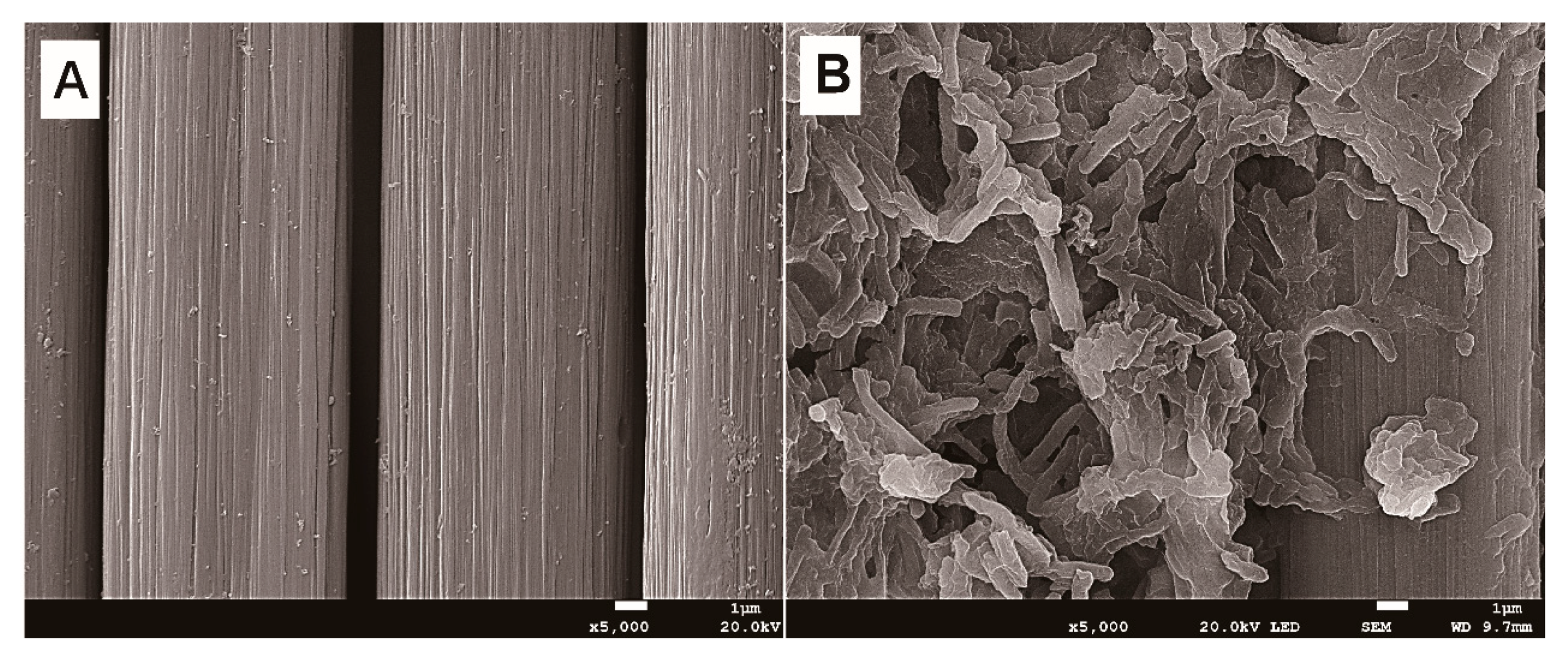
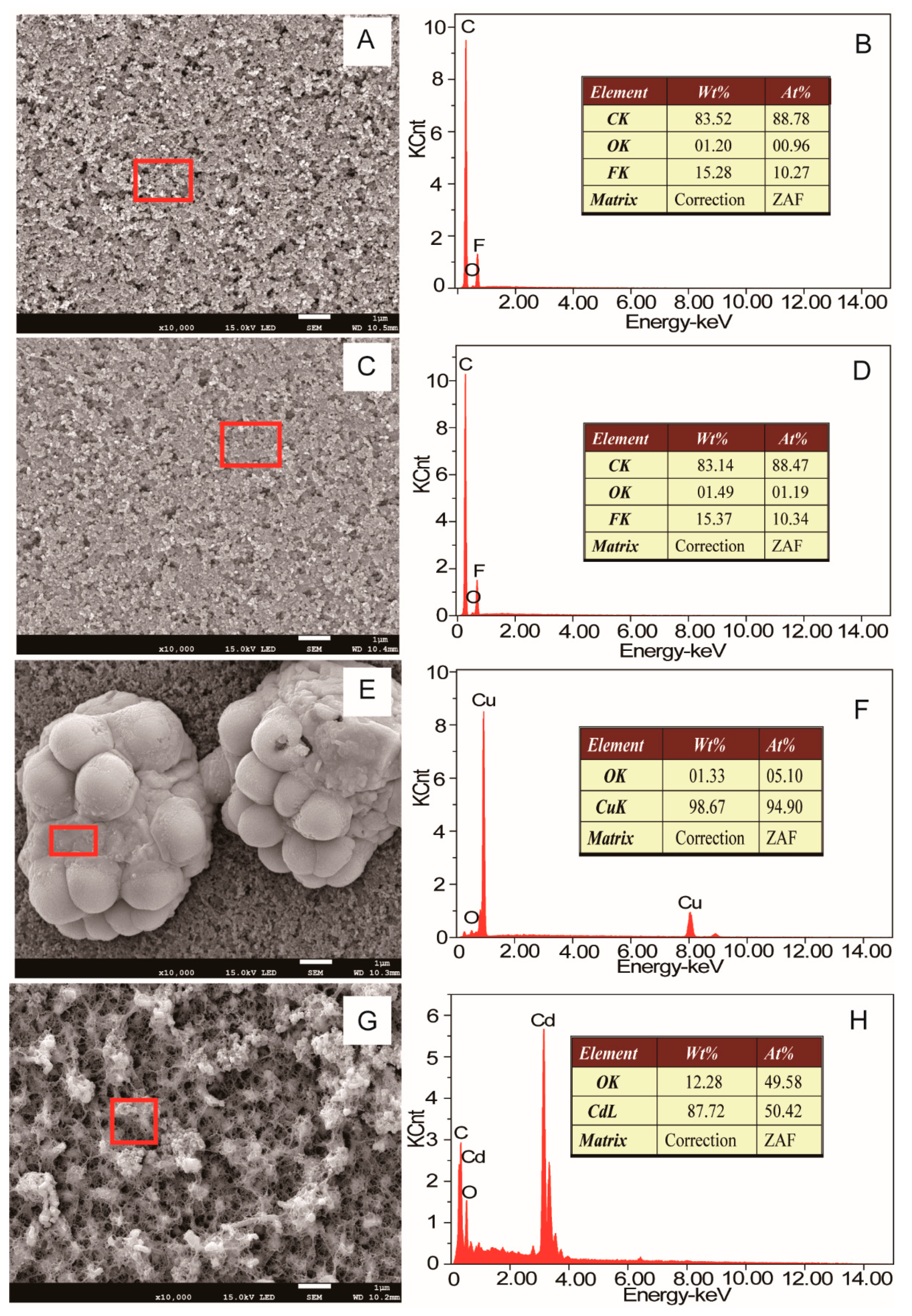
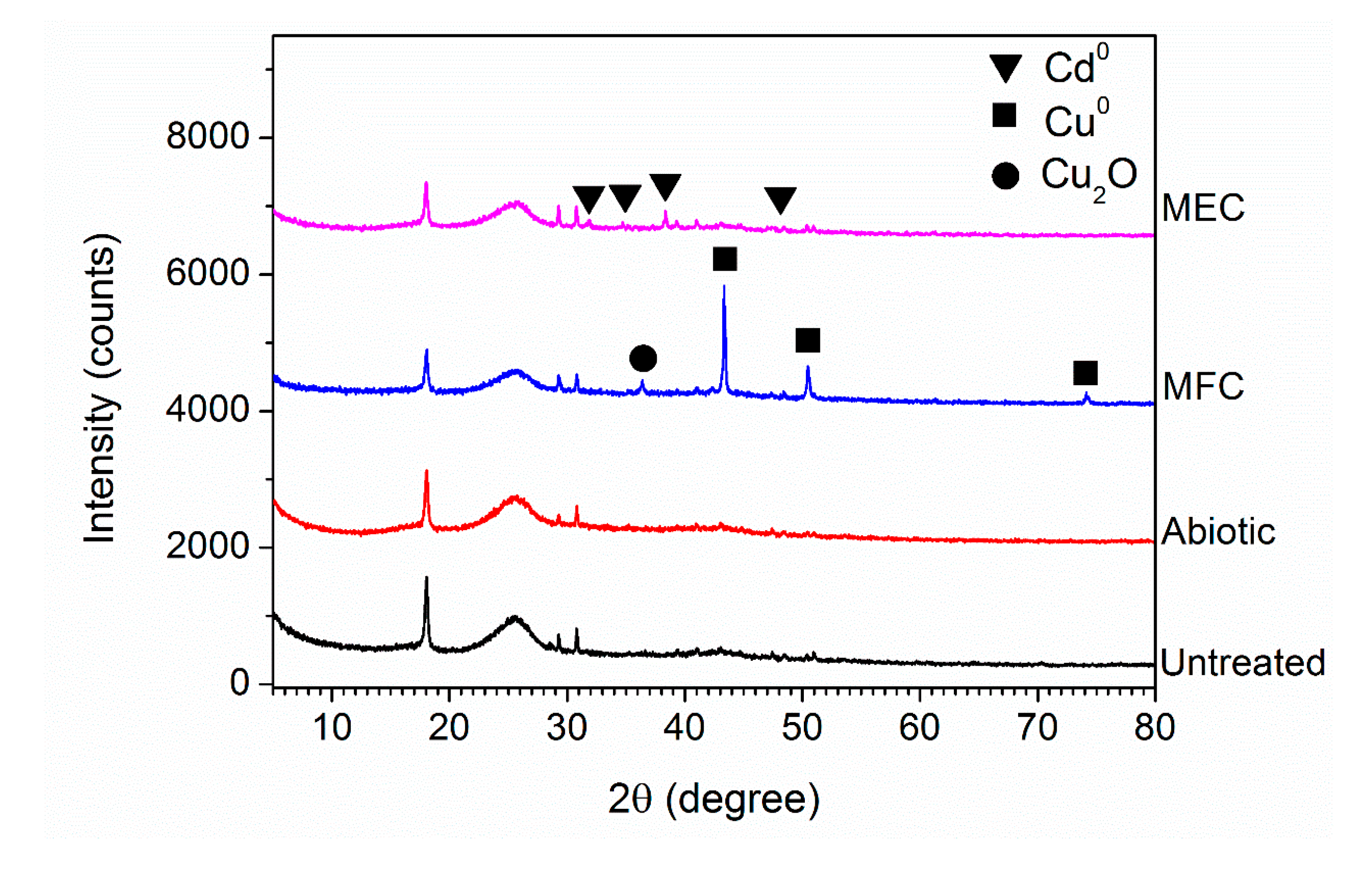
2.5. Scanning Electron Microscopy (SEM) and X-ray Diffraction (XRD) Analysis
3. Results and Discussion
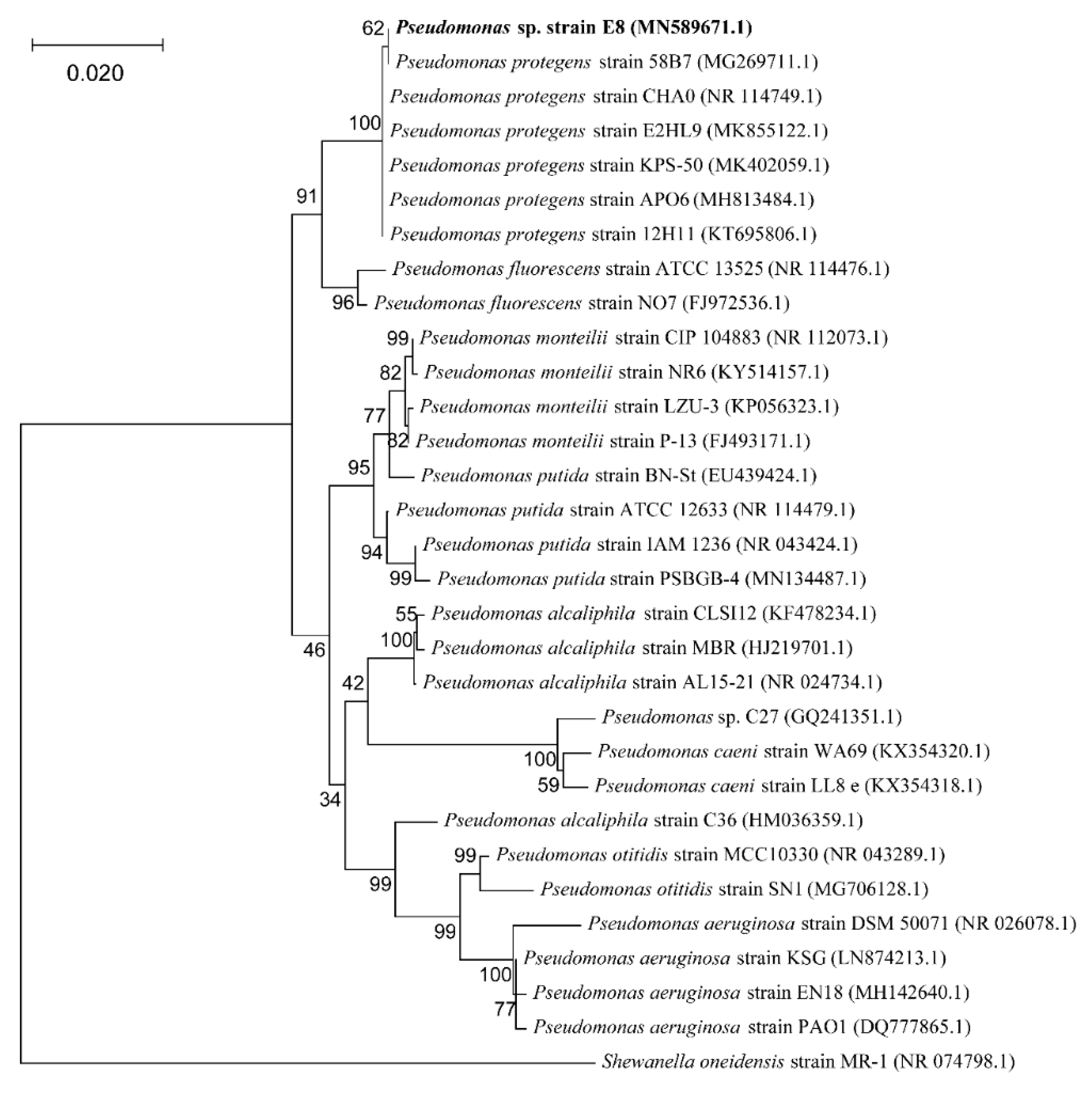
3.1. Identification of the Isolated Strain
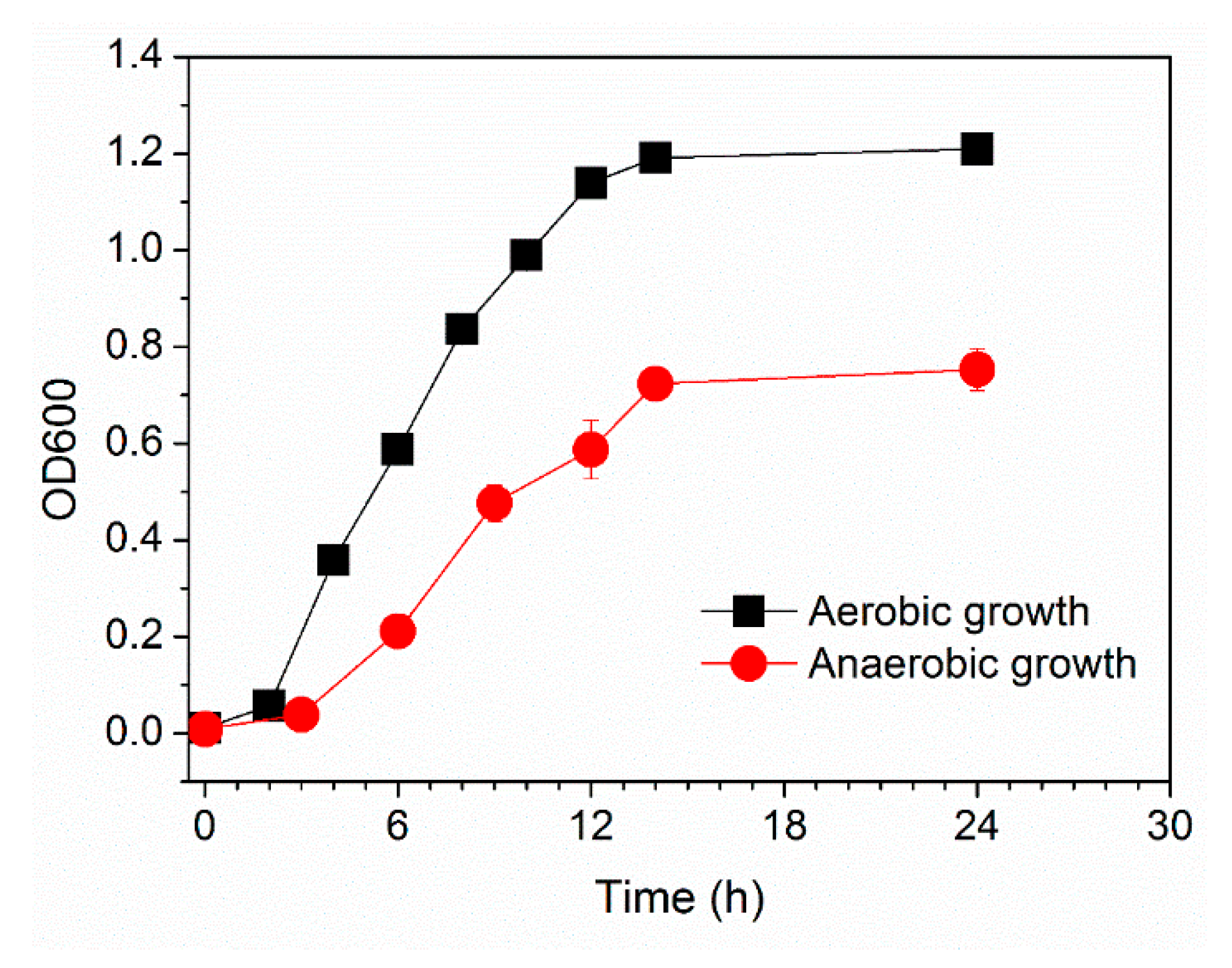
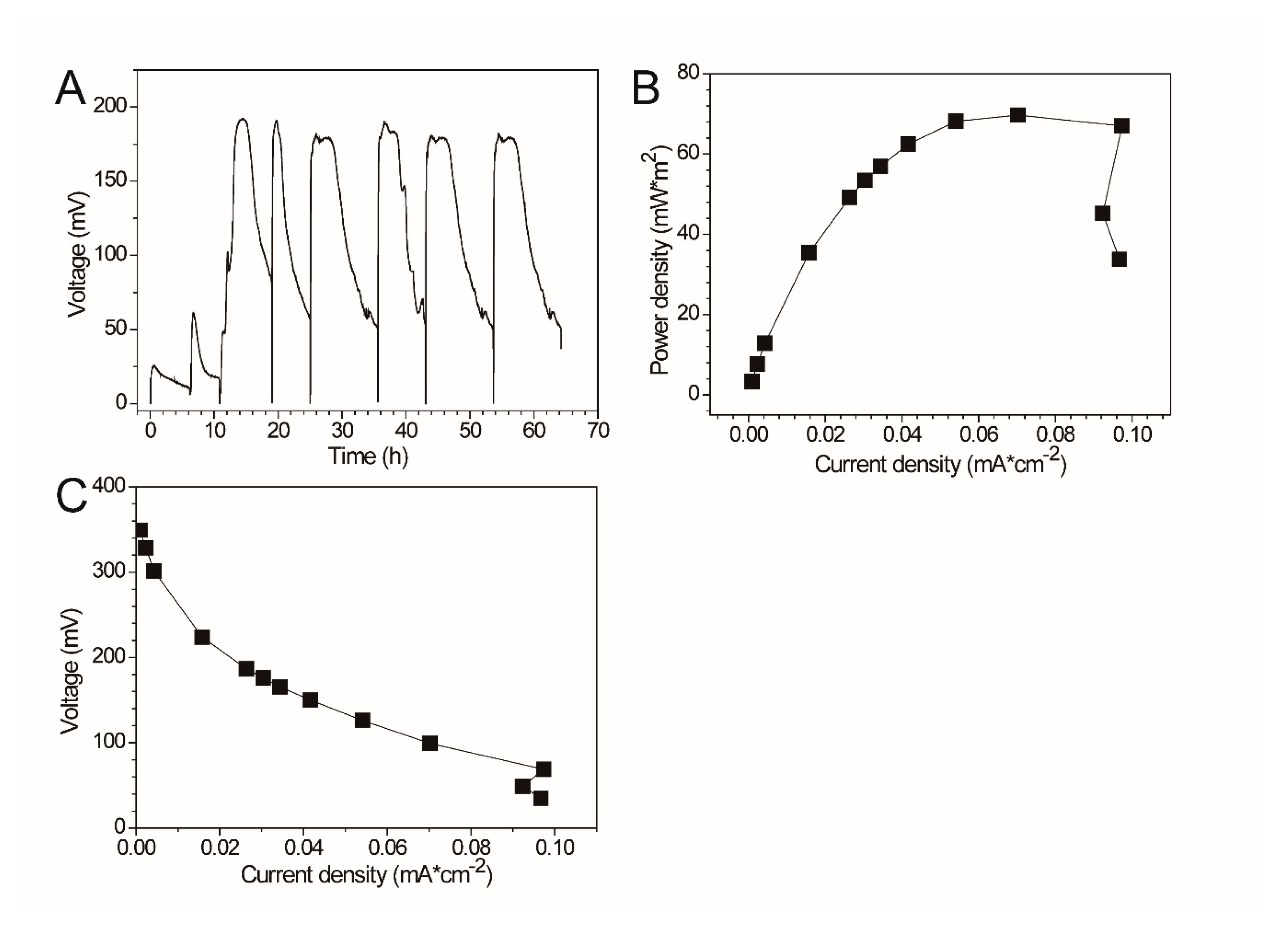
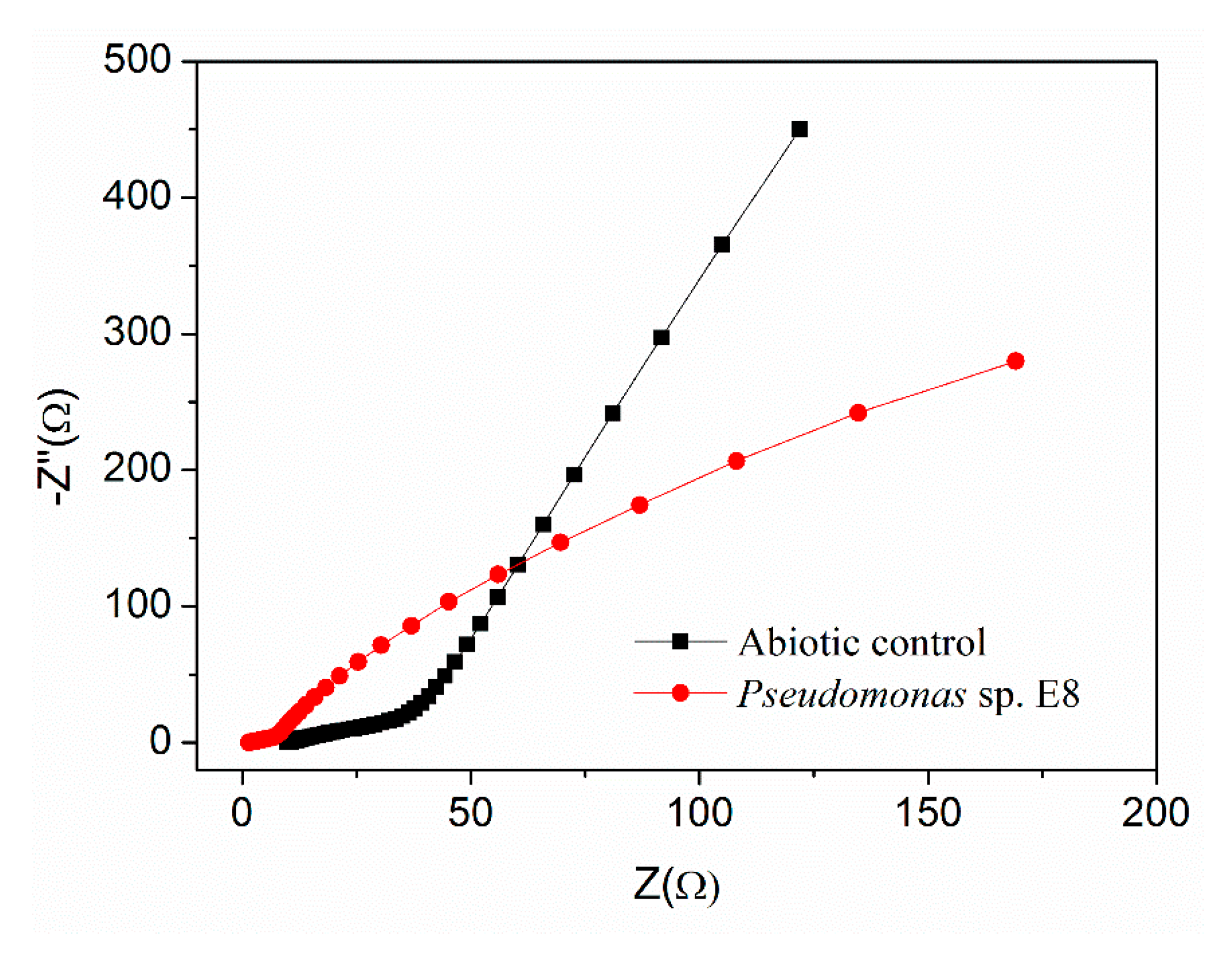
3.2. Electrochemical Properties of Pseudomonas sp. E8
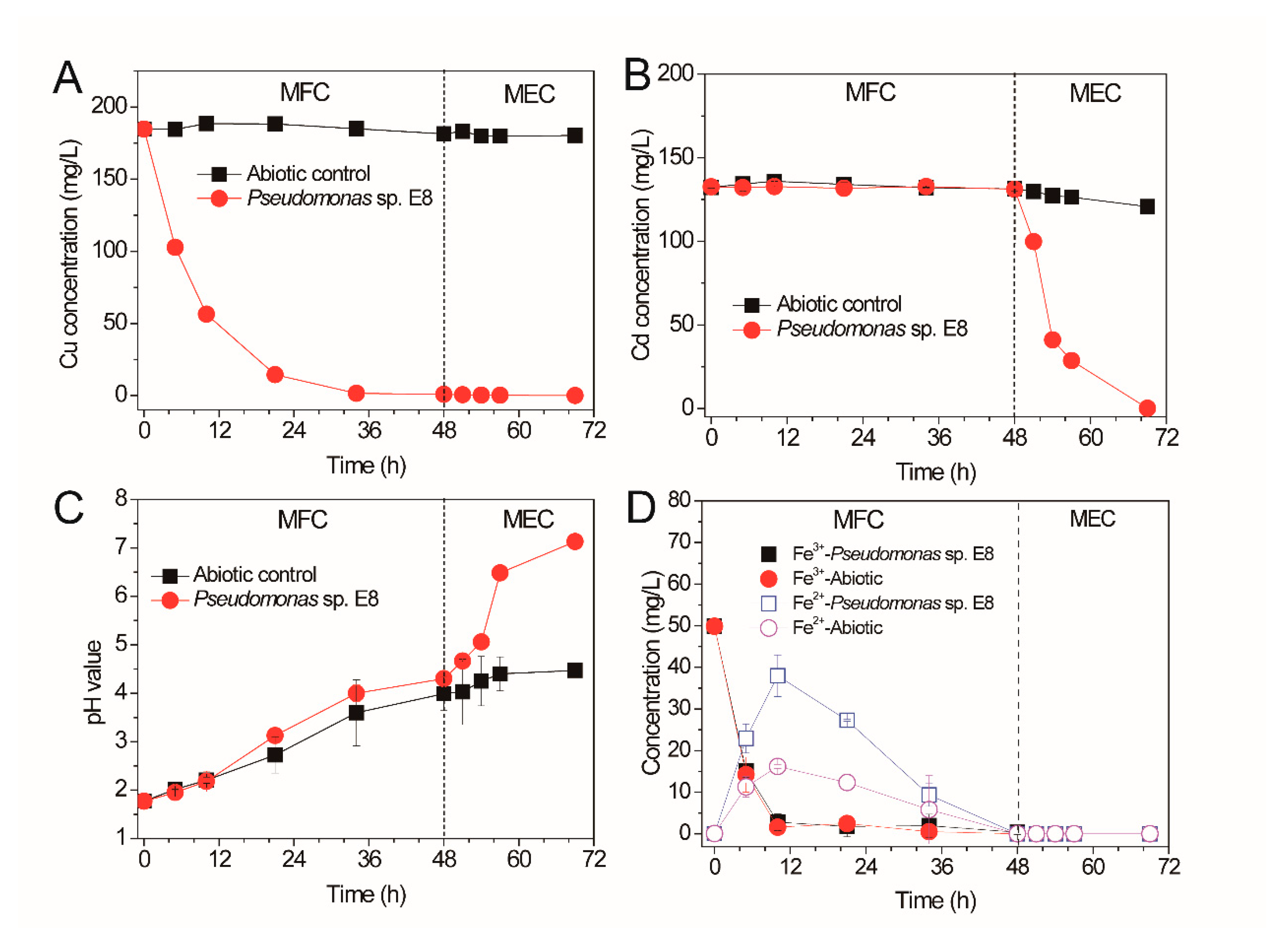
3.3. Sequential Recovery of Cu2+ and Cd2+ from Simulated AMD
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alegbe, M.J.; Ayanda, O.S.; Ndungu, P.; Nechaev, A.; Fatoba, O.O.; Petrik, L.F. Physicochemical characteristics of acid mine drainage, simultaneous remediation and use as feedstock for value added products. J. Environ. Chem. Eng. 2019, 7. [Google Scholar] [CrossRef]
- Ai, C.; Liang, Y.; Miao, B.; Chen, M.; Zeng, W.; Qiu, G. Identification and analysis of a novel gene cluster involves in Fe2+ oxidation in Acidithiobacillus ferrooxidans ATCC 23270, a typical biomining acidophile. Curr. Microbiol. 2018, 75, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Ai, C.; McCarthy, S.; Liang, Y.; Rudrappa, D.; Qiu, G.; Blum, P. Evolution of copper arsenate resistance for enhanced enargite bioleaching using the extreme thermoacidophile Metallosphaera sedula. J. Ind. Microbiol. Biotechnol. 2017, 44, 1613–1625. [Google Scholar] [CrossRef] [PubMed]
- Skousen, J.G.; Ziemkiewicz, P.F.; McDonald, L.M. Acid mine drainage formation, control and treatment: Approaches and strategies. Extr. Ind. Soc. 2019, 6, 241–249. [Google Scholar] [CrossRef]
- Ai, C.; Yan, Z.; Chai, H.; Gu, T.; Wang, J.; Chai, L.; Qiu, G.; Zeng, W. Increased chalcopyrite bioleaching capabilities of extremely thermoacidophilic Metallosphaera sedula inocula by mixotrophic propagation. J. Ind. Microbiol. Biotechnol. 2019, 46, 1113–1127. [Google Scholar] [CrossRef] [PubMed]
- Sima, M.; Dold, B.; Frei, L.; Senila, M.; Balteanu, D.; Zobrist, J. Sulfide oxidation and acid mine drainage formation within two active tailings impoundments in the Golden Quadrangle of the Apuseni Mountains, Romania. J. Hazard. Mater. 2011, 189, 624–639. [Google Scholar] [CrossRef]
- Ryu, S.; Naidu, G.; Hasan Johir, M.A.; Choi, Y.; Jeong, S.; Vigneswaran, S. Acid mine drainage treatment by integrated submerged membrane distillation-sorption system. Chemosphere 2019, 218, 955–965. [Google Scholar] [CrossRef]
- Chen, A.; Lin, C.; Lu, W.; Wu, Y.; Ma, Y.; Li, J.; Zhu, L. Well water contaminated by acidic mine water from the Dabaoshan Mine, South China: Chemistry and toxicity. Chemosphere 2007, 70, 248–255. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.-G.; Huang, P.; Zhang, W.-G.; Yuan, X.-W.; Fan, F.-X.; Wang, H.-L.; Liu, J.-S.; Wang, Z.-H. Leaching of heavy metals from Dexing copper mine tailings pond. Trans. Nonferr. Metals Soc. 2013, 23, 3068–3075. [Google Scholar] [CrossRef]
- Bonnail, E.; Cunha Lima, R.; Bautista-Chamizo, E.; Salamanca, M.J.; Cruz-Hernández, P. Biomarker responses of the freshwater clam Corbicula fluminea in acid mine drainage polluted systems. Environ. Pollut. 2018, 242, 1659–1668. [Google Scholar] [CrossRef]
- Xiang, L.; Liu, P.-H.; Jiang, X.-F.; Chen, P.-J. Health risk assessment and spatial distribution characteristics of heavy metal pollution in rice samples from a surrounding hydrometallurgy plant area in No. 721 uranium mining, East China. J. Geochem. Explor. 2019, 207, 106360. [Google Scholar] [CrossRef]
- Yilmaz, T.; Yucel, A.; Cakmak, Y.; Uyanik, S.; Yurtsever, A.; Ucar, D. Treatment of acidic mine drainage in up-flow sulfidogenic reactor: Metal recovery and the pH neutralization. J. Water Process Eng. 2019, 32, 100916. [Google Scholar] [CrossRef]
- Sephton, M.G.; Webb, J.A. The role of secondary minerals in remediation of acid mine drainage by Portland cement. J. Hazard. Mater. 2019, 367, 267–276. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, S.; Kakade, A.; Kulshreshtha, S.; Liu, P.; Li, X. A review on microbial electrocatalysis systems coupled with membrane bioreactor to improve wastewater treatment. Microorganisms 2019, 7, 372. [Google Scholar] [CrossRef] [Green Version]
- Logan Bruce, E.; Korneel, R. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [Green Version]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells from fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Wang, H.; Ren, Z.J. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 2013, 31, 1796–1807. [Google Scholar] [CrossRef]
- Yuan, S.J.; Li, W.W.; Cheng, Y.Y.; He, H.; Chen, J.J.; Tong, Z.H.; Lin, Z.Q.; Zhang, F.; Sheng, G.P.; Yu, H.Q. A plate-based electrochromic approach for the high-throughput detection of electrochemically active bacteria. Nat. Protoc. 2014, 9, 112–119. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecul. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.A.; Wang, B.S.; Wang, Y.H. Increasing efficiencies of microbial fuel cells for collaborative treatment of copper and organic wastewater by designing reactor and selecting operating parameters. Bioresour. Technol. 2013, 147, 332–337. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.K.R. Microbial fuel cells methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Herrera, L.; Ruiz, P.; Aguillon, J.C.; Fehrmann, A. A new spectrophotometric method for the determination of ferrous iron in the presence of ferric iron. J. Chem. Technol. Biotechnol. 1989, 44, 171–181. [Google Scholar] [CrossRef]
- Yong, X.-Y.; Shi, D.-Y.; Chen, Y.-L.; Jiao, F.; Lin, X.; Zhou, J.; Wang, S.-Y.; Yong, Y.-C.; Sun, Y.-M.; OuYang, P.-K.; et al. Enhancement of bioelectricity generation by manipulation of the electron shuttles synthesis pathway in microbial fuel cells. Bioresour. Technol. 2014, 152, 220–224. [Google Scholar] [CrossRef]
- Thulasinathan, B.; Nainamohamed, S.; Ebenezer Samuel, J.O.; Soorangkattan, S.; Muthuramalingam, J.; Kulanthaisamy, M.; Balasubramani, R.; Nguyen, D.D.; Chang, S.W.; Bolan, N.; et al. Comparative study on Cronobacter sakazakii and Pseudomonas otitidis isolated from septic tank wastewater in microbial fuel cell for bioelectricity generation. Fuel 2019, 248, 47–55. [Google Scholar] [CrossRef]
- Khan, A.; Chen, Z.; Zhao, S.; Ni, H.; Pei, Y.; Xu, R.; Ling, Z.; Salama, E.-S.; Liu, P.; Li, X. Micro-aeration in anode chamber promotes p-nitrophenol degradation and electricity generation in microbial fuel cell. Bioresour. Technol. 2019, 285, 121291. [Google Scholar] [CrossRef]
- Yasri, N.; Roberts, E.P.L.; Gunasekaran, S. The electrochemical perspective of bioelectrocatalytic activities in microbial electrolysis and microbial fuel cells. Energy Rep. 2019, 5, 1116–1136. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, L.; Su, W.; Gao, P.; Li, D.; He, X.; Zhang, Y. The direct electrocatalysis of phenazine-1-carboxylic acid excreted by Pseudomonas alcaliphila under alkaline condition in microbial fuel cells. Bioresour. Technol. 2011, 102, 7099–7102. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Kim, K.-Y.; Resurreccion, E.P.; Lee, W.H. Surfactant addition to enhance bioavailability of bilge water in single chamber microbial fuel cells (MFCs). J. Hazard. Mater. 2019, 368, 732–738. [Google Scholar] [CrossRef]
- Ilamathi, R.; Merline Sheela, A.; Nagendra Gandhi, N. Comparative evaluation of Pseudomonas species in single chamber microbial fuel cell with manganese coated cathode for reactive azo dye removal. Int. Biodeterior. Biodegrad. 2019, 144, 104744. [Google Scholar] [CrossRef]
- He, Z.; Mansfeld, F. Exploring the use of electrochemical impedance spectroscopy (EIS) in microbial fuel cell studies. Energy Environ. Sci. 2009, 2, 215–219. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Ho, K.-L.; Lee, D.-J.; Su, A.; Chang, J.-S. Electricity harvest from nitrate/sulfide-containing wastewaters using microbial fuel cell with autotrophic denitrifier, Pseudomonas sp. C27. Int. J. Hydrog. Energy 2012, 37, 15827–15832. [Google Scholar] [CrossRef]
- Wu, D.; Pan, Y.; Huang, L.; Zhou, P.; Quan, X.; Chen, H. Complete separation of Cu(II), Co(II) and Li(I) using self-driven MFCs–MECs with stainless steel mesh cathodes under continuous flow conditions. Sep. Purif. Technol. 2015, 147, 114–124. [Google Scholar] [CrossRef]
- Tao, H.-C.; Liang, M.; Li, W.; Zhang, L.-J.; Ni, J.-R.; Wu, W.-M. Removal of copper from aqueous solution by electrodeposition in cathode chamber of microbial fuel cell. J. Hazard. Mater. 2011, 189, 186–192. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, L.; Wu, D.; Huang, L.; Zhou, P.; Quan, X.; Chen, G. Dependency of simultaneous Cr(VI), Cu(II) and Cd(II) reduction on the cathodes of microbial electrolysis cells self-driven by microbial fuel cells. J. Power Sources 2015, 273, 1103–1113. [Google Scholar] [CrossRef]
- Colantonio, N.; Kim, Y. Cadmium (II) removal mechanisms in microbial electrolysis cells. J. Hazard. Mater. 2016, 311, 134–141. [Google Scholar] [CrossRef]
- Peng, T.; Zhou, D.; Liu, Y.; Yu, R.; Qiu, G.; Zeng, W. Effects of pH value on the expression of key iron/sulfur oxidation genes during bioleaching of chalcopyrite on thermophilic condition. Ann. Microbiol. 2019, 69, 627–635. [Google Scholar] [CrossRef]
- Ai, C.; McCarthy, S.; Eckrich, V.; Rudrappa, D.; Qiu, G.; Blum, P. Increased acid resistance of the archaeon, Metallosphaera sedula by adaptive laboratory evolution. J. Ind. Microbiol. Biotechnol. 2016, 43, 1455–1465. [Google Scholar] [CrossRef]
- Ai, C.; Yan, Z.; Zhou, H.; Hou, S.; Chai, L.; Qiu, G.; Zeng, W. Metagenomic insights into the effects of seasonal temperature variation on the activities of activated sludge. Microorganisms 2019, 7, 713. [Google Scholar] [CrossRef] [Green Version]
- Stöckl, M.; Teubner, N.C.; Holtmann, D.; Mangold, K.M.; Sand, W. Extracellular polymeric substances from Geobacter sulfurreducens biofilms in microbial fuel cells. ACS Appl. Mater. Interfaces. 2019, 11, 8961–8968. [Google Scholar] [CrossRef]

| Name of Bacterial Species | Device Type | Anode Material | Cathode Material | Exchange Membrane | Energy Substrate | Coulombic Efficiency | Maximum Power Density | Reference |
|---|---|---|---|---|---|---|---|---|
| Pseudomonas aeruginosa PAO1 | single-chamber MFC (180 mL) | Pt-loaded carbon cloth (7 cm2) | Pt-loaded carbon cloth (7 cm2) | \ | glucose | \ | 42.53 μW/cm2 | [24] |
| Pseudomonas putida ATCC 49128 | single-chamber MFC (40 mL) | a carbon fiber brush (area, 17.7 cm2) | carbonized 50 × 50 mesh stainless steel | \ | bilge water | 27.20% | 0.04 mW/m2 | [29] |
| Pseudomonas fluorescens MTCC 2269 | single-chamber MFC (180 mL) | graphite block | treated carbon cloth (9 cm2) | \ | glucose | \ | 83.04 ± 4.0 μW/m2 | [30] |
| Pseudomonas otitidis AATB4 | dual-chamber MFC (each chamber 250 mL) | graphite electrode | graphite electrode | proton exchange membrane | septic tank wastewater | \ | 218 mW/m2 | [25] |
| Pseudomonas monteilii LZU-3 | dual-chamber MFC (each chamber 240 mL) | carbon felt (16 cm2) | carbon felt (16 cm2) | proton exchange membrane | p-nitrophenol | \ | 31 mW/m2 | [26] |
| Pseudomonas sp. C27 | dual-chamber MFC (each chamber 78 mL) | carbon felt (area, 6 cm2) | carbon cloth (area, 9 cm2) | cation exchange membrane | sulfide | 25.60% | 40 mW/m2 | [32] |
| Pseudomonas alcaliphila MBR | dual-chamber MFC (each chamber 245 mL) | carbon felt (30 cm2) | carbon felt (30 cm2) | cation exchange membrane | sodium citrate | \ | \ | [28] |
| Pseudomonas sp. E8 | single-chamber MFC (28 mL) | carbon brush | carbon cloth (area, 7 cm2) | \ | LB | 12.70% | 70.4 mW/m2 | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ai, C.; Hou, S.; Yan, Z.; Zheng, X.; Amanze, C.; Chai, L.; Qiu, G.; Zeng, W. Recovery of Metals from Acid Mine Drainage by Bioelectrochemical System Inoculated with a Novel Exoelectrogen, Pseudomonas sp. E8. Microorganisms 2020, 8, 41. https://doi.org/10.3390/microorganisms8010041
Ai C, Hou S, Yan Z, Zheng X, Amanze C, Chai L, Qiu G, Zeng W. Recovery of Metals from Acid Mine Drainage by Bioelectrochemical System Inoculated with a Novel Exoelectrogen, Pseudomonas sp. E8. Microorganisms. 2020; 8(1):41. https://doi.org/10.3390/microorganisms8010041
Chicago/Turabian StyleAi, Chenbing, Shanshan Hou, Zhang Yan, Xiaoya Zheng, Charles Amanze, Liyuan Chai, Guanzhou Qiu, and Weimin Zeng. 2020. "Recovery of Metals from Acid Mine Drainage by Bioelectrochemical System Inoculated with a Novel Exoelectrogen, Pseudomonas sp. E8" Microorganisms 8, no. 1: 41. https://doi.org/10.3390/microorganisms8010041




