The Bacterial Microbiome in the Small Intestine of Hooded Seals (Cystophora cristata)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Sampling
2.2. DNA Extraction
2.3. Sequencing
2.4. Sequence Processing
2.5. Sequence Analysis
2.6. Statistical Analysis
3. Results
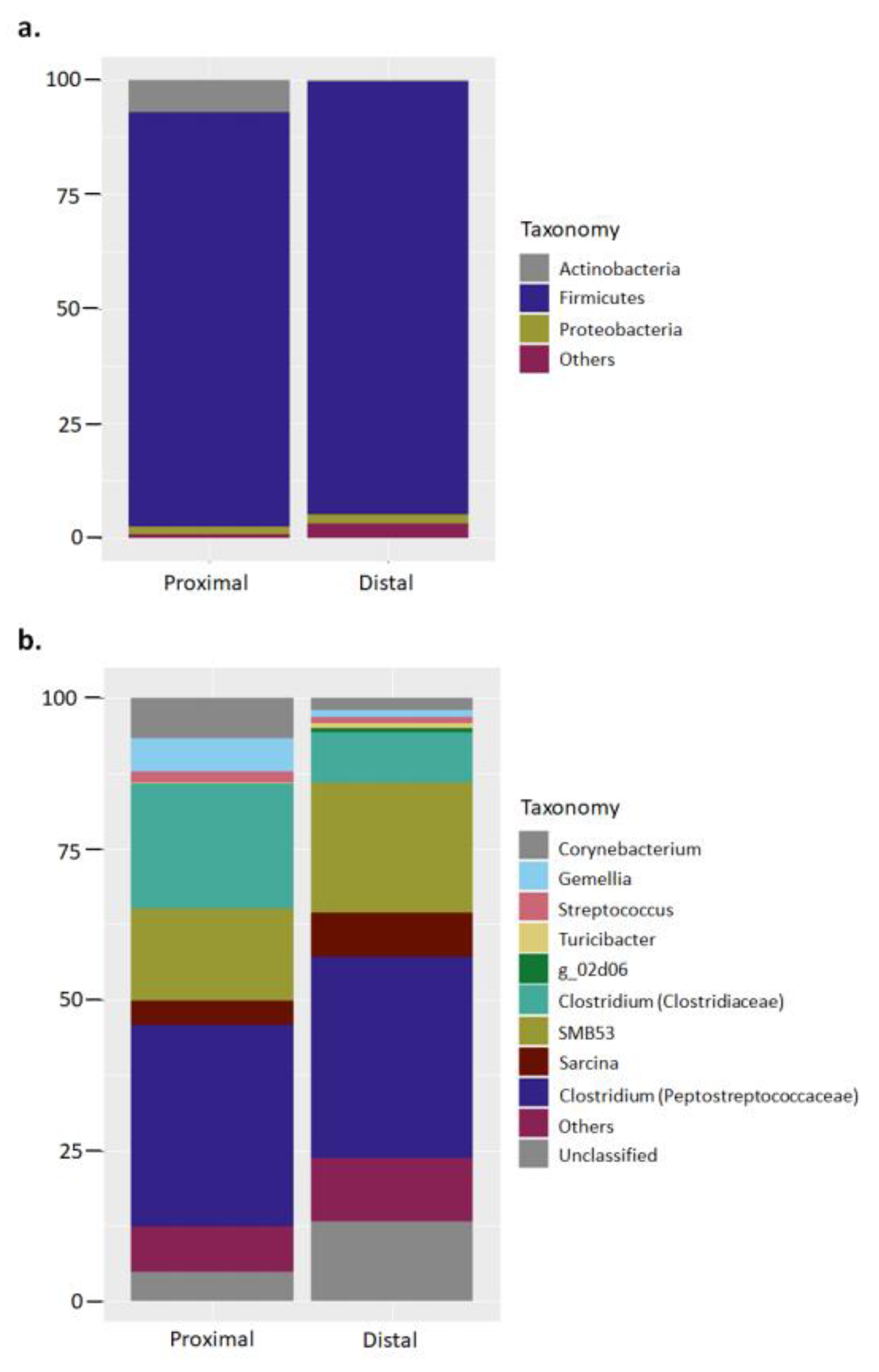
3.1. Bar Chart Plot
3.2. Group-Based Diversity Tests
3.3. Comparison with the Microbiota from Other Marine Mammals
4. Discussion
4.1. Homogenous Bacterial Microbiome Along the Small Intestine of the Hooded Seals
4.2. Comparing the Small Intestine Microbiome of the Hooded Seal to That of Other Marine Mammals
4.3. Potential Effect of Antimicrobial and Antiparasitic Treatment on the Gut Microbiota
4.4. Importance of the Gut Microbiota in Food Digestion in Carnivores
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Folkow, L.P.; Mårtensson, P.-E.; Blix, A.S. Annual distribution of hooded seals (Cystophora cristata) in the Greenland and Norwegian seas. Polar Biol. 1996, 16, 179–189. [Google Scholar] [CrossRef]
- Andersen, J.M.; Wiersma, Y.F.; Stenson, G.B.; Hammill, M.O.; Rosing-Asvid, A.; Skern-Maurizen, M. Habitat selection by hooded seals (Cystophora cristata) in the Northwest Atlantic Ocean. ICES J. Mar. Sci. 2012, 70, 173–185. [Google Scholar] [CrossRef]
- Vacquie-Garcia, J.; Lydersen, C.; Biuw, M.; Haug, T.; Fedak, M.A.; Kovacs, K.M. Hooded seal Cystophora cristata foraging areas in the Northeast Atlantic Ocean—Investigated using three complementary methods. PLoS ONE 2017, 12, e0187889. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, D.E. A rediscovered whelping population of hooded seals, Cystophora cristata Erxleben, and its possible relationship to other populations. Polarforschung 1974, 44, 1–7. [Google Scholar]
- Folkow, L.; Blix, A.S. Distribution and Diving Behaviour of Hooded Seals; Elsevier BV: Amsterdam, The Netherlands, 1995; Volume 4, pp. 193–202. [Google Scholar]
- Folkow, L.P.; Blix, A.S. Diving behavior of hooded seals (Cystophora cristata) in the Greenland and Norwegian Seas. Polar Biol. 1999, 22, 61–74. [Google Scholar] [CrossRef]
- Hammill, M.O.; Stenson, G. Abundance of Northwest Atlantic hooded seals (1960–2005). DFO Canada. Canadian Science Advisory Secretariat Research Document 2006. p. 19. Available online: http://www.dfo-mpo.gc.ca/csas/ (accessed on 30 May 2020).
- Bowen, W.D.; Oftedal, O.T.; Boness, D.J. Birth to weaning in 4 days: Remarkable growth in the hooded seal, Cystophora cristata. Can. J. Zool. 1985, 63, 2841–2846. [Google Scholar] [CrossRef]
- Lydersen, C.; Kovacs, K.M.; Hammill, M.O. Energetics during nursing and early postweaning fasting in hooded seal (Cystophora cristata) pups from the Gulf of St Lawrence, Canada. J. Comp. Physiol. B 1997, 167, 81–88. [Google Scholar] [CrossRef]
- Oftedal, O.T.; Bowen, D.; Widdowson, E.M.; Boness, D.J. Effects of Suckling and the Postsuckling Fast on Weights of the Body and Internal Organs of Harp and Hooded Seal Pups. Neonatology 1989, 56, 283–300. [Google Scholar] [CrossRef]
- Schots, P.C.; Bue, M.E.; Nordøy, E.S. Hooded seal (Cystophora cristata) pups ingest snow and seawater during their post-weaning fast. J. Comp. Physiol. B 2016, 187, 493–502. [Google Scholar] [CrossRef] [Green Version]
- Bowen, W.D.; Boness, D.J.; Oftedal, O.T. Mass transfer from mother to pup and subsequent mass loss by the weaned pup in the hooded seal, Cystophora cristata. Can. J. Zool. 1987, 65, 1–8. [Google Scholar] [CrossRef]
- Haug, T.; Nilssen, K.T.; Lindblom, L. First independent feeding of harp seal (Phoca groenlandica) and hooded seal (Cystophora cristata) pups in the Greenland Sea. NAMMCO Sci. Publ. 2000, 2, 29–39. [Google Scholar] [CrossRef]
- Olsen, M.A.; Nilssen, K.T.; Mathiesen, S.D. Gross anatomy of the gastrointestinal system of harp seals (Phoca groenlandica). J. Zool. 1996, 238, 581–589. [Google Scholar] [CrossRef]
- Christiansen, J.S.; Gildberg, A.; Nilssen, K.T.; Lindblom, C.; Haug, T. The gastric properties of free-ranging harp (Pagophilus groenlandicus (Erxleben, 1777)) and hooded (Cystophora cristata (Erxleben, 1777)) seals. ICES J. Mar. Sci. 2004, 61, 287–292. [Google Scholar] [CrossRef]
- Mårtensson, P.-E.; Nordøy, E.S.; Messelt, E.B.; Blix, A.S. Gut length, food transit time and diving habit in phocid seals. Polar Biol. 1998, 20, 213–217. [Google Scholar] [CrossRef]
- Bryden, M. Size and growth of viscer in the southern elephant seal, Mirounga leonina (L.). Aust. J. Zool. 1971, 19, 103. [Google Scholar] [CrossRef]
- Helm, R.C. Intestinal length of three California pinniped species. J. Zool. 2009, 199, 297–304. [Google Scholar] [CrossRef]
- Krockenberger, M.B.; Bryden, M.M. Rate of passage of digesta through the alimentary tract of southern elephan seals (Mirounga leonina) (Carnivora: Phocidae). J. Zool. Lond. 1994, 234, 229–237. [Google Scholar] [CrossRef]
- Kapel, F. Feeding habits of harp and hooded seals in Greenland waters. NAMMCO Sci. Publ. 2000, 2, 50–64. [Google Scholar] [CrossRef] [Green Version]
- Tucker, S.; Bowen, W.; Iverson, S.; Blanchard, W.; Stenson, G. Sources of variation in diets of harp and hooded seals estimated from quantitative fatty acid signature analysis (QFASA). Mar. Ecol. Prog. Ser. 2009, 384, 287–302. [Google Scholar] [CrossRef]
- Wang, T.; Hung, C.C.Y.; Randall, D.J. The comparative physiology of food deprivation: From Feast to Famine. Annu. Rev. Physiol. 2006, 68, 223–251. [Google Scholar] [CrossRef]
- Glad, T.; Kristiansen, V.F.; Nielsen, K.M.; Brusetti, L.; Wright, A.-D.G.; Sundset, M.A. Ecological characterisation of the colonic microbiota in arctic and sub-arctic seals. Microb. Ecol. 2010, 60, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Numberger, D.; Herlemann, D.P.R.; Jürgens, K.; Dehnhardt, G.; Schulz-Vogt, H. Comparative analysis of the fecal bacterial community of five harbor seals (Phoca vitulina). Microbiology 2016, 5, 782–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, T.M. Factor Influencing the gut microbiota of antartic seals. Ph.D. Thesis, University of New South Wales, Sydney, Australia, 2012. [Google Scholar]
- Nelson, T.M.; Rogers, T.L.; Carlini, A.R.; Brown, M.V. Diet and phylogeny shape the gut microbiota of Antarctic seals: A comparison of wild and captive animals. Environ. Microbiol. 2012, 15, 1132–1145. [Google Scholar] [CrossRef] [PubMed]
- Kastl, A.J.; Terry, N.A.; Wu, G.D.; Albenberg, L.G. The structure and function of the human small intestinal microbiota: Current understanding and future directions. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 33–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Morrison, M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechnology 2004, 36, 808–812. [Google Scholar] [CrossRef]
- Ishaq, L.S.; Wright, A. High-throughput DNA sequencing of the ruminal bacteria from moose (Alces alces) in Vermont, Alaska, and Norway. Microb. Ecol. 2014, 68, 185–195. [Google Scholar] [CrossRef]
- Ovreås, L.; Forney, L.; Daae, F.L.; Torsvik, V. Distribution of bacterioplankton in meromictic Lake Saelenvannet, as determined by denaturing gradient gel electrophoresis of PCR-amplified gene fragments coding for 16S rRNA. Appl. Environ. Microbiol. 1997, 63, 3367–3373. [Google Scholar] [CrossRef] [Green Version]
- Salgado-Flores, A.; Hagen, L.H.; Ishaq, S.L.; Zamanzadeh, M.; Wright, A.-D.G.; Pope, P.B.; Sundset, M.A. Rumen and Cecum Microbiomes in Reindeer (Rangifer tarandus tarandus) Are Changed in Response to a Lichen Diet and May Affect Enteric Methane Emissions. PLoS ONE 2016, 11, e0155213. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; DeSantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 2009, 26, 266–267. [Google Scholar] [CrossRef] [Green Version]
- Cole, J.R. The Ribosomal Database Project (RDP-II): Previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res. 2003, 31, 442–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; A Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efrancino, M.P. Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front. Microbiol. 2016, 6, 1543. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.C.; Chalker, A.; Dewar, M.L.; Arnould, J.P.Y. Age-related differences revealed in Australian fur seal Arctocephalus pusillus doriferus gut microbiota. FEMS Microbiol. Ecol. 2013, 86, 246–255. [Google Scholar] [CrossRef] [Green Version]
- Bik, E.M.; Costello, E.K.; Switzer, A.D.; Callahan, B.J.; Holmes, S.P.; Wells, R.S.; Carlin, K.P.; Jensen, E.D.; Venn-Watson, S.; Relman, D.A. Marine mammals harbour unique microbiotas shaped by and yet distinct from the sea. Nature Comm. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Delport, T.C.; Power, M.L.; Harcourt, R.G.; Webster, K.N.; Tetu, S.G. Colony location and captivity influence the gut microbial community composition of the Australian sea lion (Neophoc cinerea). AEM Appl. Environ. Microbiol. 2016. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, A.W.; Giongo, A.; Valdez, F.P.; de Amorin, D.B.; Tavares, M.; d’Awevedo, P.A.; Franco, A.C.; Frazzon, J.; Frazzon, A.P.G. Characterization of the faecal bacterial community of wild young South American (Arctocephalus australis) and Subantarctic fur seals (Arctocephalus tropicalis). FEMS Microbiol. Ecol. 2016, 92. [Google Scholar] [CrossRef]
- McLaughlin, R.W.; Chen, M.; Zheng, J.; Zhao, Q.; Wang, D. Analysis of the bacterial diversity in the fecal material of the endangered Yangtze finless porpoise, Neophocaena phocaenoides asiaeorientalis. Mol. Biol. Rep. 2012, 39, 5669–5676. [Google Scholar] [CrossRef]
- Zoetendal, E.G.; Raes, J.; Bogert, B.V.D.; Arumugam, M.; Booijink, C.C.G.M.; Troost, F.J.; Bork, P.; Wels, M.; De Vos, W.M.; Kleerebezem, M. The human small intestinal microbiota is driven by rapid uptake and conversion of simple carbohydrates. ISME J. 2012, 6, 1415–1426. [Google Scholar] [CrossRef]
- De Oliveira, M.N.V.; Jewell, K.A.; Freitas, F.S.; Benjamin, L.A.; Tótola, M.R.; Borges, A.C.; Moraes, C.A.; Suen, G. Characterizing the microbiota across the gastrointestinal tract of a Brazilian Nelore steer. Vet. Microbiol. 2013, 164, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Huang, X.; Fang, S.; Xin, W.; Huang, L.; Chen, C. Uncovering the composition of microbial community structure and metagenomics among three gut locations in pigs with distinct fatness. Sci. Rep. 2016, 6, 27427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenzie, V.J.; Song, S.J.; Delsuc, F.; Prest, T.L.; Oliverio, A.M.; Korpita, T.M.; Alexiev, A.; Amato, K.R.; Metcalf, J.L.; Kowalewski, M.; et al. The Effects of Captivity on the Mammalian Gut Microbiome. Integr. Comp. Biol. 2017, 57, 690–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Cao, J.; Li, J.-R.; Yang, F.; Li, Z.; Li, L. Comparative analysis of the gastrointestinal microbial communities of bar-headed goose (Anser indicus) in different breeding patterns by high-throughput sequencing. Microbiol. Res. 2016, 182, 59–67. [Google Scholar] [CrossRef]
- Horie, M.; Miura, T.; Hirakata, S.; Hosoyama, A.; Sugino, S.; Umeno, A.; Murotomi, K.; Yoshida, Y.; Koike, T. Comparative analysis of the intestinal flora in type 2 diabetes and nondiabetic mice. Exp. Anim. 2017, 66, 405–416. [Google Scholar] [CrossRef] [Green Version]
- Menke, S.; Wasimuddin; Meier, M.; Melzheimer, J.; Mfune, J.K.E.; Heinrich, S.; Thalwitzer, S.; Wachter, B.; Sommer, S. Oligotyping reveals differences between gut microbiomes of free-ranging sympatric Namibian carnivores (Acinonyx jubatus, Canis mesomelas) on a bacterial species-like level. Front. Microbiol. 2014, 5, 526. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, X.; Zhang, Y.; Zheng, K.; Xiang, Q.; Chen, N.; Chen, Z.; Zhang, N.; Zhu, J.; He, Q. Antibiotic-Induced Disruption of Gut Microbiota Alters Local Metabolomes and Immune Responses. Front. Cell. Infect. Microbiol. 2019, 9, 99. [Google Scholar] [CrossRef]
- Easton, A.V.; Quiñones, M.; Vujkovic-Cvijin, I.; Oliveira, R.G.; Kepha, S.; Odiere, M.R.; Anderson, R.M.; Belkaid, Y.; Nutman, T.B. The impact of anthelmintic treatment on human gut microbiota based on cross-sectional and pre- and postdeworming comparisons in Western Kenya. mBio 2019, 10, e005191-9. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, T.P.; Formenti, F.; Castro, C.; Piubelli, C.; Perandin, F.; Buonfrate, D.; Otranto, D.; Griffin, J.L.; Krause, L.; Bisoffi, Z.; et al. A comprehensive analysis of the fecal microbiome and metabolome of Strongyloides stercoralis infected volunteers from a non-endemic area. Sci. Rep. 2018, 8, 15651. [Google Scholar] [CrossRef] [Green Version]
- Crotch-Harvey, L.; Thomas, L.-A.; Worgan, H.J.; Douglas, J.-L.; Gilby, D.E.; McEwan, N.R. The effect of administration of fenbendazole on the microbial hindgut population of the horse. J. Equine Sci. 2018, 29, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Semova, I.; Carten, J.D.; Stombaugh, J.; Mackey, L.C.; Knight, R.; Farber, S.A.; Rawls, J.F. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe 2012, 12, 277–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Guryn, K.; Hubert, N.; Frazier, K.; Urlass, S.; Musch, M.W.; Ojeda, P.; Pierre, J.F.; Miyoshi, J.; Sontag, T.J.; Cham, C.M.; et al. Small intestine microbiota regulate host digestive and absorptive adaptive responses to dietary lipids. Cell Host Microbe 2018, 23, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nat. Cell Biol. 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Schwiertz, A.; Taras, D.; Schaefer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef]
- Jensen, K.N.; Jacobsen, C.; Nielsen, H.H. Fatty acid composition of herring (Clupea harengus L.): Influence of time and place of catch on n-3 PUFA content. J. Sci. Food Agric. 2007, 87, 710–718. [Google Scholar] [CrossRef]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of omega-3 fatty acids on the gut microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef] [Green Version]

| Species | Latin Name | Location—Diet | Samples | Bacterial Community | Method | Reference |
|---|---|---|---|---|---|---|
| Hooded seal | Cystophora cristata | Wild (Greenland Sea)—Halibut, herring, cod, squid, crustaceans | Colon | Bacteroidetes (68%) Firmicutes (22%) Proteobacteria (9%) | BigDye—Sanger sequencing | [23] |
| Harbor seal | Phoca vitulina | Wild (Ringvassøy, Troms)—saithe, cod, herring, sculpin | Colon | Firmicutes (50%) Bacteroidetes (49%) | BigDye—Sanger sequencing | [23] |
| Gray seal | Halichoerus grypus | Wild (Ringvassøy, Troms)—Cod, saithe, herring, sandeel, catfish | Colon | Firmicutes (76%) Bacteroidetes (24%) | BigDye—Sanger sequencing | [23] |
| Harbor seals | Phoca vitulina | Captive – herring, sprat, crustaceans | Feces | Firmicutes (33 ± 11%) Bacteroidetes (27 ± 7%) Fusobacteria (26 ± 4%) Proteobacteria (13 ± 5%) | 454 pyrosequencing | [24] |
| Southern elephant seals | Mirounga leonina | Wild (Western Antarctica)—squid, fish | Rectal swab | Firmicutes (42 ± 10%) Bacteroidetes (22 ± 5%) Fusobacteria (20 ± 8) Proteobacteria (16 ± 8%) | 454 pyrosequencing | [26] |
| Leopard seals | Hydrurga leptonyx | Wild (Western Antarctica)—seals, krill, penguins, fish/captive (Taronga zoo)—fish | Rectal swab | Firmicutes (45 ± 13%) Proteobacteria (33 ± 12%) Fusobacteria (14 ± 8%) Firmicutes (60 ± 32%) Fusobacteria (34 ± 13%) | 454 pyrosequencing | [26] |
| Australian fur seals | Arctocephalus pusillus doriferus | Wild (Kanowna island, Australia)—fish and cephalops | Feces | Firmicutes (67%) Bacteroidetes (15%) Actinobacteria (3%) | FISH | [38] |
| California sea lion | Zalophus californianus | Captive—fed fish and squid | Rectal swab | Bacteroidetes (40 ± 21%) Firmicutes (29 ± 20%) Fusobacteria (25 ± 9) | Sanger sequencing | [39] |
| Australian sea lion | Neophoca cinerea | Wild—benthic fish, squid, lobster, small crustacean)/captive—frozen fish | Feces | Firmicutes (74 ± 28%) Proteobacteria (10 ± 20%) Bacteroidetes (9 ± 16%) Firmicutes (58 ± 33%) Proteobacteria (30 ± 35.9%) Bacteroidetes (5 ± 8.6%) | Illumina MiSeq sequencing | [40] |
| South American fur seals | Arctocephalus australis | Wild dead animal (Southern coast of Brazil)—fish, cephalopods, crustaceans | Feces | Firmicutes (89 ± 6%) Proteobacteria (6 ± 6%) Actinobacteria (3 ± 2%) | Ion Torrent | [41] |
| Subantarctic fur seals | Arctocephalus tropicalis | Wild dead animal (Southern coast of Brazil)—fish, cephalopods, crustaceans, rock hoper penguins | Feces | Firmicutes (84 ± 6%) Actinobacteria (11 ± 3%) | Ion Torrent | [41] |
| Yangtze porpoise | Neophocaena phocaenoides asiaeorientalis | Wild (Jianxi, China)—fish, crustaceans, cephalopods | Feces | Firmicutes (51.3%) Tenericutes (17.9%) Proteobacteria (15.4%) Actinobacteria (7.7%) | Sanger sequencing | [42] |
| Hooded seals | Cystophora cristata | Captive—herring and diet supplements | Small intestine | Firmicutes (93 ± 9%) Actinobacteria (4 ± 9%) Proteobacteria (2 ± 3%) | Illumina HiSeq sequencing | Current study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acquarone, M.; Salgado-Flores, A.; Sundset, M.A. The Bacterial Microbiome in the Small Intestine of Hooded Seals (Cystophora cristata). Microorganisms 2020, 8, 1664. https://doi.org/10.3390/microorganisms8111664
Acquarone M, Salgado-Flores A, Sundset MA. The Bacterial Microbiome in the Small Intestine of Hooded Seals (Cystophora cristata). Microorganisms. 2020; 8(11):1664. https://doi.org/10.3390/microorganisms8111664
Chicago/Turabian StyleAcquarone, Mario, Alejandro Salgado-Flores, and Monica Alterskjær Sundset. 2020. "The Bacterial Microbiome in the Small Intestine of Hooded Seals (Cystophora cristata)" Microorganisms 8, no. 11: 1664. https://doi.org/10.3390/microorganisms8111664





