Enhanced Anaerobic Digestion of Long Chain Fatty Acid by Adding Magnetite and Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inoculum Preparation
2.2. Experimental
2.3. Analysis and Calculations
2.4. Microbial Community Analysis
3. Results and Discussion
3.1. CH4 Production At Different Concentrations of Oleic Acid
3.2. Conductive Material-Based Broth Change
3.3. Conductive Material-Based Microbial Community Change
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yan, W.; Shen, N.; Xiao, Y.; Chen, Y.; Sun, F.; Kumar Tyagi, V.; Zhou, Y. The role of conductive materials in the start-up period of thermophilic anaerobic system. Bioresour. Technol. 2017, 239, 336–344. [Google Scholar]
- Park, J.H.; Kang, H.J.; Park, K.H.; Park, H.D. Direct interspecies electron transfer via conductive materials: A perspective for anaerobic digestion applications. Bioresour. Technol. 2018, 254, 300–311. [Google Scholar]
- Shen, L.; Zhao, Q.; Wu, X.; Li, X.; Li, Q.; Wang, Y. Interspecies electron transfer in syntrophic methanogenic consortia: From cultures to bioreactors. Renew. Sustain. Energy Rev. 2016, 54, 1358–1367. [Google Scholar]
- Lin, R.; Cheng, J.; Zhang, J.; Zhou, J.; Cen, K.; Murphy, J.D. Boosting biomethane yield and production rate with graphene: The potential of direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 239, 345–352. [Google Scholar]
- Zhao, Z.; Zhang, Y.; Li, Y.; Dang, Y.; Zhu, T.; Quan, X. Potentially shifting from interspecies hydrogen transfer to direct interspecies electron transfer for syntrophic metabolism to resist acidic impact with conductive carbon cloth. Chem. Eng. J. 2017, 313, 10–18. [Google Scholar]
- Zhao, Z.; Zhang, Y.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Enhancing syntrophic metabolism in up-flow anaerobic sludge blanket reactors with conductive carbon materials. Bioresour. Technol. 2015, 191, 140–145. [Google Scholar]
- Zhao, Z.; Zhang, Y.; Wang, L.; Quan, X. Potential for direct interspecies electron transfer in an electric- anaerobic system to increase methane production from sludge digestion. Sci. Rep. 2015, 5, 1–12. [Google Scholar]
- Alves, M.M.; Pereira, M.A.; Sousa, D.Z.; Cavaleiro, A.J.; Picavet, M.; Smidt, H.; Stams, A.J.M. Waste lipids to energy: How to optimize methane production from long-chain fatty acids (LCFA). Microb. Biotechnol. 2009, 2, 538–550. [Google Scholar]
- Cavaleiro, A.J.; Salvador, A.F.; Alves, J.I.; Alves, M. Continuous high rate anaerobic treatment of oleic acid based wastewater is possible after a step feeding start-up. Environ. Sci. Technol. 2009, 43, 2931–2936. [Google Scholar]
- Pereira, M.A.; Sousa, D.Z.; Mota, M.; Alves, M.M. Mineralization of LCFA Associated With Anaerobic Sludge: Kinetics, Enhancement of Methanogenic Activity, and Effect of VFA. Biotechnol. Bioeng. 2004, 88, 502–511. [Google Scholar]
- Sousa, D.Z.; Salvador, A.F.; Ramos, J.; Guedes, A.P.; Barbosa, S.; Stams, A.J.M.; Alves, M.M.; Pereira, M.A. Activity and viability of methanogens in anaerobic digestion of unsaturated and saturated long-chain fatty acids. Appl. Environ. Microbiol. 2013, 79, 4239–4245. [Google Scholar]
- Sharma, P.; Khardenavis, A.; Purohit, H. Metabolism of Long-Chain Fatty Acids (LCFAs) in Methanogenesis. In Microbial Factories; Springer: New Delhi, India, 2015; ISBN 978-81-322-2594-2. [Google Scholar]
- Harris, P.W.; Schmidt, T.; McCabe, B.K. Evaluation of chemical, thermobaric and thermochemical pre-treatment on anaerobic digestion of high-fat cattle slaughterhouse waste. Bioresour. Technol. 2017, 244, 605–610. [Google Scholar]
- Wu, L.J.; Kobayashi, T.; Li, Y.Y.; Xu, K.Q.; Lv, Y. Determination and abatement of methanogenic inhibition from oleic and palmitic acids. Int. Biodeterior. Biodegrad. 2017, 123, 10–16. [Google Scholar]
- Battimelli, A.; Carrère, H.; Delgenès, J. Saponification of fatty slaughterhouse wastes for enhancing anaerobic biodegradability. Bioresour. Technol. 2009, 100, 3695–3700. [Google Scholar]
- Ahn, J.H.; Do, T.H.; Kim, S.D.; Hwang, S. The effect of calcium on the anaerobic digestion treating swine wastewater. Biochem. Eng. J. 2006, 30, 33–38. [Google Scholar]
- Oh, S.T.; Martin, A.D. Long chain fatty acids degradation in anaerobic digester: Thermodynamic equilibrium consideration. Process Biochem. 2010, 45, 335–345. [Google Scholar]
- Duarte, M.S.; Silva, S.A.; Salvador, A.F.; Cavaleiro, A.J.; Stams, A.J.M.; Alves, M.M.; Pereira, M.A. Insight into the Role of Facultative Bacteria Stimulated by Microaeration in Continuous Bioreactors Converting LCFA to Methane. Environ. Sci. Technol. 2018, 52, 6497–6507. [Google Scholar]
- Viggi, C.C.; Rossetti, S.; Fazi, S.; Paiano, P.; Majone, M.; Aulenta, F.; Cruz Viggi, C.; Rossetti, S.; Fazi, S.; Paiano, P.; et al. Magnetite particles triggering a faster and more robust syntrophic pathway of methanogenic propionate degradation. Environ. Sci. Technol. 2014, 48, 7536–7543. [Google Scholar]
- Im, S.; Yun, Y.-M.M.; Song, Y.-C.C.; Kim, D.-H.H. Enhanced anaerobic digestion of glycerol by promoting DIET reaction. Biochem. Eng. J. 2019, 142, 18–26. [Google Scholar]
- Zhang, J.; Zhaoa, W.; Zhang, H.; Wang, Z.; Fan, C.; Zang, L.; Zhao, W.; Zhang, H.; Wang, Z.; Fan, C.; et al. Recent achievements in enhancing anaerobic digestion with carbon- based functional materials. Bioresour. Technol. J. 2018, 266, 13. [Google Scholar]
- Peng, H.; Zhang, Y.; Tan, D.; Zhao, Z.; Zhao, H.; Quan, X. Roles of magnetite and granular activated carbon in improvement of anaerobic sludge digestion. Bioresour. Technol. 2018, 249, 666–672. [Google Scholar]
- Liu, F.; Rotaru, A.; Shrestha, P.M.; Malvankar, N.S.; Nevin, K.P.; Lovley, D.R. Magnetite compensates for the lack of a pilin-associated c-type cytochrome in extracellular electron exchange. Environ. Microbial. 2015, 17, 648–655. [Google Scholar]
- Dang, Y.; Holmes, D.E.; Zhao, Z.; Woodard, T.L.; Zhang, Y.; Sun, D.; Wang, L.Y.; Nevin, K.P.; Lovley, D.R. Enhancing anaerobic digestion of complex organic waste with carbon-based conductive materials. Bioresour. Technol. 2016, 220, 516–522. [Google Scholar]
- Liu, F.; Rotaru, A.E.; Shrestha, P.M.; Malvankar, N.S.; Nevin, K.P.; Lovley, D.R. Promoting direct interspecies electron transfer with activated carbon. Energy Environ. Sci. 2012, 5, 8982–8989. [Google Scholar]
- Zhao, Z.; Zhang, Y.; Holmes, D.E.; Dang, Y.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Potential enhancement of direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate with biochar in up-flow anaerobic sludge blanket reactors. Bioresour. Technol. 2016, 209, 148–156. [Google Scholar]
- Yin, Q.; Miao, J.; Li, B.; Wu, G. Enhancing electron transfer by ferroferric oxide during the anaerobic treatment of synthetic wastewater with mixed organic carbon. Int. Biodeterior. Biodegrad. 2017, 119, 104–110. [Google Scholar]
- Barua, S.; Zakaria, B.S.; Dhar, B.R. Enhanced methanogenic co-degradation of propionate and butyrate by anaerobic microbiome enriched on conductive carbon fibers. Bioresour. Technol. 2018, 266, 259–266. [Google Scholar]
- Zhuang, L.; Tang, J.; Wang, Y.; Hu, M.; Zhou, S. Conductive iron oxide minerals accelerate syntrophic cooperation in methanogenic benzoate degradation. J. Hazard. Mater. 2015, 293, 37–45. [Google Scholar]
- Dang, Y.; Sun, D.; Woodard, T.L.; Wang, L.Y.; Nevin, K.P.; Holmes, D.E. Stimulation of the anaerobic digestion of the dry organic fraction of municipal solid waste (OFMSW) with carbon-based conductive materials. Bioresour. Technol. 2017, 238, 30–38. [Google Scholar]
- Baek, G.; Kim, J.; Cho, K.; Bae, H.; Lee, C. The biostimulation of anaerobic digestion with (semi) conductive ferric oxides: Their potential for enhanced biomethanation. Appl. Microbial. Biotechnol. 2015, 99, 10355–10366. [Google Scholar]
- Baek, G.; Kim, J.; Lee, C. Influence of ferric oxyhydroxide addition on biomethanation of waste activated sludge in a continuous reactor. Bioresour. Technol. 2014, 166, 596–601. [Google Scholar]
- Wang, T.; Zhang, D.; Dai, L.; Dong, B.; Dai, X. Magnetite Triggering Enhanced Direct Interspecies Electron Transfer: A Scavenger for the Blockage of Electron Transfer in Anaerobic Digestion of High-Solids Sewage Sludge. Environ. Sci. Technol. 2018, 52, 7160–7169. [Google Scholar]
- Hwu, C.S.; Tseng, S.K.; Yuan, C.Y.; Kulik, Z.; Lettinga, G. Biosorption of long-chain fatty acids in UASB treatment process. Water Res. 1998, 32, 1571–1579. [Google Scholar]
- Kim, D.H.; Lee, M.K.; Hwang, Y.; Im, W.T.; Yun, Y.M.; Park, C.; Kim, M.S. Microbial Granulation for Lactic Acid Production. Biotechnol. Bioeng. 2016, 113, 101–111. [Google Scholar]
- APHA Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005.
- Wang, X.; Zhang, L.; Peng, Y.; Zhang, Q.; Li, J.; Yang, S. Enhancing the digestion of waste activated sludge through nitrite addition: Insight on mechanism through profiles of extracellular polymeric substances (EPS) and microbial communities. J. Hazard. Mater. 2019, 369, 164–170. [Google Scholar]
- Moon, C.; Jang, S.; Yun, Y.M.; Lee, M.K.; Kim, D.H.; Kang, W.S.; Kwak, S.S.; Kim, M.S. Effect of the Accuracy of pH Control on Hydrogen Fermentation. Bioresour. Technol. 2015, 179, 595–601. [Google Scholar]
- Na, J.G.; Lee, M.K.; Yun, Y.M.; Moon, C.; Kim, M.S.; Kim, D.H. Microbial community analysis of anaerobic granules in phenol-degrading UASB by next generation sequencing. Biochem. Eng. J. 2016, 112, 241–248. [Google Scholar]
- Hanaki, K.; Matsuo, T.; Nagase, M. Mechanism of Inhibition Caused by Long-Chain Fatty Acids in Anaerobic Digestion Process. Biotechnol. Bioeng. 1981, 23, 1591–1610. [Google Scholar]
- Hwu, C.S.; Donlon, B.; Lettinga, G. Comparative toxicity of long-chain fatty acid to anaerobic sludges from various origins. Water Sci. Technol. 1996, 34, 351–358. [Google Scholar]
- Shin, H.S.; Kim, S.H.; Lee, C.Y.; Nam, S.Y. Inhibitory effects of long-chain fatty acids on VFA degradation and beta-oxidation. Water Sci. Technol. 2003, 47, 139–146. [Google Scholar]
- Zhang, Y.; Feng, Y.; Yu, Q.; Xu, Z.; Quan, X. Enhanced high-solids anaerobic digestion of waste activated sludge by the addition of scrap iron. Bioresour. Technol. 2014, 159, 297–304. [Google Scholar]
- Salvador, A.F.; Martins, G.; Melle-franco, M.; Serpa, R.; Stams, A.J.M.; Cavaleiro, A.J.; Pereira, M.A.; Alves, M.M. Carbon nanotubes accelerate methane production in pure cultures of methanogens and in a syntrophic coculture. Environ. Microbiol. 2017, 19, 2727–2739. [Google Scholar]
- Rasit, N.; Idris, A.; Harun, R.; Azlina, W.; Ab, W.; Ghani, K.; Wan Ab Karim Ghani, W.A.; Azlina, W.; Ab, W.; Ghani, K. Effects of lipid inhibition on biogas production of anaerobic digestion from oily effluents and sludges: An overview. Renew. Sustain. Energy Rev. 2015, 45, 351–358. [Google Scholar]
- Yan, W.; Lu, D.; Liu, J.; Zhou, Y. The interactive effects of ammonia and carbon nanotube on anaerobic digestion. Chem. Eng. J. 2019, 372, 332–340. [Google Scholar]
- Zhang, J.; Lu, Y. Conductive Fe3O4 Nanoparticles Accelerate Syntrophic Methane Production from Butyrate Oxidation in Two Different Lake Sediments. Front. Microbiol. 2016, 7, 1–9. [Google Scholar]
- Zhao, Z.; Li, Y.; Quan, X.; Zhang, Y. Towards engineering application: Potential mechanism for enhancing anaerobic digestion of complex organic waste with different types of conductive materials. Water Res. 2017, 115, 266–277. [Google Scholar]
- Yin, Q.; Yang, S.; Wang, Z.; Xing, L.; Wu, G. Clarifying electron transfer and metagenomic analysis of microbial community in the methane production process with the addition of ferroferric oxide. Chem. Eng. J. 2018, 333, 216–225. [Google Scholar]
- Zhao, Z.; Zhang, Y. Application of ethanol-type fermentation in establishment of direct interspecies electron transfer: A practical engineering case study. Renew. Energy 2019, 136, 846–855. [Google Scholar]
- Zhuang, H.; Zhu, H.; Shan, S.; Zhang, L.; Fang, C.; Shi, Y. Potential enhancement of direct interspecies electron transfer for anaerobic degradation of coal gasification wastewater using up-flow anaerobic sludge blanket (UASB) with nitrogen doped sewage sludge carbon assisted. Bioresour. Technol. 2018, 270, 230–235. [Google Scholar]
- Jin, Z.; Zhao, Z.; Zhang, Y. Potential of direct interspecies electron transfer in synergetic enhancement of methanogenesis and sulfate removal in an up-flow anaerobic sludge blanket reactor with magnetite. Sci. Total Environ. 2019, 677, 299–306. [Google Scholar]
- Yan, W.; Sun, F.; Liu, J.; Zhou, Y. Enhanced anaerobic phenol degradation by conductive materials via EPS and microbial community alteration. Chem. Eng. J. 2018, 352, 1–9. [Google Scholar]
- Vella, L.; Emerson, D. Electrical Properties of Magnetite- and Hematite-Rich Rocks and Ores. ASEG Ext. Abstr. 2012, 2012, 1–4. [Google Scholar]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon N. Y. 2001, 39, 507–514. [Google Scholar]
- Iconaru, S.L.; Guégan, R.; Popa, C.L.; Motelica-Heino, M.; Ciobanu, C.S.; Predoi, D. Magnetite (Fe3O4) nanoparticles as adsorbents for As and Cu removal. Appl. Clay Sci. 2016, 134, 128–135. [Google Scholar]
- Chen, M.; Sun, Y.; Liang, J.; Zeng, G.; Li, Z.; Tang, L.; Zhu, Y.; Jiang, D.; Song, B. Understanding the influence of carbon nanomaterials on microbial communities. Environ. Int. 2019, 126, 690–698. [Google Scholar]
- Kato, S.; Hashimoto, K.; Watanabe, K. Methanogenesis facilitated by electric syntrophy via (semi) conductive iron-oxide minerals. Environ. Microbiol. 2011, 14, 1646–1654. [Google Scholar]
- Ivanov, V.N.; Stabnikova, E.V.; Stabnikov, V.P.; Kim, I.S.; Zubair, A. Effects of Iron Compounds on the Treatment of Fat-Containing Wastewaters. Appl. Biochem. Microbiol. 2002, 38, 255–258. [Google Scholar]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence Excitation—Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ. Sci. Technol 2003, 37, 5701–5710. [Google Scholar]
- Li, S.; Cao, Y.; Zhao, Z.; Zhang, Y. Regulating Secretion of Extracellular Polymeric Substances through Dosing Magnetite and Zerovalent Iron Nanoparticles To Affect Anaerobic Digestion Mode. ACS Sustain. Chem. Eng. 2019, 7, 9655–9662. [Google Scholar]
- Tian, T.; Qiao, S.; Yu, C.; Zhou, J. Bio-electrochemically assisting low-temperature anaerobic digestion of low-organic strength wastewater. Chem. Eng. J. 2018, 335, 657–664. [Google Scholar]
- Xu, S.; Han, R.; Zhang, Y.; He, C.; Liu, H. Differentiated stimulating effects of activated carbon on methanogenic degradation of acetate, propionate and butyrate. Waste Manag. 2018, 76, 394–403. [Google Scholar]
- Zhao, Z.; Sun, C.; Li, Y.; Peng, H.; Zhang, Y. Upgrading Current Method of Anaerobic Co-digestion of Waste Activated Sludge for High-efficiency Methanogenesis: Establishing Direct Interspecies Electron Transfer via Ethanol-type Fermentation. Renew. Energy 2020, 148, 523–533. [Google Scholar]
- Cavaleiro, A.J.; Sousa, D.Z.; Alves, M.M. Methane production from oleate: Assessing the bioaugmentation potential of Syntrophomonas zehnderi. Water Res. 2010, 44, 4940–4947. [Google Scholar]
- Baena, S.; Fardeau, M.L.; Labat, M.; Ollivier, B.; Garcia, J.L.; Patel, B.K.C. Aminobacterium mobile sp. nov., a new anaerobic amino-acid-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2000, 50, 259–264. [Google Scholar]
- Khemkhao, M.; Techkarnjanaruk, S.; Phalakornkule, C. Simultaneous treatment of raw palm oil mill effluent and biodegradation of palm fiber in a high-rate CSTR. Bioresour. Technol. 2015, 177, 17–27. [Google Scholar]
- Zheng, H.; Chen, T.; Rudolph, V.; Golding, S.D. Biogenic methane production from Bowen Basin coal waste materials. Int. J. Coal Geol. 2017, 169, 22–27. [Google Scholar]
- Nepomnyashchaya, Y.N.; Slobodkina, G.B.; Baslerov, R.V.; Chernyh, N.A.; Bonch-Osmolovskaya, E.A.; Netrusov, A.I.; Slobodkin, A.I. Moorella humiferrea sp. nov., a thermophilic, anaerobic bacterium capable of growth via electron shuttling between humic acid and Fe(III). Int. J. Syst. Evol. Microbiol. 2012, 62, 613–617. [Google Scholar]



| Oleic Acid Concentration (g COD/L) | Additives | CH4 Potential (mL) | CH4 Production Rate (mL/d) | Lag Period (d) |
|---|---|---|---|---|
| 0.10 | Control | 4.5 | 1.9 | 7.1 |
| nFe3O4 | 4.9 | 1.0 | 5.3 | |
| CNT | 5.1 | 1.6 | 6.0 | |
| 0.25 | Control | 10.4 | 1.4 | 6.2 |
| nFe3O4 | 14.2 | 3.8 | 6.5 | |
| CNT | 13.4 | 2.3 | 6.4 | |
| 0.50 | Control | 23.3 | 9.8 | 7.0 |
| nFe3O4 | 26.7 | 6.7 | 7.1 | |
| CNT | 28.1 | 8.0 | 7.1 | |
| 1.00 | Control | 24.0 | 5.9 | 8.0 |
| nFe3O4 | 40.2 | 12.5 | 6.1 | |
| CNT | 49.2 | 9.5 | 6.6 | |
| 2.00 | Control | 46.9 | 5.4 | 6.9 |
| nFe3O4 | 79.6 | 5.6 | 6.2 | |
| CNT | 96.2 | 28.6 | 7.1 | |
| 4.00 | Control | 64.7 | 6.5 | 7.2 |
| nFe3O4 | 138.7 | 26.8 | 7.4 | |
| CNT | 171.9 | 23.6 | 7.1 |
| Microorganism | Accession Number | Similarity (%) | Control (%) | nFe₃O₄-Supplemented (%) | CNT-Supplemented (%) |
|---|---|---|---|---|---|
| Methanothrix concilii | NR_102903.1 | 99 | 57 | 58 | 71 |
| Methanosarcina flavescens | NR_148758.1 | 99 | 8 | 29 | 11 |
| Methanoculleus receptaculi | NR_043961.1 | 99 | 8 | 3 | 2 |
| Methanospirillum hungatei | NR_074177.1 | 98 | 7 | 2 | 2 |
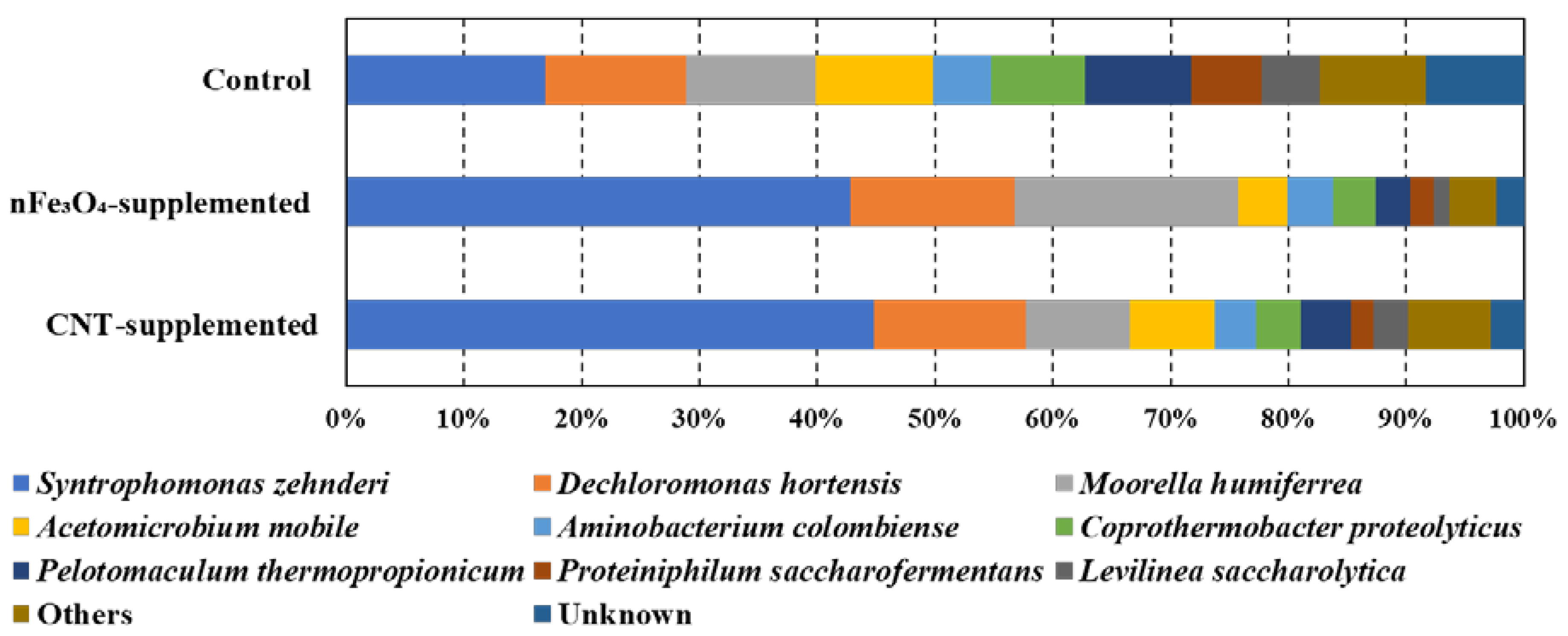
| Microorganism | Accession Number | Similarity (%) | Control (%) | nFe₃O₄-Supplemented (%) | CNT-Supplemented (%) |
|---|---|---|---|---|---|
| Syntrophomonas zehnderi | NR_044008.1 | 98 | 17 | 43 | 45 |
| Dechloromonas hortensis | NR_042819.1 | 98 | 12 | 14 | 13 |
| Moorella humiferrea | NR_108634.1 | 98 | 11 | 19 | 9 |
| Acetomicrobium mobile | NR_102954.1 | 99 | 10 | 4 | 7 |
| Aminobacterium colombiense | NR_074624.1 | 99 | 5 | 4 | 4 |
| Coprothermobacter proteolyticus | NR_074653.1 | 100 | 8 | 4 | 4 |
| Pelotomaculum thermopropionicum | NR_074685.1 | 98 | 9 | 3 | 4 |
| Proteiniphilum saccharofermentans | NR_148807.1 | 99 | 6 | 2 | 2 |
| Levilinea saccharolytica | NR_040972.1 | 99 | 5 | 1 | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostafa, A.; Im, S.; Song, Y.-C.; Kang, S.; Kim, D.-H. Enhanced Anaerobic Digestion of Long Chain Fatty Acid by Adding Magnetite and Carbon Nanotubes. Microorganisms 2020, 8, 333. https://doi.org/10.3390/microorganisms8030333
Mostafa A, Im S, Song Y-C, Kang S, Kim D-H. Enhanced Anaerobic Digestion of Long Chain Fatty Acid by Adding Magnetite and Carbon Nanotubes. Microorganisms. 2020; 8(3):333. https://doi.org/10.3390/microorganisms8030333
Chicago/Turabian StyleMostafa, Alsayed, Seongwon Im, Young-Chae Song, Seoktae Kang, and Dong-Hoon Kim. 2020. "Enhanced Anaerobic Digestion of Long Chain Fatty Acid by Adding Magnetite and Carbon Nanotubes" Microorganisms 8, no. 3: 333. https://doi.org/10.3390/microorganisms8030333








