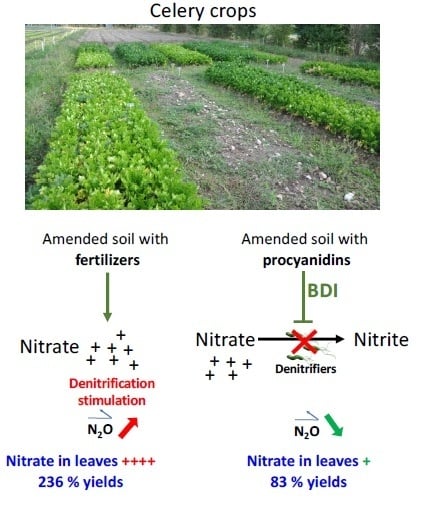
Does Biological Denitrification Inhibition (BDI) in the Field Induce an Increase in Plant Growth and Nutrition in Apium graveolens L. Grown for a Long Period?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth and Experimental Design
2.2. Denitrification Enzyme Activity (DEA)
2.3. Quantification of Total Bacteria and Denitrifier Abundance
2.4. Nitrate Concentrations in Soil
2.5. Measurement of Celery Traits
2.6. Plant N Content
2.7. Colorimetric Measurement of Nitrogen Stress
2.8. Data and Statistical Analyses
3. Results
3.1. Biological Denitrification Inhibition
3.2. Abundance of Total and Denitrifying Bacterial Communities
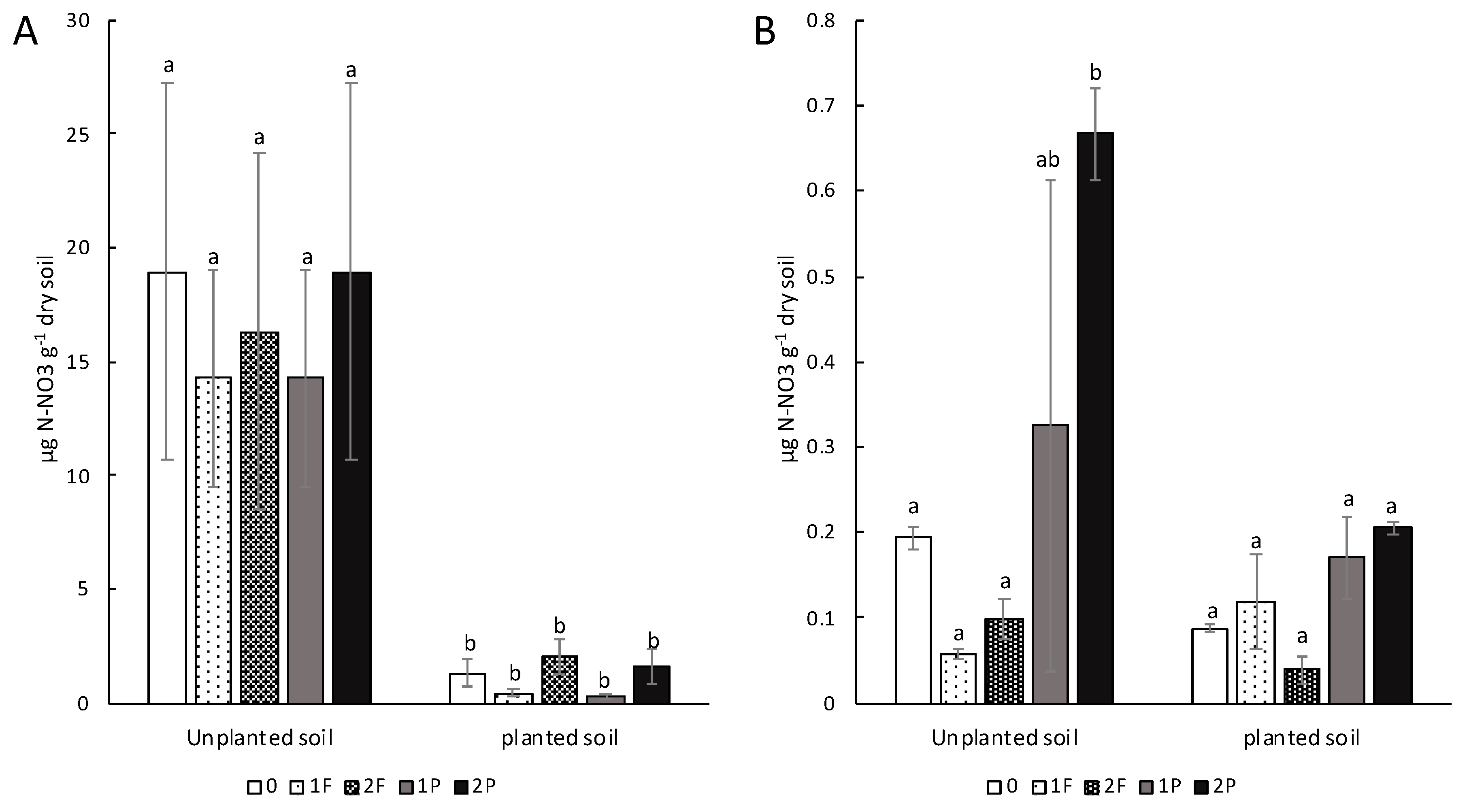
3.3. Effects of Amendments on Soil Nitrate Content
3.4. Effect of Amendments on Celery Traits
3.5. Effect of Amendments on Plant N Content
3.6. Effect of Amendments on Colorimetric Measurements of N Stress
4. Discussion
4.1. Procyanidin Amendment to Celery Plots Induced an Increase in BDI and Changes in Denitrifiers Abundance
4.2. The Addition of Procyanidins to Celery Plots Modifies the Soil Nitrate Concentration
4.3. The Addition of Procyanidins to Celery Plots Improves Celery Growth Traits
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alston, J.M.; Babcock, B.A.; Pardey, P.G. The Shifting Patterns of Agricultural Production and Productivity Worldwide; Midwest Agribusiness Trade Research and Information Center: Ames, IA, USA, 2010. [Google Scholar]
- Borrás, L.; Slafer, G.A.; Otegui, M.E. Seed dry weight response to source–sink manipulations in wheat, maize and soybean: A quantitative reappraisal. Field Crops Res. 2004, 86, 131–146. [Google Scholar] [CrossRef]
- Oerke, E.-C.; Dehne, H.-W. Safeguarding production—losses in major crops and the role of crop protection. Crop Prot. 2004, 23, 275–285. [Google Scholar] [CrossRef]
- Wittwer, S.H.; Castilla, N. Protected cultivation of horticultural crops worldwide. HortTechnology 1995, 5, 6–23. [Google Scholar] [CrossRef] [Green Version]
- Harris, R.W. Arboriculture: Integrated Management of Landscape Trees, Shrubs, and Vines; Prentice-Hall International: Engelwood Cliffs, NJ, USA, 1992. [Google Scholar]
- Cassman, K.G.; Dobermann, A.; Walters, D.T. Agroecosystems, nitrogen-use efficiency, and nitrogen management. AMBIO J. Hum. Environ. 2002, 31, 132–141. [Google Scholar] [CrossRef]
- Glass, A.D. Nitrogen use efficiency of crop plants: Physiological constraints upon nitrogen absorption. Crit. Rev. Plant Sci. 2003, 22, 453–470. [Google Scholar] [CrossRef]
- Pollack, S.L. Consumer demand for fruit and vegetables: The US example. Chang. Struct. Glob. Food Consum. Trade 2001, 6, 49–54. [Google Scholar]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671. [Google Scholar] [CrossRef]
- Davis, K.F.; Gephart, J.A.; Emery, K.A.; Leach, A.M.; Galloway, J.N.; D’Odorico, P. Meeting future food demand with current agricultural resources. Glob. Environ. Change 2016, 39, 125–132. [Google Scholar] [CrossRef]
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Bloom, A.J. The increasing importance of distinguishing among plant nitrogen sources. Curr. Opin. Plant Biol. 2015, 25, 10–16. [Google Scholar] [CrossRef]
- Mulvaney, R.L.; Khan, S.A.; Ellsworth, T.R. Synthetic nitrogen fertilizers deplete soil nitrogen: A global dilemma for sustainable cereal production. J. Environ. Qual. 2009, 38, 2295–2314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorup-Kristensen, K. Root growth and soil nitrogen depletion by onion, lettuce, early cabbage and carrot. In Proceedings of the International Conference on Environmental Problems Associated with Nitrogen Fertilisation of Field Grown Vegetable Crops, Potsdam, Germany, 30 August–1 September 1999; pp. 201–206. [Google Scholar]
- Kuzyakov, Y.; Xu, X. Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytol. 2013, 198, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Shoun, H.; Kim, D.-H.; Uchiyama, H.; Sugiyama, J. Denitrification by fungi. FEMS Microbiol. Lett. 1992, 94, 277–281. [Google Scholar] [CrossRef]
- Philippot, L. Denitrifying genes in bacterial and archaeal genomes. Biochim. Biophys. Acta BBA-Gene Struct. Expr. 2002, 1577, 355–376. [Google Scholar] [CrossRef]
- Van der Salm, C.; Dolfing, J.; Heinen, M.; Velthof, G.L. Estimation of nitrogen losses via denitrification from a heavy clay soil under grass. Agric. Ecosyst. Environ. 2007, 119, 311–319. [Google Scholar] [CrossRef]
- Kuypers, M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263. [Google Scholar] [CrossRef]
- Oenema, O.; Witzke, H.P.; Klimont, Z.; Lesschen, J.P.; Velthof, G.L. Integrated assessment of promising measures to decrease nitrogen losses from agriculture in EU-27. Agric. Ecosyst. Environ. 2009, 133, 280–288. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C. Nitrate leaching in temperate agroecosystems: Sources, factors and mitigating strategies. Nutr. Cycl. Agroecosyst. 2002, 64, 237–256. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhao, X.; Teng, Y.; Li, X.; Zhang, J.; Wu, J.; Zuo, R. Groundwater nitrate pollution and human health risk assessment by using HHRA model in an agricultural area, NE China. Ecotoxicol. Environ. Saf. 2017, 137, 130–142. [Google Scholar] [CrossRef]
- De Ponti, T.; Rijk, B.; Van Ittersum, M.K. The crop yield gap between organic and conventional agriculture. Agric. Syst. 2012, 108, 1–9. [Google Scholar] [CrossRef]
- Haynes, R. Mineral Nitrogen in the Plant-Soil System; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Bardon, C.; Piola, F.; Bellvert, F.; Haichar, F.Z.; Comte, G.; Meiffren, G.; Pommier, T.; Puijalon, S.; Tsafack, N.; Poly, F. Evidence for biological denitrification inhibition (BDI) by plant secondary metabolites. New Phytol. 2014, 204, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Iwashina, T. The structure and distribution of the flavonoids in plants. J. Plant Res. 2000, 113, 287–299. [Google Scholar] [CrossRef]
- Galland, W.; Piola, F.; Burlet, A.; Mathieu, C.; Nardy, M.; Poussineau, S.; Blazère, L.; Gervaix, J.; Puijalon, S.; Simon, L.; et al. Biological denitrification inhibition (BDI) in the field: A strategy to improve plant nutrition and growth. Soil Biol. Biochem. 2019, 136, 107513. [Google Scholar] [CrossRef]
- Burns, I.G. Nitrogen supply, growth and development. In Ecological aspects of Vegetable Fertigation in Integrated crop Production in the Field; Hähndel, R., Wichmann, W., Eds.; International Society for Horticultural Science: Neustadt an der Weinstrasse, Germany, 1996; Volume 428; pp. 21–30. [Google Scholar]
- Charles, K.S.; Ngouajio, M.; Warncke, D.D.; Poff, K.L.; Hausbeck, M.K. Integration of cover crops and fertilizer rates for weed management in celery. Weed Sci. 2006, 54, 326–334. [Google Scholar] [CrossRef]
- Anjana, S.U.; Iqbal, M. Nitrate accumulation in plants, factors affecting the process, and human health implications. A review. Agron. Sustain. Dev. 2007, 27, 45–57. [Google Scholar] [CrossRef]
- Santamaria, P. Nitrate in vegetables: Toxicity, content, intake and EC regulation. J. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Green, L.C.; De Luzuriaga, K.R.; Wagner, D.A.; Rand, W.; Istfan, N.; Young, V.R.; Tannenbaum, S.R. Nitrate biosynthesis in man. Proc. Natl. Acad. Sci. USA 1981, 78, 7764–7768. [Google Scholar] [CrossRef] [Green Version]
- Van Velzen, A.G.; Sips, A.J.; Schothorst, R.C.; Lambers, A.C.; Meulenbelt, J. The oral bioavailability of nitrate from nitrate-rich vegetables in humans. Toxicol. Lett. 2008, 181, 177–181. [Google Scholar] [CrossRef]
- Wright, R.O.; Lewander, W.J.; Woolf, A.D. Methemoglobinemia: Etiology, pharmacology, and clinical management. Ann. Emerg. Med. 1999, 34, 646–656. [Google Scholar] [CrossRef]
- Sindelar, J.J.; Milkowski, A.L. Human safety controversies surrounding nitrate and nitrite in the diet. Nitric Oxide 2012, 26, 259–266. [Google Scholar] [CrossRef]
- Preece, D.A. RA Fisher and experimental design: A review. Biometrics 1990, 46, 925–935. [Google Scholar] [CrossRef]
- Guyonnet, J.P.; Vautrin, F.; Meiffren, G.; Labois, C.; Cantarel, A.A.; Michalet, S.; Comte, G.; Haichar, F.Z. The effects of plant nutritional strategy on soil microbial denitrification activity through rhizosphere primary metabolites. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef] [PubMed]
- Zerulla, W.; Barth, T.; Dressel, J.; Erhardt, K.; Horchler von Locquenghien, K.; Pasda, G.; Rädle, M.; Wissemeier, A. 3,4-Dimethylpyrazole phosphate (DMPP)—a new nitrification inhibitor for agriculture and horticulture. Biol. Fertil. Soils 2001, 34, 79–84. [Google Scholar] [CrossRef]
- Sun, R.; Guo, X.; Wang, D.; Chu, H. Effects of long-term application of chemical and organic fertilizers on the abundance of microbial communities involved in the nitrogen cycle. Appl. Soil Ecol. 2015, 95, 171–178. [Google Scholar] [CrossRef]
- Tatti, E.; Goyer, C.; Zebarth, B.J.; Burton, D.L.; Giovannetti, L.; Viti, C. Short-term effects of mineral and organic fertilizer on denitrifiers, nitrous oxide emissions and denitrification in long-term amended vineyard soils. Soil Sci. Soc. Am. J. 2013, 77, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Venterea, R.T.; Halvorson, A.D.; Kitchen, N.; Liebig, M.A.; Cavigelli, M.A.; Grosso, S.J.D.; Motavalli, P.P.; Nelson, K.A.; Spokas, K.A.; Singh, B.P. Challenges and opportunities for mitigating nitrous oxide emissions from fertilized cropping systems. Front. Ecol. Environ. 2012, 10, 562–570. [Google Scholar] [CrossRef]
- Bardon, C.; Poly, F.; el Zahar Haichar, F.; Le Roux, X.; Simon, L.; Meiffren, G.; Comte, G.; Rouifed, S.; Piola, F. Biological denitrification inhibition (BDI) with procyanidins induces modification of root traits, growth and N status in Fallopia x bohemica. Soil Biol. Biochem. 2017, 107, 41–49. [Google Scholar] [CrossRef]
- Santamaria, P.; Elia, A.; Serio, F.; Gonnella, M.; Parente, A. Comparison between nitrate and ammonium nutrition in fennel, celery, and Swiss chard. J. Plant Nutr. 1999, 22, 1091–1106. [Google Scholar] [CrossRef]
- Burns, I.G. Short-and long-term effects of a change in the spatial distribution of nitrate in the root zone on N uptake, growth and root development of young lettuce plants. Plant Cell Environ. 1991, 14, 21–33. [Google Scholar] [CrossRef]
- Van Wassenhove, F.A.; Dirinck, P.J.; Schamp, N.M.; Vulsteke, G.A. Effect of nitrogen fertilizers on celery volatiles. J. Agric. Food Chem. 1990, 38, 220–226. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Jezek, M.; Mühling, K.-H.; Geilfus, C.-M. Magnesium deficiency in maize and effectiveness of nutrient supply through MgSO4 leaf-application. Ph.D. Thesis, Christian-Albrechts-Universität zu Kiel, Kiel, Germany, July 2016. [Google Scholar]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galland, W.; Piola, F.; Mathieu, C.; Bouladra, L.; Simon, L.; Haichar, F.e.Z. Does Biological Denitrification Inhibition (BDI) in the Field Induce an Increase in Plant Growth and Nutrition in Apium graveolens L. Grown for a Long Period? Microorganisms 2020, 8, 1204. https://doi.org/10.3390/microorganisms8081204
Galland W, Piola F, Mathieu C, Bouladra L, Simon L, Haichar FeZ. Does Biological Denitrification Inhibition (BDI) in the Field Induce an Increase in Plant Growth and Nutrition in Apium graveolens L. Grown for a Long Period? Microorganisms. 2020; 8(8):1204. https://doi.org/10.3390/microorganisms8081204
Chicago/Turabian StyleGalland, William, Florence Piola, Céline Mathieu, Lyna Bouladra, Laurent Simon, and Feth el Zahar Haichar. 2020. "Does Biological Denitrification Inhibition (BDI) in the Field Induce an Increase in Plant Growth and Nutrition in Apium graveolens L. Grown for a Long Period?" Microorganisms 8, no. 8: 1204. https://doi.org/10.3390/microorganisms8081204