Ultrasound-Attenuated Microorganisms Inoculated in Vegetable Beverages: Effect of Strains, Temperature, Ultrasound and Storage Conditions on the Performances of the Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
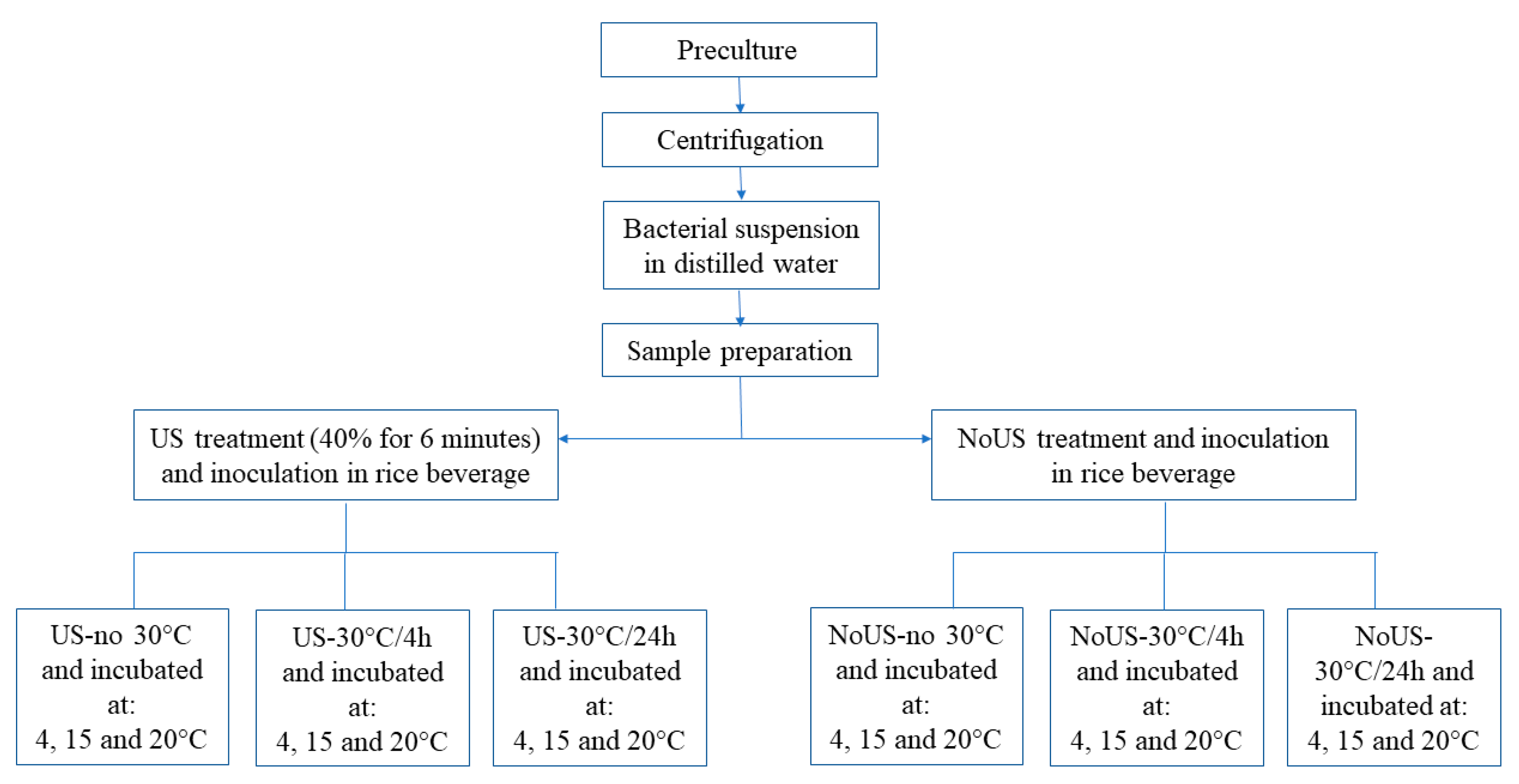
2.2. Ultrasound Treatments
2.3. Viability and Acidification of Attenuated Strains in Vegetable Beverages
2.4. Effect of Thermal Abuse on Lactiplantibacillus plantarum c16 and c19 Attenuated by US and Inoculated in Rice Beverage
3. Results
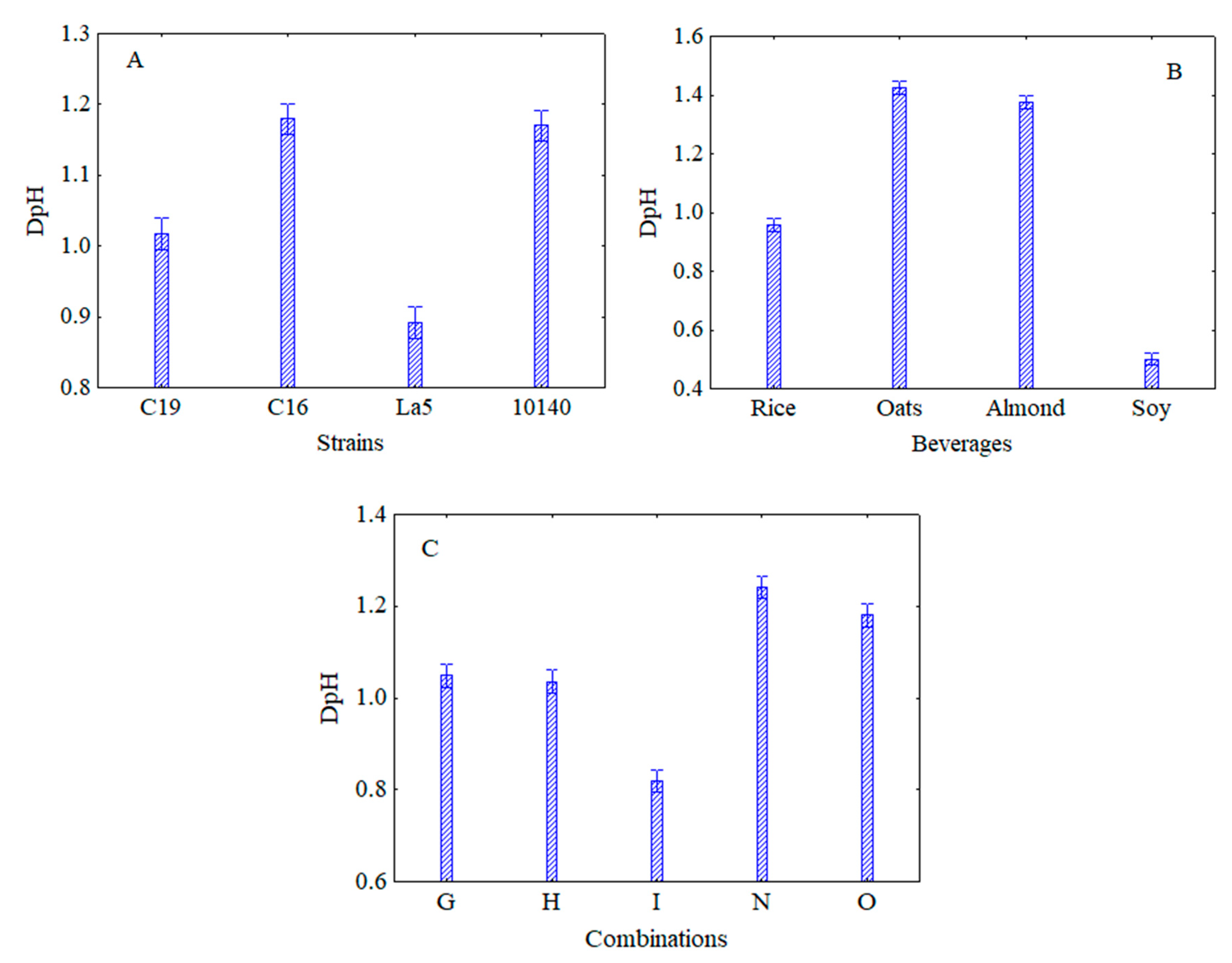
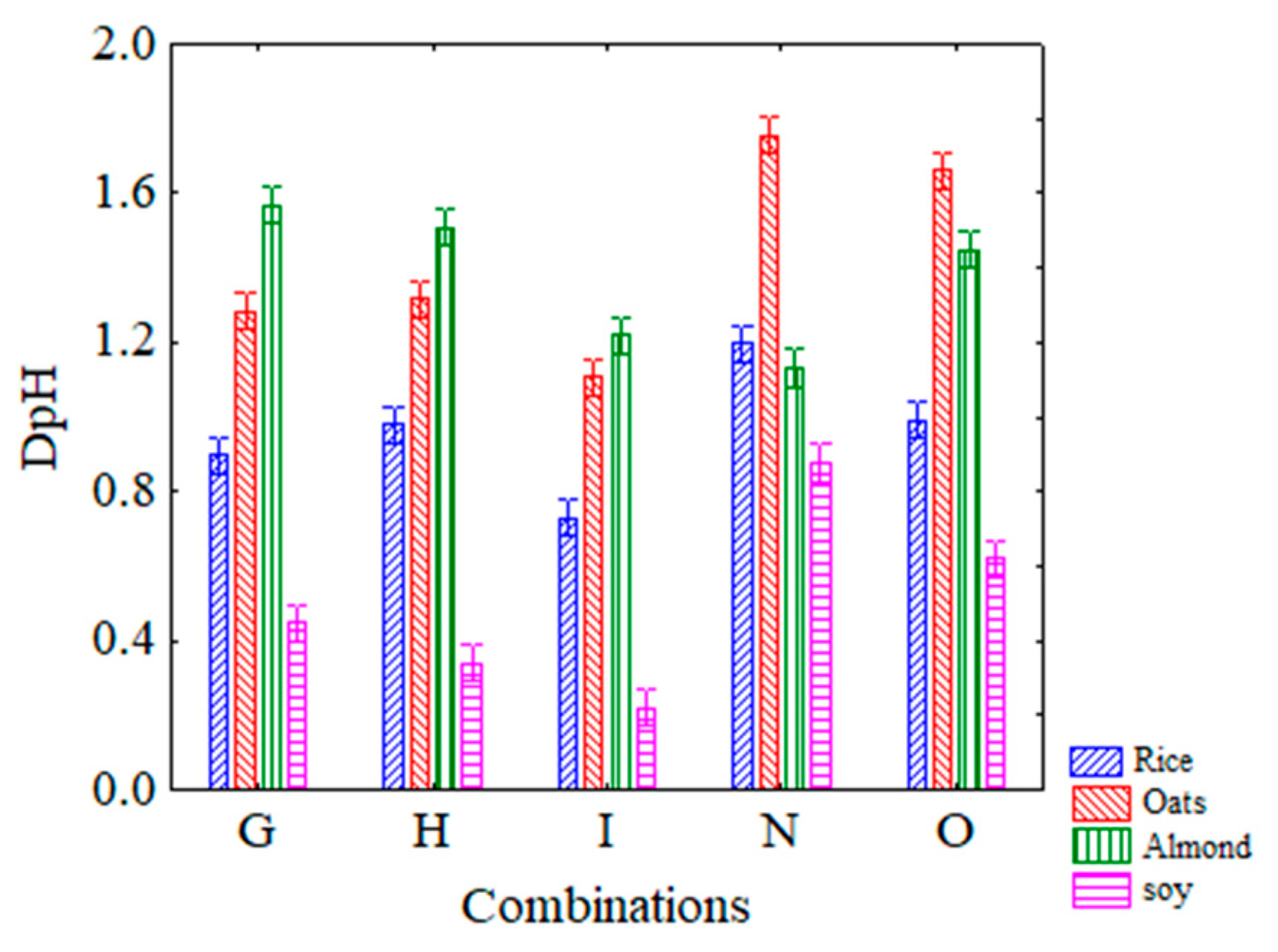
3.1. Viability and Acidifying Capability of Attenuated Strains in Vegetable Beverages
3.2. Effect of Thermal Abuse on Lactiplantibacillus plantarum c16 and c19 Attenuated by US and Inoculated in Rice Beverage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Lb. | Lactobacillus |
| L. | Lactiplantibacillus |
| Lc. | Lacticaseibacillus |
| Li. | Limosilactobacillus |
References
- Aspri, M.; Papademas, P.; Tsaltas, D. Review on Non-Dairy Probiotics and Their Use in Non-Dairy Based Products. Fermentation 2020, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Bernat, N.; Cháfer, M.; Chiralt, A.; Laparra, J.M.; González-Martínez, C. Almond milk fermented with different potentially probiotic bacteria improves iron uptake by intestinal epithelial (Caco-2) cells. Int. J. Food Stud. 2015, 4, 49–60. [Google Scholar] [CrossRef]
- Martins, E.M.F.; Ramos, A.M.; Vanzela, E.S.L.; Stringheta, P.C.; de Oliveira Pinto, C.L.; Martins, J.M. Products of vegetable origin: A new alternative for the consumption of probiotic bacteria. Food Res. Int. 2013, 51, 764–770. [Google Scholar] [CrossRef]
- Kun, S.; Rezessy-Szabò, J.M.; Nguyen, Q.D.; Goston-Hoschke, A. Changes of microbial population and some components in carrot juice during fermentation with selected Bifidobacterium strains. Process Biochem. 2008, 43, 816–821. [Google Scholar] [CrossRef]
- Profir, A.; Vizireanu, C. Effect of the preservation processes on the storage stability of juice made from carrot, celery and beetroot. J. Agroaliment. Process. Technol. 2013, 19, 99–104. [Google Scholar]
- Han, X.; Zhang, L.; Du, M.; Yi, H.; Li, Y.; Zhang, L. Effects of copper on the post-acidification of fermented milk by Streptococcus thermophilus. J. Food Sci. 2012, 71, M25–M28. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, A.; Campaniello, D.; Speranza, B.; Altieri, C.; Sinigaglia, M.; Corbo, M.R. Two Nonthermal Technologies for Food Safety and Quality—Ultrasound and High Pressure Homogenization: Effects on Microorganisms, Advances, and Possibilities: A Review. J. Food Protect. 2019, 82, 2049–2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speranza, B.; Campaniello, D.; Petruzzi, L.; Altieri, C.; Sinigaglia, M.; Bevilacqua, A.; Corbo, M.R. The Inoculation of Probiotics In Vivo Is a Challenge: Strategies to Improve Their Survival, to Avoid Unpleasant Changes, or to Enhance Their Performances in Beverages. Beverages 2020, 6, 20. [Google Scholar] [CrossRef] [Green Version]
- Bevilacqua, A.; Casanova, F.P.; Petruzzi, L.; Sinigaglia, M.; Corbo, M.R. Using physical approaches for the attenuation of lactic acid bacteria in an organic rice beverage. Food Microbiol. 2016, 53, 1–8. [Google Scholar] [CrossRef]
- Racioppo, A.; Corbo, M.R.; Piccoli, C.; Sinigaglia, M.; Speranza, B.; Bevilacqua, A. Ultrasound attenuation of lactobacilli and bifidobacteria: Effect on some technological and probiotic properties. Int. J. Food Microbiol. 2017, 243, 78–83. [Google Scholar] [CrossRef]
- Perricone, M.; Corbo, M.R.; Sinigaglia, M.; Speranza, B.; Bevilacqua, A. Viability of Lactobacillus reuteri in fruit juices. J. Funct. Foods 2014, 10, 421–426. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Sist. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Altieri, C.; Corbo, M.R.; Sinigaglia, M.; Ouoba, L.I.I. Characterization of lactic acid bacteria isolated from Italian Bella di Cerignola table olives: Selection of potential multifunctional starter cultures. J. Food Sci. 2010, 75, 536–544. [Google Scholar] [CrossRef]
- Valero-Cases, E.; Cerdá-Bernad, D.; Pastor, J.J.; Frutos, M.J. Non-dairy fermented beverages as potential carriers to ensure probiotics, prebiotics, and bioactive compounds arrival to the gut and their health benefits. Nutrients 2020, 12, 1666. [Google Scholar] [CrossRef]
- Kentish, S.; Feng, H. Applications of power ultrasound in food processing. Annu. Rev. Food Sci. Technol. 2014, 5, 263–284. [Google Scholar] [CrossRef]
- Hashemi, S.M.B. Effect of pulsed ultrasound treatment compared to continuous mode on microbiological and quality of Mirabelle plum during postharvest storage. Int. J. Food Sci. Technol. 2018, 53, 564–570. [Google Scholar] [CrossRef]
- Yeo, S.K.; Liong, M.T. Effect of Ultrasound on the Growth of Probiotics and Bioconversion of Isoflavones in Prebiotic-Supplemented Soymilk. J. Agric. Food Chem. 2011, 59, 885–897. [Google Scholar] [CrossRef]
- Ojha, K.S.; Mason, T.J.; O’Donnell, C.P.; Kerry, J.P.; Tiwari, B.K. Ultrasound technology for food fermentation applications. Ultrason. Sonochem. 2017, 34, 410–417. [Google Scholar] [CrossRef]
- Casanova, F.P.; Bevilacqua, A.; Sinigaglia, M.; Corbo, M.R. Food design e innovazione: Le bevande a base di cereali. Ingred. Aliment. 2013, 71, 1–10. [Google Scholar]
- Bevilacqua, A.; Racioppo, A.; Sinigaglia, M.; Speranza, B.; Campaniello, D.; Corbo, M.R. A low-power ultrasound attenuation improves the stability of biofilm and hydrophobicity of Propionibacterium freudenreichii subsp. freudenreichii DSM 20271 and Acidipropionibacterium jensenii DSM 20535. Food Microbiol. 2019, 78, 104–109. [Google Scholar] [CrossRef]

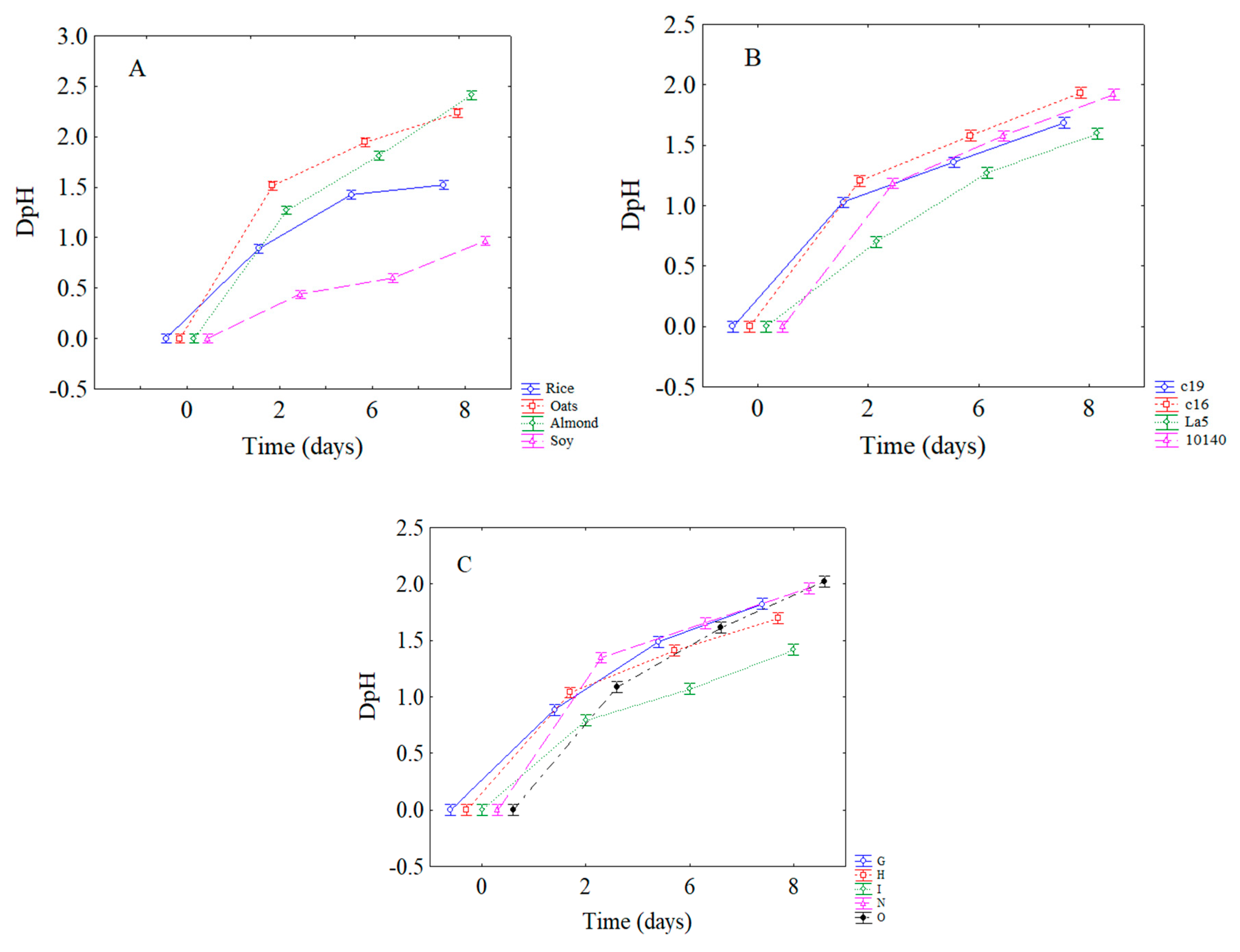
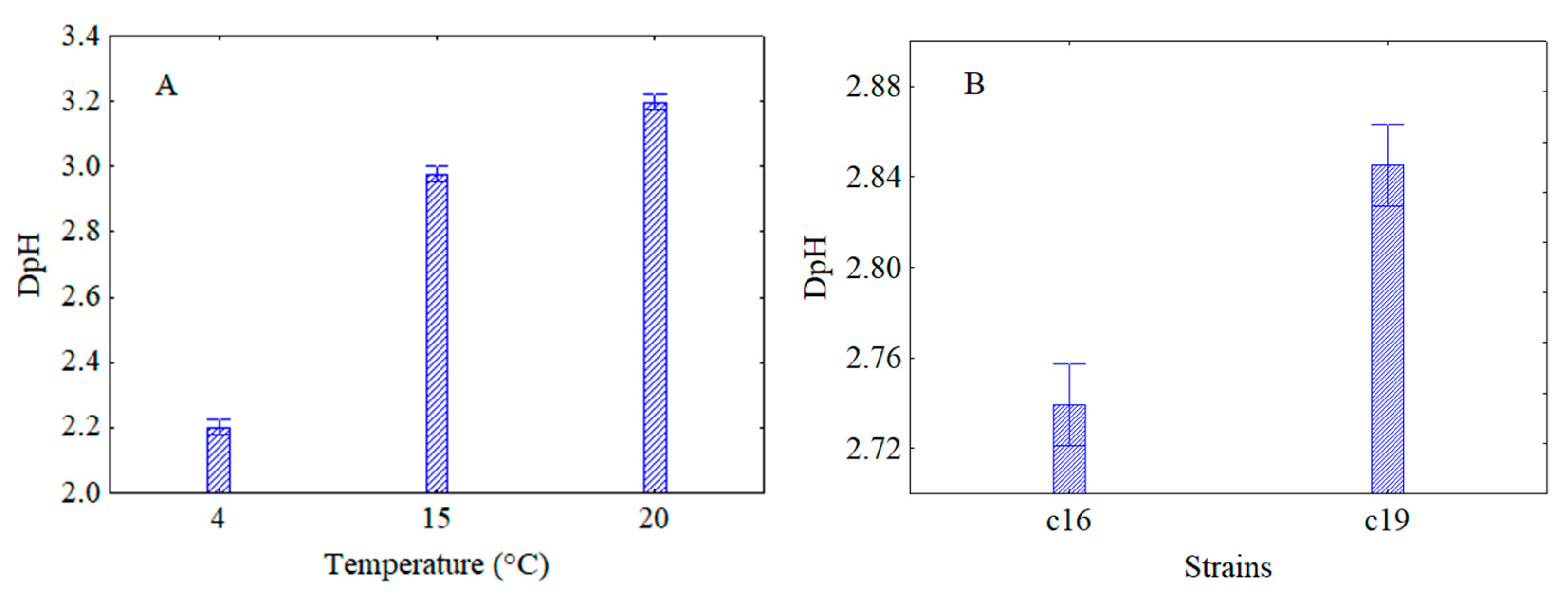
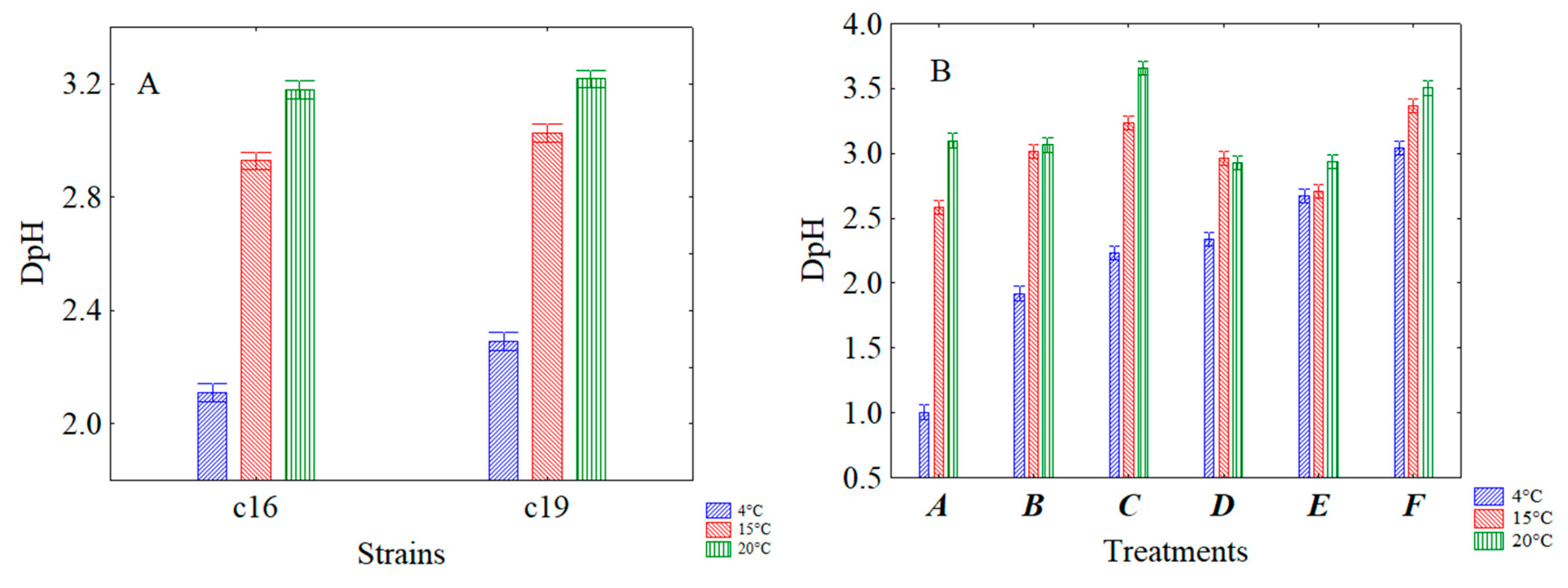

| Combinations | Power (%) | Time (Minutes) | Pulse (Seconds) |
|---|---|---|---|
| G | 40 | 2 | 2 |
| H | 40 | 4 | 2 |
| I | 40 | 6 | 2 |
| O | 60 | 2 | 2 |
| N (Control) | - | - | - |
| Code | Treatments |
|---|---|
| A | US treatment |
| B | Control |
| C | US treatment; storage at 30 °C for 4 h |
| D | Control; storage at 30 °C for 4 h |
| E | US treatment; storage at 30 °C for 24 h |
| F | Control; storage at 30 °C for 24 h |
| SS | Degree of Freedom | MS | F | p | |
|---|---|---|---|---|---|
| Intercept | 725.69 | 1 | 725.69 | 36,596.77 | 0.00 |
| {1} strains | 9.027 | 3 | 3.01 | 151.75 | 0.00 |
| {2} combinations | 13.53 | 4 | 3.38 | 170.54 | 0.00 |
| {3} beverages | 88.85 | 3 | 29.62 | 1493.53 | 0.00 |
| {4} time | 287.64 | 3 | 95.88 | 4835.34 | 0.00 |
| Strains x combinations | 9.12 | 12 | 0.76 | 38.31 | 0.00 |
| Strains x beverages | 68.52 | 9 | 7.61 | 383.94 | 0.00 |
| Combinations x beverages | 12.69 | 12 | 1.06 | 53.32 | 0.00 |
| Strains x time | 3.86 | 9 | 0.43 | 21.65 | 0.00 |
| Combinations x time | 6.66 | 12 | 0.56 | 28.01 | 0.00 |
| Beverages x time | 35.55 | 9 | 3.95 | 199.19 | 0.00 |
| Strains x combinations x beverages | 19.99 | 36 | 0.55 | 28.01 | 0.00 |
| Strains x combinations x time | 4.87 | 36 | 0.13 | 6.82 | 0.00 |
| Strains x beverages x time | 35.26 | 27 | 1.31 | 65.86 | 0.00 |
| Combinations x beverages x time | 10.22 | 36 | 0.28 | 14.32 | 0.00 |
| 1 × 2 × 3 × 4 | 21.66 | 108 | 0.20 | 10.11 | 0.00 |
| Error | 6.34 | 320 | 0.02 |
| SS | Degr. of Freedom | MS | F | p | |
|---|---|---|---|---|---|
| Intercept | 3368.37 | 1 | 3368.37 | 184,038.30 | 0.00 |
| {1}temperature | 79.04 | 2 | 39.52 | 2159.20 | 0.00 |
| {2}time | 702.23 | 5 | 140.45 | 7673.60 | 0.00 |
| {3}treatment | 47.63 | 5 | 9.53 | 520.50 | 0.00 |
| {4}strains | 1.21 | 1 | 1.21 | 66.20 | 0.00 |
| Temperature x time | 16.79 | 10 | 1.68 | 91.70 | 0.00 |
| Temperature x treatment | 33.49 | 10 | 3.35 | 183.00 | 0.00 |
| Time x treatment | 14.28 | 25 | 0.57 | 31.20 | 0.00 |
| Temperature x strains | 0.36 | 2 | 0.18 | 9.90 | 0.00 |
| Time x strains | 17.41 | 5 | 3.48 | 190.30 | 0.00 |
| Treatment x strains | 11.97 | 5 | 2.39 | 130.80 | 0.00 |
| Temperature x time x treatment | 9.97 | 50 | 0.20 | 10.90 | 0.00 |
| Temperature x time x strains | 1.14 | 10 | 0.11 | 6.20 | 0.00 |
| Temperature x treatment x strains | 1.58 | 10 | 0.16 | 8.60 | 0.00 |
| Time x treatment x strains | 4.96 | 25 | 0.20 | 10.80 | 0.00 |
| 1 × 2 × 3 × 4 | 3.20 | 50 | 0.06 | 3.50 | 0.00 |
| Error | 3.95 | 216 | 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campaniello, D.; Corbo, M.R.; Speranza, B.; Sinigaglia, M.; Bevilacqua, A. Ultrasound-Attenuated Microorganisms Inoculated in Vegetable Beverages: Effect of Strains, Temperature, Ultrasound and Storage Conditions on the Performances of the Treatment. Microorganisms 2020, 8, 1219. https://doi.org/10.3390/microorganisms8081219
Campaniello D, Corbo MR, Speranza B, Sinigaglia M, Bevilacqua A. Ultrasound-Attenuated Microorganisms Inoculated in Vegetable Beverages: Effect of Strains, Temperature, Ultrasound and Storage Conditions on the Performances of the Treatment. Microorganisms. 2020; 8(8):1219. https://doi.org/10.3390/microorganisms8081219
Chicago/Turabian StyleCampaniello, Daniela, Maria Rosaria Corbo, Barbara Speranza, Milena Sinigaglia, and Antonio Bevilacqua. 2020. "Ultrasound-Attenuated Microorganisms Inoculated in Vegetable Beverages: Effect of Strains, Temperature, Ultrasound and Storage Conditions on the Performances of the Treatment" Microorganisms 8, no. 8: 1219. https://doi.org/10.3390/microorganisms8081219