Coxsackievirus-B4 Infection Can Induce the Expression of Human Endogenous Retrovirus W in Primary Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus
2.2. Human Serum
2.3. Human Pancreatic Cells
2.4. Peripheral Blood Mononuclear Cells
2.5. Human Macrophages
2.6. Viability Measurement
2.7. Viral Titration
2.8. RNA Extraction
2.9. Quantitative RT-PCR
2.9.1. HERV-W ENV mRNA
2.9.2. Enteroviral RNA
2.10. Protein Extraction
2.11. Quantification of HERV-W ENV Protein
3. Results
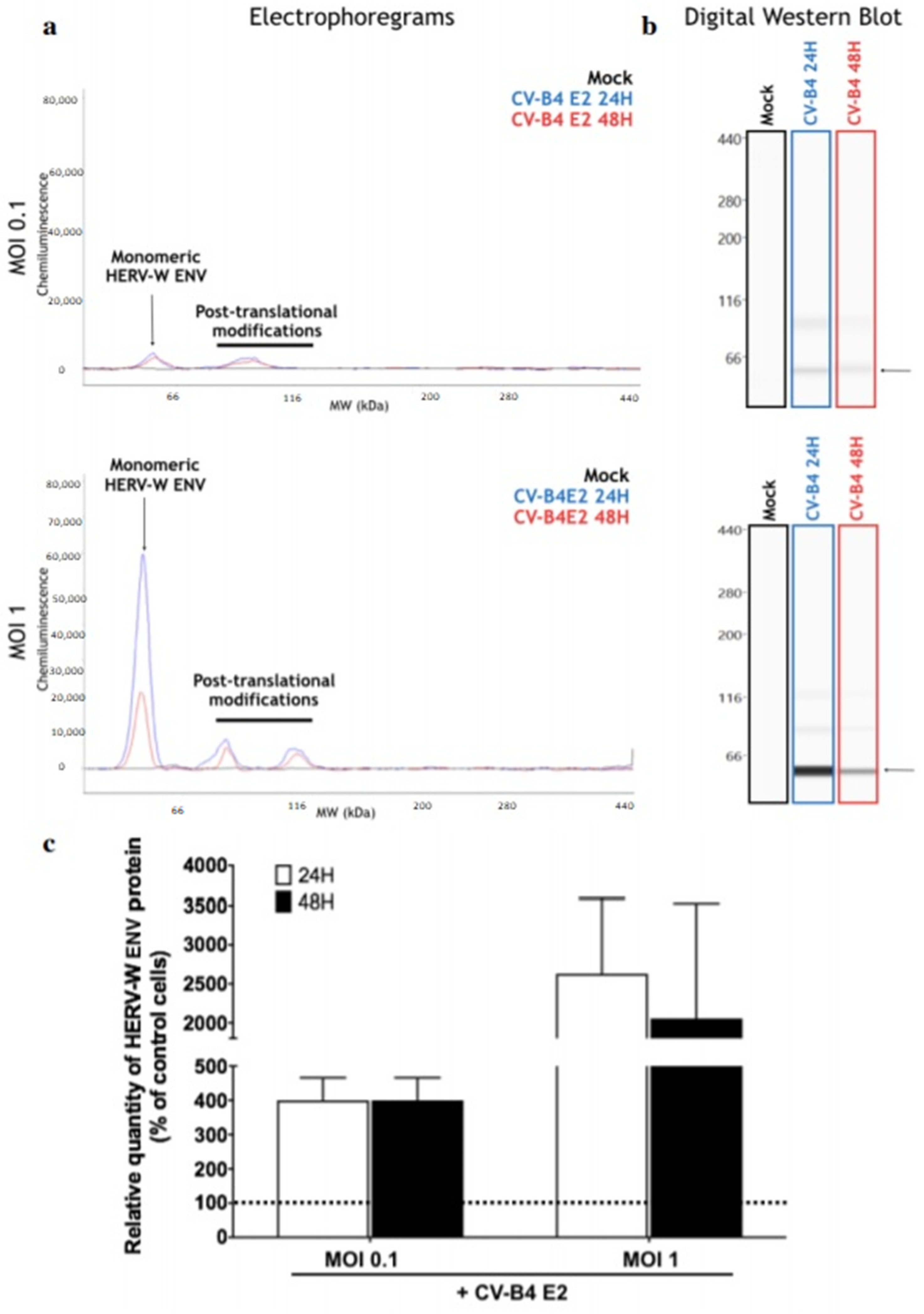
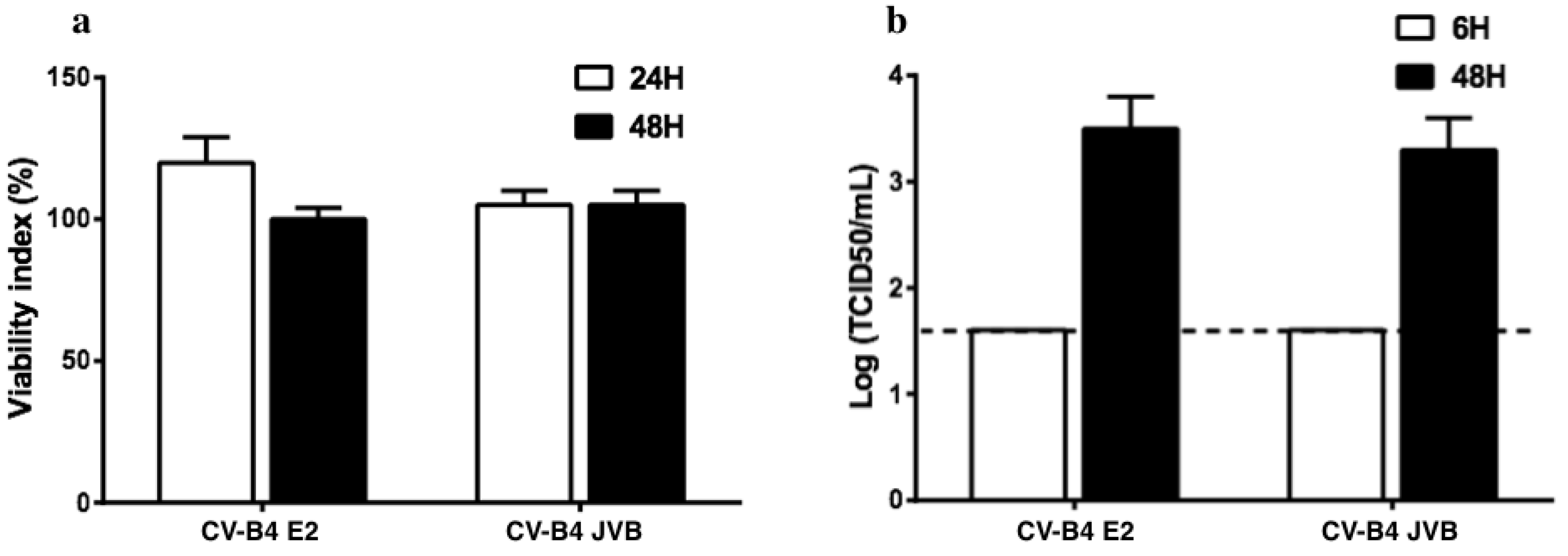
3.1. CVB4 Can Promote the Expression of HERV-W ENV in Primary Pancreatic Ductal Cells
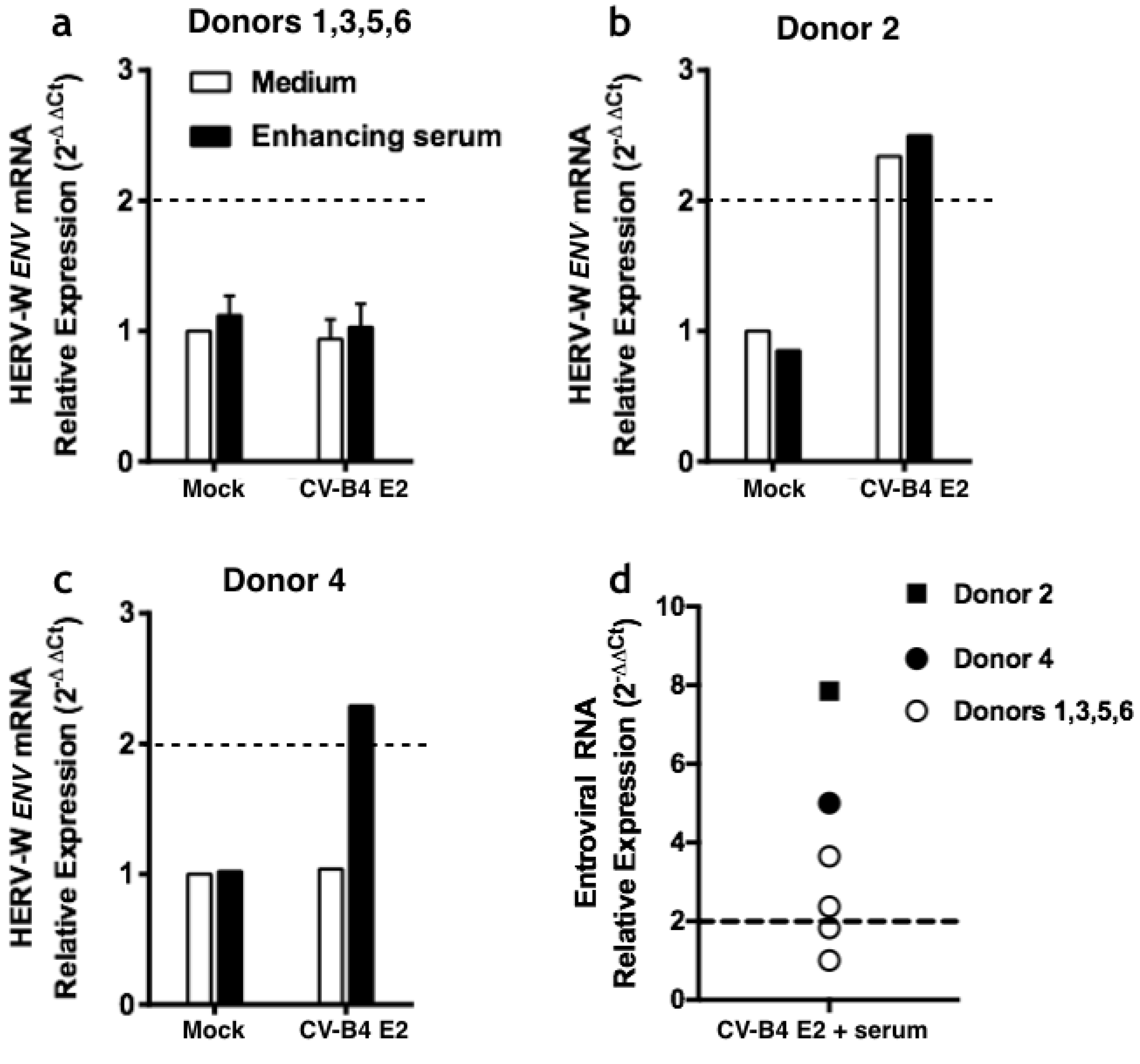
3.2. CVB4 Can Promote the Expression of HERV-W ENV mRNA in PBMCs and Macrophages
3.2.1. Infection of PBMCs
3.2.2. Infection of Macrophages
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grandi, N.; Cadeddu, M.; Blomberg, J.; Tramontano, E. Contribution of type W human endogenous retroviruses to the human genome: Characterization of HERV-W proviral insertions and processed pseudogenes. Retrovirology 2016, 13, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belshaw, R.; Katzourakis, A.; Paces, J.; Burt, A.; Tristem, M. High copy number in human endogenous retrovirus families is associated with copying mechanisms in addition to reinfection. Mol. Biol. Evol. 2005, 22, 814–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levet, S.; Medina, J.; Joanou, J.; Demolder, A.; Queruel, N.; Réant, K.; Normand, M.; Seffals, M.; Dimier, J.; Germi, R.; et al. An ancestral retroviral protein identified as a therapeutic target in type-1 diabetes. JCI Insight 2017, 2, e94387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frendo, J.L.; Olivier, D.; Cheynet, V.; Blond, J.L.; Bouton, O.; Vidaud, M.; Rabreau, M.; Evain-Brion, D.; Mallet, F. Direct involvement of HERV-W Env glycoprotein in human trophoblast cell fusion and differentiation. Mol. Cell Biol. 2003, 23, 3566–3574. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.; Perron, H.; Feschotte, C. Variation in proviral content among human genomes mediated by LTR recombination. Mob. DNA 2018, 9, 36. [Google Scholar] [CrossRef] [Green Version]
- Wildschutte, J.H.; Williams, Z.H.; Monesion, M.; Subramanian, R.P.; Kidd, J.M.; Coffin, J.M. Discovery of unfixed endogenous retrovirus insertions in diverse human populations. Proc. Natl. Acad. Sci. USA 2016, 113, E2326–E2334. [Google Scholar] [CrossRef] [Green Version]
- Levet, S.; Charvet, B.; Bertin, A.; Deschaumes, A.; Perron, H.; Hober, D. Human endogenous retroviruses and type 1 diabetes. Curr. Diabetes Rep. 2019, 19, 141. [Google Scholar] [CrossRef] [Green Version]
- Grandi, N.; Tramontano, E. HERV envelope proteins: Physiological role and pathogenic potential in cancer and autoimmunity. Front. Microbiol. 2018, 9, 462. [Google Scholar] [CrossRef]
- Brudek, T.; Luhdorf, P.; Christensen, T.; Hansen, H.J.; Moller-Larsen, A. Activation of endogenous retrovirus reverse transcriptase in multiple sclerosis patient lymphocytes by inactivated HSV-1, HHV- 6 and VZV. J. Neuroimmunol. 2007, 187, 147–155. [Google Scholar] [CrossRef]
- Ruprecht, K.; Obojes, K.; Wengel, V.; Gronen, F.; Kim, K.S.; Perron, H.; Schneider-Schaulies, J.; Rieckmann, P. Regulation of human endogenous retrovirus W protein expression by herpes simplex virus type 1: Implications for multiple sclerosis. J. Neurovirol. 2006, 12, 65–71. [Google Scholar] [CrossRef]
- Charvet, B.; Reynaud, J.M.; Gourru-Lesimple, G.; Perron, H.; Marche, P.N.; Horvat, B. Induction of proinflammatory multiple sclerosis-associated retrovirus envelope protein by human herpesvirus- 6A and CD46 receptor engagement. Front. Immunol. 2018, 9, 2803. [Google Scholar] [CrossRef]
- Yeung, W.C.; Rawlinson, W.D.; Craig, M.E. Enterovirus infection and type 1 diabetes mellitus: Systematic review and meta-analysis of observational molecular studies. BMJ 2011, 342, d35. [Google Scholar] [CrossRef] [Green Version]
- Vehik, K.; Lynch, K.F.; Wong, M.C.; Tian, X.; Ross, M.C.; Gibbs, R.A.; Ajami, N.J.; Petrosino, J.F.; Rewers, M.; Toppari, J.; et al. Prospective virome analyses in young children at increased genetic risk for type 1 diabetes. Nat. Med. 2019, 25, 1865–1872. [Google Scholar] [CrossRef]
- Jaïdane, H.; Hober, D. Role of coxsackievirus B4 in the pathogenesis of type 1 diabetes. Diabetes Metab. 2008, 34, 537–548. [Google Scholar] [CrossRef]
- Zell, R.; Delwart, E.; Gorbalenya, A.E.; Hovi, T.; King, A.M.Q.; Knowles, N.J.; Lindberg, A.M.; Pallansch, M.A.; Palmenberg, A.C.; Reuter, G.; et al. ICTV Virus Taxonomy Profile: “Picornaviridae”. J. Gen. Virol. 2017, 98, 2421–2422. [Google Scholar]
- Hober, D.; Sauter, P. Pathogenesis of type 1 diabetes mellitus: Interplay between enterovirus and host. Nat. Rev. Endocrinol. 2010, 6, 279–289. [Google Scholar] [CrossRef]
- Riabi, S.; Harrath, R.; Gaaloul, I.; Bouslama, L.; Nasri, D.; Aouni, M.; Pillet, S.; Pozzetto, B. Study of Coxsackie B viruses interactions with Coxsackie Adenovirus receptor and decay-accelerating factor using human CaCo-2 cell line. J. Biomed. Sci. 2014, 21, 50. [Google Scholar] [CrossRef]
- Krogvold, L.; Edwin, B.; Buanes, T.; Frisk, G.; Skog, O.; Anagandula, M.; Undlien, D.; Eike, M.C.; Richardson, S.J.; Leete, P.; et al. Detection of a low-grade enteroviral infection in the islets of langerhans of living patients newly diagnosed with type 1 diabetes. Diabetes 2015, 64, 1682–1687. [Google Scholar] [CrossRef] [Green Version]
- Alidjinou, E.K.; Sane, F.; Lefevre, C.; Baras, A.; Moumna, I.; Engelmann, I.; Vantyghem, M.C.; Hober, D. Enteroviruses in blood of patients with type 1 diabetes detected by integrated cell culture and reverse transcription quantitative real-time PCR. Acta Diabetol. 2017, 54, 1025–1029. [Google Scholar] [CrossRef]
- Ylipaasto, P.; Klingel, K.; Lindberg, A.M.; Otonkoski, T.; Kandolf, R.; Hovi, T.; Roivainen, M. Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia 2004, 47, 225–239. [Google Scholar] [CrossRef] [Green Version]
- Alidjinou, E.K.; Engelmann, I.; Bossu, J.; Villenet, C.; Figeac, M.; Romond, M.-B.; Sane, F.; Hober, D. Persistence of Coxsackievirus B4 in pancreatic ductal-like cells results in cellular and viral changes. Virulence 2017, 8, 1229–1244. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, I.; Alidjinou, E.K.; Bertin, A.; Bossu, J.; Villenet, C.; Figeac, M.; Sane, F.; Hober, D. Persistent coxsackievirus B4 infection induces microRNA dysregulation in human pancreatic cells. Cell Mol. Life Sci. 2017, 74, 3851–3861. [Google Scholar] [CrossRef]
- Chehadeh, W.; Lobert, P.E.; Sauter, P.; Goffard, A.; Lucas, B.; Weill, J.; Vantyghem, M.C.; Alm, G.; Pigny, P.; Hober, D. Viral protein VP4 is a target of human antibodies enhancing coxsackievirus B4- and B3-induced synthesis of alpha interferon. J. Virol. 2005, 79, 13882–13891. [Google Scholar] [CrossRef] [Green Version]
- Hober, D.; Chehadeh, W.; Bouzidi, A.; Wattré, P. Antibody-dependent enhancement of coxsackievirus B4 infectivity of human peripheral blood mononuclear cells results in increased interferon-α synthesis. J. Infect. Dis. 2001, 184, 1098–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alidjinou, E.K.; Sané, F.; Engelmann, I.; Hober, D. Serum-dependent enhancement of coxsackievirus B4-induced production of IFNα, IL-6 and TNFα by peripheral blood mononuclear cells. J. Mol. Biol. 2013, 425, 5020–5031. [Google Scholar] [CrossRef]
- Alidjinou, E.K.; Chehadeh, W.; Weill, J.; Vantyghem, M.C.; Stuckens, C.; Decoster, A.; Hober, C.; Hober, D. Monocytes of patients with type 1 diabetes harbour enterovirus RNA. Eur. J. Clin. Investig. 2015, 45, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Alidjinou, E.K.; Sané, F.; Trauet, J.; Copin, M.C.; Hober, D. Coxsackievirus B4 can infect human peripheral blood-derived macrophages. Viruses 2015, 7, 6067–6079. [Google Scholar] [CrossRef] [Green Version]
- Benkahla, M.A.; Elmastour, F.; Sane, F.; Vreulx, A.C.; Engelmann, I.; Desailloud, R.; Jaidane, H.; Alidjinou, E.K.; Hober, D. Coxsackievirus-B4E2 can infect monocytes and macrophages in vitro and in vivo. Virology 2018, 522, 271–280. [Google Scholar] [CrossRef]
- Ricordi, C.; Lacy, P.E.; Scharp, D.W. Automated islet isolation from human pancreas. Diabetes 1989, 38, 140–142. [Google Scholar] [CrossRef]
- Sane, F.; Caloone, D.; Gmyr, V.; Engelmann, I.; Belaich, S.; Kerr-Conte, J.; Pattou, F.; Desailloud, R.; Hober, D. Coxsackievirus B4 can infect human pancreas ductal cells and persist in ductal-like cell cultures which results in inhibition of Pdx1 expression and disturbed formation of islet-like cell aggregates. Cell Mol. Life Sci. 2013, 70, 4169–4180. [Google Scholar] [CrossRef]
- Bertin, A.; Sane, F.; Gmyr, V.; Lobert, D.; Dechaumes, A.; Kerr-Conte, J.; Pattou, F.; Hober, D. Coxsackievirus-B4 infection of human primary pancreatic ductal cell cultures results in impairment of differentiation into insulin-producing cells. Viruses 2019, 11, 597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charvet, B.; Pierquin, J.; Brunel, J.; Gorter, R.; Quétard, C.; Horvat, B.; Amor, S.; Perron, H.; Portoukalian, J. Multiple sclerosis demyelinating lesions contain unique human endogenous retrovirus type W envelope. 2020; Under revision. [Google Scholar]
- Hober, D.; Chehadeh, W.; Weill, J.; Hober, C.; Vantyghem, M.; Gronnier, P.; Wattré, P. Circulating and cell-bound antibodies increase coxsackievirus B4-induced production of IFN-α by peripheral blood mononuclear cells from patients with type 1 diabetes. J. Gen. Virol. 2002, 83, 2169–2176. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.D.; Mei, B.; Cohn, Z.A. The separation, long-term cultivation, and maturation of the human monocyte. J. Exp. Med. 1977, 146, 1613–1626. [Google Scholar] [CrossRef] [Green Version]
- Wajima, S.; Andreas, K.; Andreas, N.; Hans-Henning, E.; Jaroslav, P. Differentiation of human CD14+ monocytes: An experimental investigation of the optimal culture medium and evidence of a lack of differentiation along the endothelial line. Exp. Mol. Med. 2016, 48, e227. [Google Scholar]
- Schmitt, K.; Richter, C.; Backes, C.; Meese, E.; Ruprecht, K.; Mayer, J. Comprehensive analysis of human endogenous retrovirus group HERV-W locus transcription in multiple sclerosis brain lesions by high-throughput amplicon sequencing. J. Virol. 2013, 87, 13837–13852. [Google Scholar] [CrossRef] [Green Version]
- Mameli, G.; Poddighe, L.; Mei, A.; Uleri, E.; Sotgiu, S.; Serra, C.; Manetti, R.; Dolei, A.l. Expression and activation by Epstein Barr virus of human endogenous retroviruses-W in blood cells and astrocytes: Inference for multiple sclerosis. PLoS ONE 2012, 7, e44991. [Google Scholar] [CrossRef]
- Mameli, G.; Madeddu, G.; Mei, A.; Uleri, E.; Poddighe, L.; Delogu, L.G.; Maida, I.; Babudieri, S.; Serra, C.; Manetti, R.; et al. Activation of MSRV-type endogenous retroviruses during infectious mononucleosis and Epstein-Barr virus latency: The missing link with multiple sclerosis? PLoS ONE 2013, 8, e78474. [Google Scholar] [CrossRef]
- Nellåker, C.; Yao, Y.; Jones-Brando, L.; Mallet, F.; Yolken, R.H.; Karlsson, H. Transactivation of elements in the human endogenous retrovirus W family by viral infection. Retrovirology 2006, 3, 44. [Google Scholar] [CrossRef]
- Li, F.; Nellåker, C.; Sabunciyan, S.; Sabunciyan, S.; Yolken, R.H.; Jones-Brando, L.; Johansson, A.S.; Owe-Larsson, B.; Karlsson, H. Transcriptional derepression of the ERVWE1 locus following influenza A virus infection. J. Virol. 2014, 88, 4328–4337. [Google Scholar] [CrossRef] [Green Version]
- Perron, H.; Suh, M.; Lalande, B.; Gratacap, B.; Laurent, A.; Stoebner, P.; Seigneurin, J.M. Herpes simplex virus ICP0 and ICP4 immediate early proteins strongly enhance expression of a retrovirus harboured by a leptomeningeal cell line from a patient with multiple sclerosis. J. Gen. Virol. 1993, 74, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Tovo, P.A.; Rabbone, I.; Tinti, D.; Galliano, I.; Trada, M.; Dapra, V.; Cerutti, F.; Bergallo, M. Enhanced expression of human endogenous retroviruses in new-onset type 1 diabetes: Potential pathogenetic and therapeutic implications. Autoimmunity 2020, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Perron, H.; Jouvin-Marche, E.; Michel, M.; Ounanian-Paraz, A.; Camelo, S.; Dumon, A.; Jolivet-Reynaud, C.; Marcel, F.; Souillet, Y.; Borel, E.; et al. Multiple sclerosis retrovirus particles and recombinant envelope trigger an abnormal immune response in vitro, by inducing polyclonal Vbeta16 T-lymphocyte activation. Virology 2001, 287, 321–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafon, M.; Jouvin-Marche, E.; Marche, P.N.; Perron, H. Human viral superantigens: To be or not to be transactivated? Trends Immunol. 2002, 23, 238–239. [Google Scholar] [CrossRef]
- Ramasamy, R.; Mohammed, F.; Meier, U.C. HLA DR2b-binding peptides from human endogenous retrovirus envelope, Epstein-Barr virus and brain proteins in the context of molecular mimicry in multiple sclerosis. Immunol. Lett. 2020, 217, 15–24. [Google Scholar] [CrossRef] [PubMed]



| Oligonucleotide Primers | Sequence (5′–3′) | Number of Bases |
|---|---|---|
| HERV-W ENV_Forward | GTATGTCTGATGGGGGTGGAG | 21 |
| HERV-W ENV_Reverse | CTAGTCCTTTGTAGGGGCTAGAG | 23 |
| beta-actin_Forward | TTGCCGACAGGATGCAGAA | 19 |
| beta-actin_Reverse | GCCGATCCACACGGAGTACT | 20 |
| Oligonucleotide Primers and Probe | Sequence (5′–3′) | Number of Bases |
|---|---|---|
| ENT_Forward | CCCTGAATGCGGCTAATC | 18 |
| ENT_Reverse | ATTGTCACCATAAGCAGC | 18 |
| Probe | AACCGACTACTTTGGGTGTCCGTGTTT | 27 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dechaumes, A.; Bertin, A.; Sane, F.; Levet, S.; Varghese, J.; Charvet, B.; Gmyr, V.; Kerr-Conte, J.; Pierquin, J.; Arunkumar, G.; et al. Coxsackievirus-B4 Infection Can Induce the Expression of Human Endogenous Retrovirus W in Primary Cells. Microorganisms 2020, 8, 1335. https://doi.org/10.3390/microorganisms8091335
Dechaumes A, Bertin A, Sane F, Levet S, Varghese J, Charvet B, Gmyr V, Kerr-Conte J, Pierquin J, Arunkumar G, et al. Coxsackievirus-B4 Infection Can Induce the Expression of Human Endogenous Retrovirus W in Primary Cells. Microorganisms. 2020; 8(9):1335. https://doi.org/10.3390/microorganisms8091335
Chicago/Turabian StyleDechaumes, Arthur, Antoine Bertin, Famara Sane, Sandrine Levet, Jennifer Varghese, Benjamin Charvet, Valéry Gmyr, Julie Kerr-Conte, Justine Pierquin, Govindakarnavar Arunkumar, and et al. 2020. "Coxsackievirus-B4 Infection Can Induce the Expression of Human Endogenous Retrovirus W in Primary Cells" Microorganisms 8, no. 9: 1335. https://doi.org/10.3390/microorganisms8091335




