6S-Like scr3559 RNA Affects Development and Antibiotic Production in Streptomyces coelicolor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains, Media and Conditions of Cultivation
2.2. Construction of High-Copy-Number Vector Overexpression Strains
2.3. Mapping of 5′ Ends
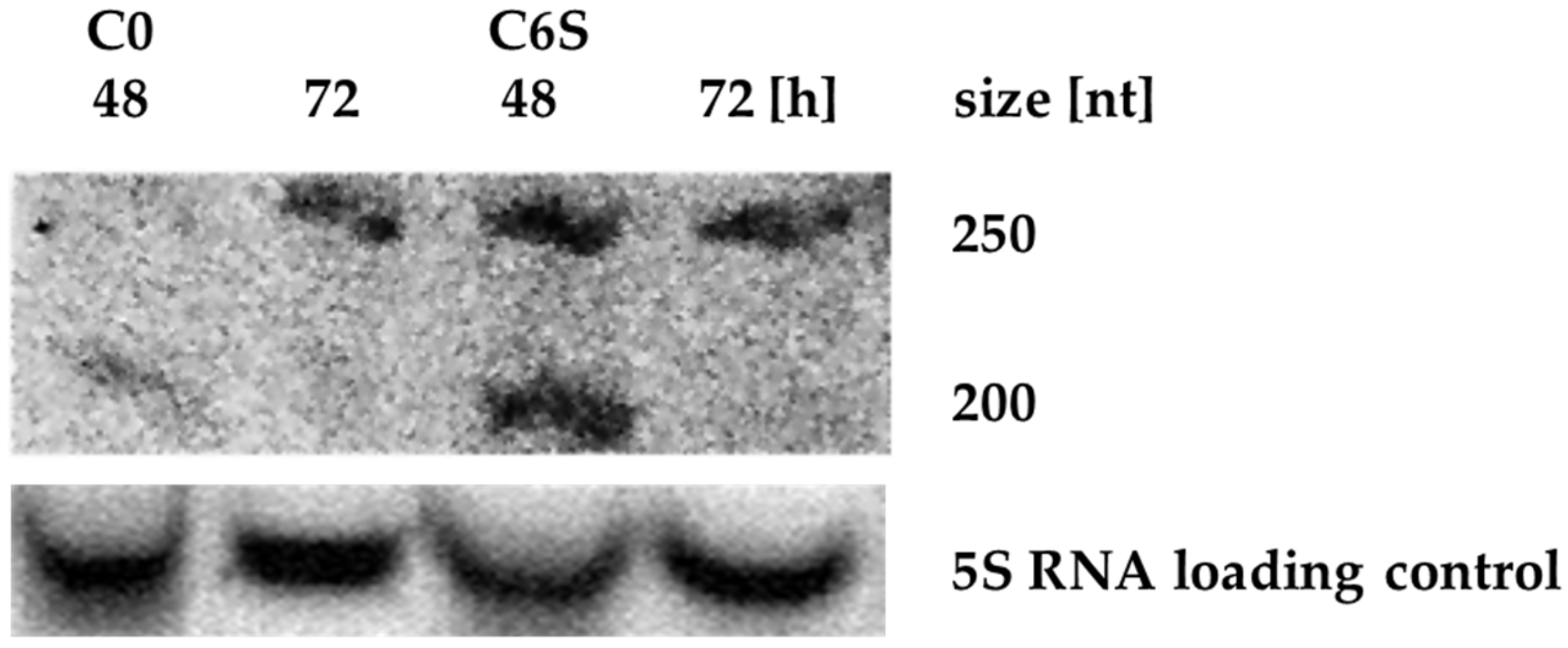
2.4. Differential Expression Analyses (Northern Blot)
2.5. Secondary Metabolite Production Assays
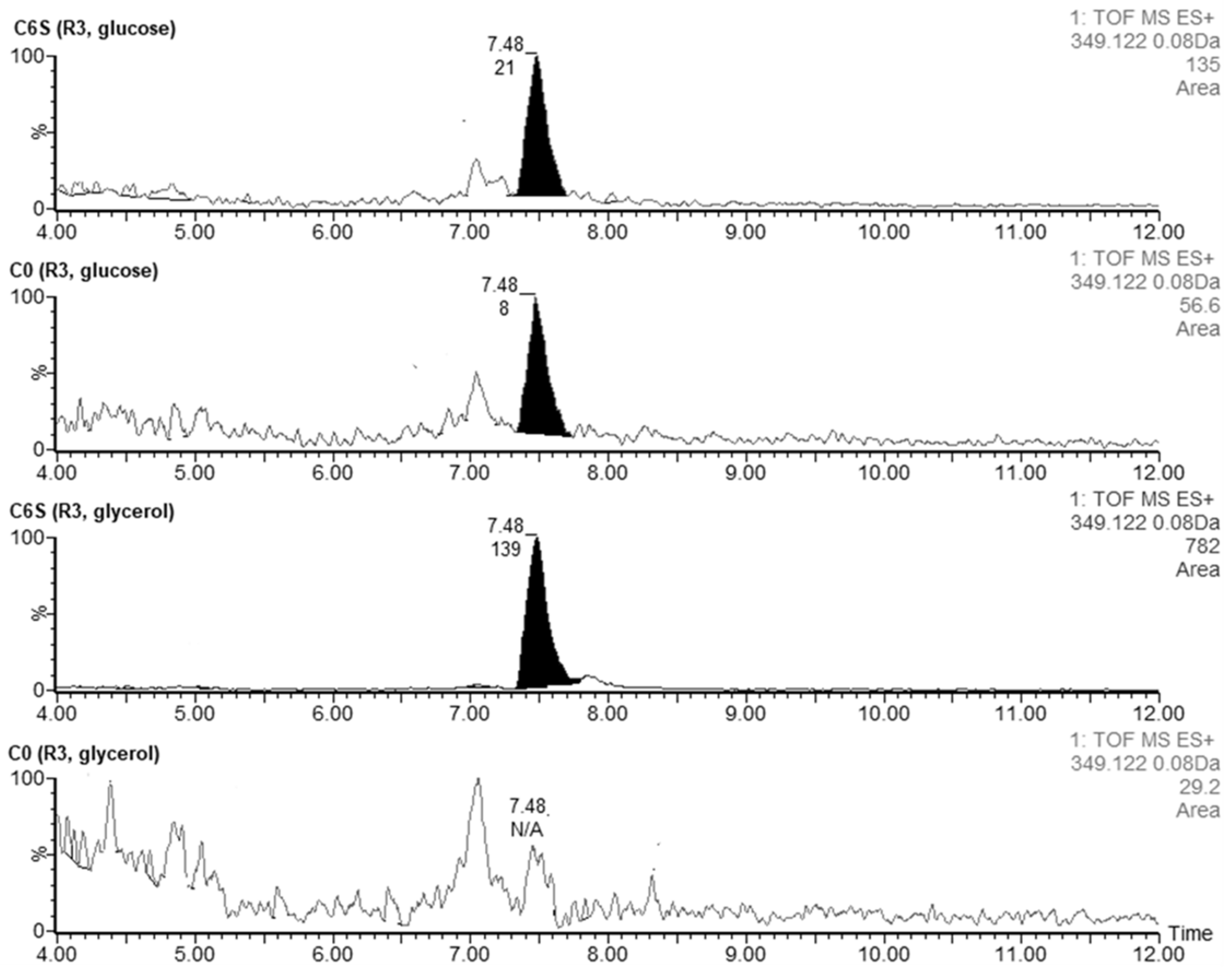
2.6. Liquid Chromatography-Mass Spectrometry (LC-MS) Assay
2.7. Microarrays
3. Results
3.1. Size and Gene Position of the scr3559 Transcript
3.2. scr3559 RNA Expression Profiles (Northern Blotting)
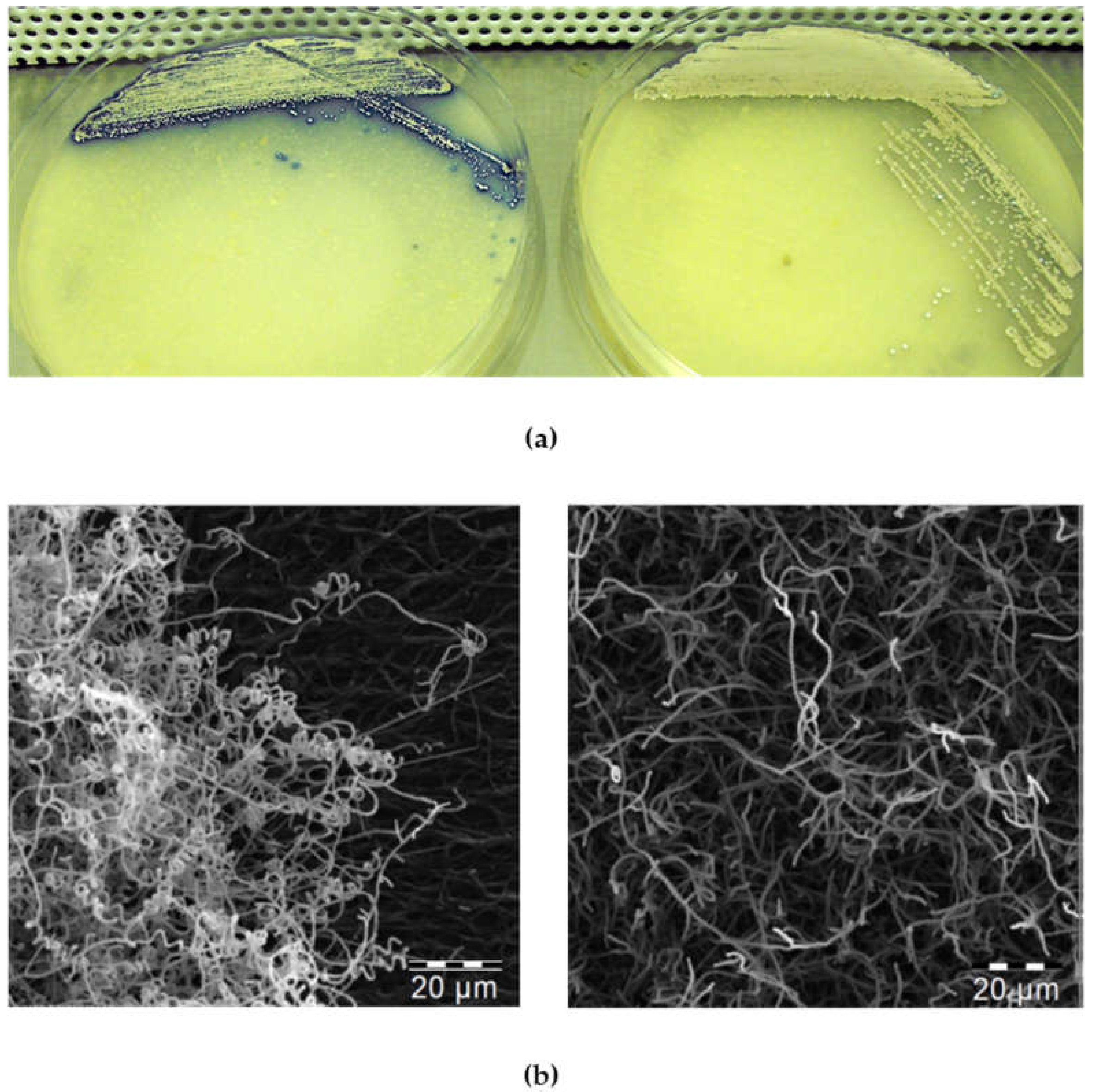
3.3. Phenotypic Analyses
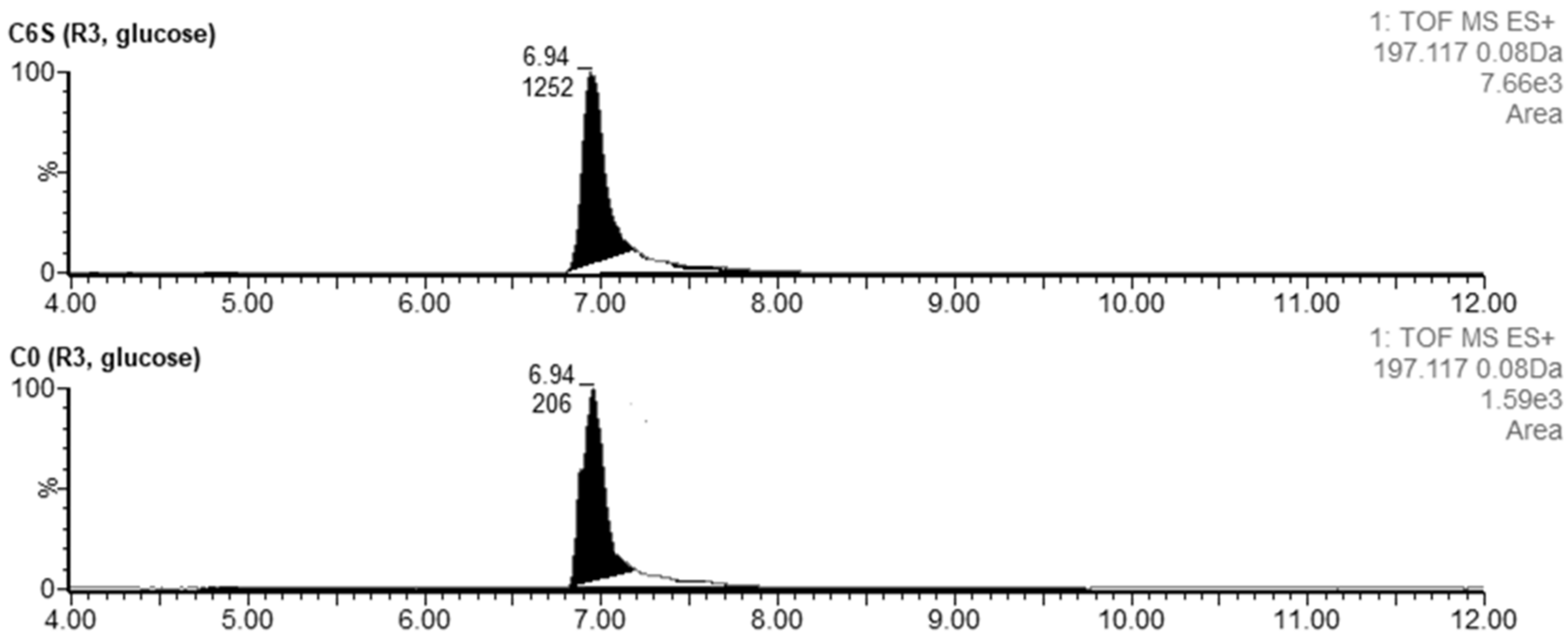
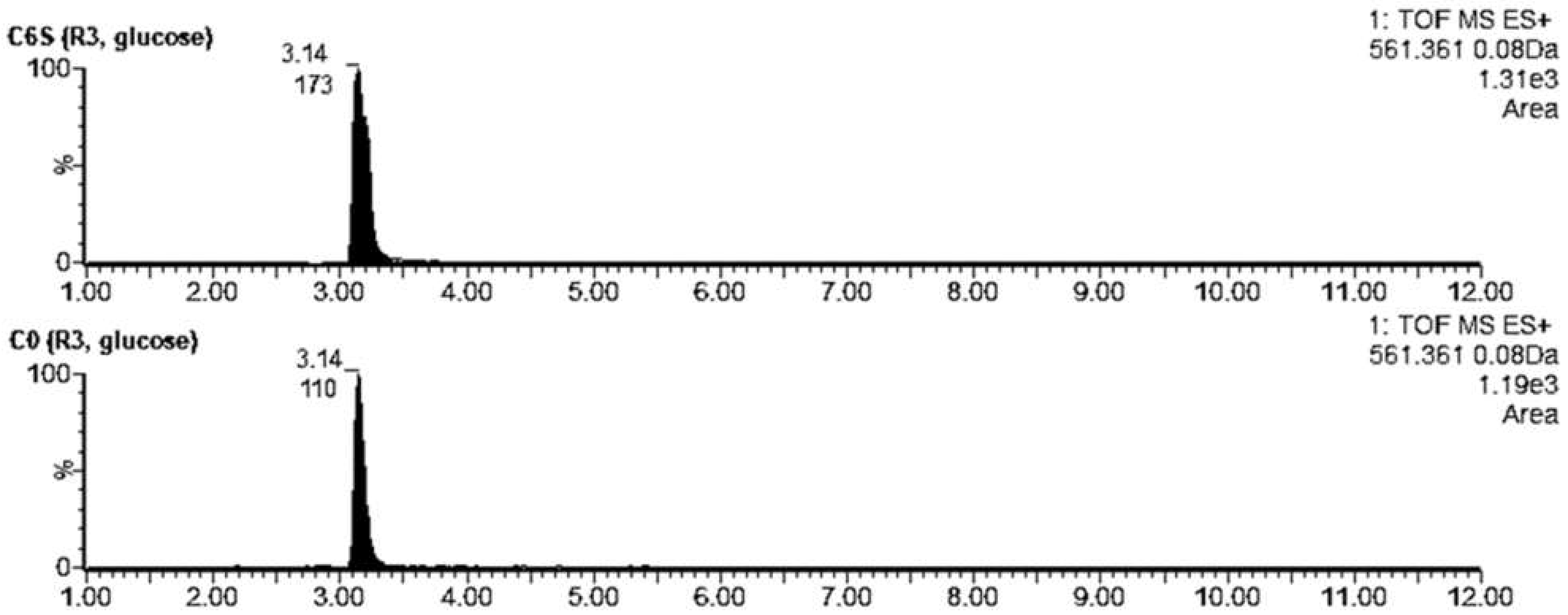
3.4. LC-MS Analysis of Other Metabolite Production
3.4.1. Germicidins
3.4.2. Desferrioxaomines
3.4.3. Coelimycin P1
3.5. Microarray Transcriptional Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelemen, G.H.; Buttner, M.J. Initiation of aerial mycelium formation in Streptomyces. Curr. Opin. Microbiol. 1998, 1, 656–662. [Google Scholar] [CrossRef]
- Flardh, K.; Buttner, M.J. Streptomyces morphogenetics: Dissecting differentiation in a filamentous bacterium. Nat. Rev. Microbiol. 2009, 7, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Flardh, K.; Richards, D.M.; Hempel, A.M.; Howard, M.; Buttner, M.J. Regulation of apical growth and hyphal branching in Streptomyces. Curr. Opin. Microbiol. 2012, 15, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Bobek, J.; Strakova, E.; Zikova, A.; Vohradsky, J. Changes in activity of metabolic and regulatory pathways during germination of S. coelicolor. BMC Genomics 2014, 15, 1173. [Google Scholar] [CrossRef] [Green Version]
- McCormick, J.R.; Flardh, K. Signals and regulators that govern Streptomyces development. FEMS Microbiol. Rev. 2012, 36, 206–231. [Google Scholar] [CrossRef] [Green Version]
- Hopwood, D.A. Forty years of genetics with Streptomyces: From in vivo through in vitro to in silico. Microbiology 1999, 145 Pt 9, 2183–2202. [Google Scholar] [CrossRef] [Green Version]
- Claessen, D.; de Jong, W.; Dijkhuizen, L.; Wosten, H.A. Regulation of Streptomyces development: Reach for the sky! Trends Microbiol. 2006, 14, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Majdalani, N.; Vanderpool, C.K.; Gottesman, S. Bacterial small RNA regulators. Crit. Rev. Biochem. Mol. Biol. 2005, 40, 93–113. [Google Scholar] [CrossRef] [Green Version]
- Wassarman, K.M. 6S RNA, a global regulator of transcription. Microbiol. Spectr. 2018, 6, 355–367. [Google Scholar] [CrossRef]
- Gildehaus, N.; Neusser, T.; Wurm, R.; Wagner, R. Studies on the function of the riboregulator 6S RNA from E. coli: RNA polymerase binding, inhibition of in vitro transcription and synthesis of RNA-directed de novo transcripts. Nucleic Acids Res. 2007, 35, 1885–1896. [Google Scholar] [CrossRef] [Green Version]
- Wassarman, K.M.; Saecker, R.M. Synthesis-mediated release of a small RNA inhibitor of RNA polymerase. Science 2006, 314, 1601–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willkomm, D.K.; Minnerup, J.; Huttenhofer, A.; Hartmann, R.K. Experimental rnomics in aquifex aeolicus: Identification of small non-coding RNAs and the putative 6S RNA homolog. Nucleic Acids Res. 2005, 33, 1949–1960. [Google Scholar] [CrossRef] [Green Version]
- Barrick, J.E.; Sudarsan, N.; Weinberg, Z.; Ruzzo, W.L.; Breaker, R.R. 6S RNA is a widespread regulator of eubacterial RNA polymerase that resembles an open promoter. RNA 2005, 11, 774–784. [Google Scholar] [CrossRef] [Green Version]
- Panek, J.; Bobek, J.; Mikulik, K.; Basler, M.; Vohradsky, J. Biocomputational prediction of small non-coding RNAs in Streptomyces. BMC Genomics 2008, 9, 217. [Google Scholar] [CrossRef] [Green Version]
- Panek, J.; Krasny, L.; Bobek, J.; Jezkova, E.; Korelusova, J.; Vohradsky, J. The suboptimal structures find the optimal RNAs: Homology search for bacterial non-coding RNAs using suboptimal RNA structures. Nucleic Acids Res. 2011, 39, 3418–3426. [Google Scholar] [CrossRef]
- Mikulik, K.; Bobek, J.; Zidkova, J.; Felsberg, J. 6S RNA modulates growth and antibiotic production in Streptomyces coelicolor. Appl. Microbiol. Biotechnol. 2014, 98, 7185–7197. [Google Scholar] [CrossRef]
- Sambrook, J.R.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001; p. A2.2. [Google Scholar]
- Kieser, T.; Bibb, M.J.; Buttner, M.J.; Chater, K.F.; Hopwood, D.A. Practical Streptomyces Genetics, 2nd ed.; John Innes Foundation: Norwich, UK, 2000; ISBN 0-7084-0623-8. [Google Scholar]
- Shima, J.; Hesketh, A.; Okamoto, S.; Kawamoto, S.; Ochi, K. Induction of actinorhodin production by rpsl (encoding ribosomal protein s12) mutations that confer streptomycin resistance in Streptomyces lividans and Streptomyces coelicolor A3(2). J. Bacteriol. 1996, 178, 7276–7284. [Google Scholar] [CrossRef] [Green Version]
- MacNeil, D.J.; Occi, J.L.; Gewain, K.M.; MacNeil, T.; Gibbons, P.H.; Ruby, C.L.; Danis, S.J. Complex organization of the Streptomyces avermitilis genes encoding the avermectin polyketide synthase. Gene 1992, 115, 119–125. [Google Scholar] [CrossRef]
- Janssen, G.R.; Bibb, M.J. Derivatives of pUC18 that have BglII sites flanking a modified multiple cloning site and that retain the ability to identify recombinant clones by visual screening of Escherichia coli colonies. Gene 1993, 124, 133–134. [Google Scholar] [CrossRef]
- Wilkinson, C.J.; Hughes-Thomas, Z.A.; Martin, C.J.; Bohm, I.; Mironenko, T.; Deacon, M.; Wheatcroft, M.; Wirtz, G.; Staunton, J.; Leadlay, P.F. Increasing the efficiency of heterologous promoters in actinomycetes. J. Mol. Microbiol. Biotechnol. 2002, 4, 417–426. [Google Scholar]
- Redenbach, M.; Kieser, H.M.; Denapaite, D.; Eichner, A.; Cullum, J.; Kinashi, H.; Hopwood, D.A. A set of ordered cosmids and a detailed genetic and physical map for the 8 Mb Streptomyces coelicolor A3(2) chromosome. Mol. Microbiol. 1996, 21, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Swiercz, J.P.; Bobek, J.; Haiser, H.J.; Di Berardo, C.; Tjaden, B.; Elliot, M.A. Small non-coding RNAs in Streptomyces coelicolor. Nucleic Acids Res. 2008, 36, 7240–7251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setinova, D.; Smidova, K.; Pohl, P.; Music, I.; Bobek, J. RNase III-binding-mRNAs revealed novel complementary transcripts in Streptomyces. Front. Microbiol. 2017, 8, 2693. [Google Scholar] [CrossRef]
- Kang, S.G.; Jin, W.; Bibb, M.; Lee, K.J. Actinorhodin and undecylprodigiosin production in wild-type and relA mutant strains of Streptomyces coelicolor A3(2) grown in continuous culture. FEMS Microbiol. Lett. 1998, 168, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Cihak, M.; Kamenik, Z.; Smidova, K.; Bergman, N.; Benada, O.; Kofronova, O.; Petrickova, K.; Bobek, J. Secondary metabolites produced during the germination of Streptomyces coelicolor. Front. Microbiol. 2017, 8, 2495. [Google Scholar] [CrossRef] [Green Version]
- Kamenik, Z.; Hadacek, F.; Mareckova, M.; Ulanova, D.; Kopecky, J.; Chobot, V.; Plhackova, K.; Olsovska, J. Ultra-high-performance liquid chromatography fingerprinting method for chemical screening of metabolites in cultivation broth. J. Chromatogr. A 2010, 1217, 8016–8025. [Google Scholar] [CrossRef]
- Gruber, A.R.; Lorenz, R.; Bernhart, S.H.; Neubock, R.; Hofacker, I.L. The Vienna RNA websuite. Nucleic Acids Res. 2008, 36, W70–W74. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Escribano, J.P.; Bibb, M.J. Streptomyces coelicolor as an expression host for heterologous gene clusters. Methods Enzymol. 2012, 517, 279–300. [Google Scholar]
- Nishiyama, T.; Sakemi, H.; Sumi, H.; Tokunaga, S.; Doi, K.; Ogata, S. A chromosomal locus encoding a phosphoserine phosphatase- and a truncated mind-like protein affects differentiation in Streptomyces azureus ATCC14921. FEMS Microbiol. Lett. 2000, 190, 133–139. [Google Scholar] [CrossRef]
- Vockenhuber, M.P.; Sharma, C.M.; Statt, M.G.; Schmidt, D.; Xu, Z.; Dietrich, S.; Liesegang, H.; Mathews, D.H.; Suess, B. Deep sequencing-based identification of small non-coding RNAs in Streptomyces coelicolor. RNA Biol. 2011, 8, 468–477. [Google Scholar] [CrossRef] [Green Version]
- Mai, J.; Rao, C.; Watt, J.; Sun, X.; Lin, C.; Zhang, L.; Liu, J. Mycobacterium tuberculosis 6C sRNA binds multiple mRNA targets via C-rich loops independent of RNA chaperones. Nucleic Acids Res. 2019, 47, 4292–4307. [Google Scholar] [CrossRef]
- Swiercz, J.P.; Elliot, M.A. McMaster University, Hamilton, ON, Canada. Unpublished work. 2009. [Google Scholar]
- Corre, C.; Song, L.; O’Rourke, S.; Chater, K.F.; Challis, G.L. 2-alkyl-4-hydroxymethylfuran-3-carboxylic acids, antibiotic production inducers discovered by Streptomyces coelicolor genome mining. Proc. Natl. Acad. Sci. USA 2008, 105, 17510–17515. [Google Scholar] [CrossRef] [Green Version]
- Paleckova, P.; Bobek, J.; Felsberg, J.; Mikulik, K. Activity of translation system and abundance of tmRNA during development of Streptomyces aureofaciens producing tetracycline. Folia Microbiol. 2006, 51, 517–524. [Google Scholar] [CrossRef]
- Mikulik, K.; Paleckova, P.; Felsberg, J.; Bobek, J.; Zidkova, J.; Halada, P. SsrA genes of streptomycetes and association of proteins to the tmRNA during development and cellular differentiation. Proteomics 2008, 8, 1429–1441. [Google Scholar] [PubMed]
- Engel, F.; Ossipova, E.; Jakobsson, P.J.; Vockenhuber, M.P.; Suess, B. sRNA scr5239 involved in feedback loop regulation of Streptomyces coelicolor central metabolism. Front. Microbiol. 2019, 10, 3121. [Google Scholar] [CrossRef]
- Moody, M.J.; Young, R.A.; Jones, S.E.; Elliot, M.A. Comparative analysis of non-coding RNAs in the antibiotic-producing Streptomyces bacteria. BMC Genomics 2013, 14, 558. [Google Scholar] [CrossRef] [Green Version]
- Yi, J.S.; Kim, M.W.; Kim, M.; Jeong, Y.; Kim, E.J.; Cho, B.K.; Kim, B.G. A novel approach for gene expression optimization through native promoter and 5′ UTR combinations based on RNA-seq, Ribo-seq, and TSS-seq of Streptomyces coelicolor. ACS Synth. Biol. 2017, 6, 555–565. [Google Scholar]
- Kim, K.S.; Lee, Y. Regulation of 6S RNA biogenesis by switching utilization of both sigma factors and endoribonucleases. Nucleic Acids Res. 2004, 32, 6057–6068. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhu, L.; Yu, Z.; Liu, L.; Chou, S.H.; Wang, J.; He, J. 6S-1 RNA contributes to sporulation and parasporal crystal formation in Bacillus thuringiensis. Front. Microbiol. 2020, 11, 604458. [Google Scholar] [CrossRef]
- Behra, P.R.K.; Pettersson, B.M.F.; Das, S.; Dasgupta, S.; Kirsebom, L.A. Comparative genomics of Mycobacterium mucogenicum and Mycobacterium neoaurum clade members emphasizing tRNA and non-coding RNA. BMC Evol. Biol. 2019, 19, 124. [Google Scholar] [CrossRef] [PubMed]
- Hnilicova, J.; Jirat Matejckova, J.; Sikova, M.; Pospisil, J.; Halada, P.; Panek, J.; Krasny, L. Ms1, a novel sRNA interacting with the RNA polymerase core in mycobacteria. Nucleic Acids Res. 2014, 42, 11763–11776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikova, M.; Janouskova, M.; Ramaniuk, O.; Palenikova, P.; Pospisil, J.; Bartl, P.; Suder, A.; Pajer, P.; Kubickova, P.; Pavlis, O.; et al. Ms1 RNA increases the amount of RNA polymerase in Mycobacterium smegmatis. Mol. Microbiol. 2019, 111, 354–372. [Google Scholar]
- Trotochaud, A.E.; Wassarman, K.M. 6S RNA function enhances long-term cell survival. J. Bacteriol. 2004, 186, 4978–4985. [Google Scholar] [CrossRef] [Green Version]
- Wassarman, K.M.; Storz, G. 6S RNA regulates E. coli RNA polymerase activity. Cell 2000, 101, 613–623. [Google Scholar] [CrossRef] [Green Version]
- Cavanagh, A.T.; Klocko, A.D.; Liu, X.; Wassarman, K.M. Promoter specificity for 6S RNA regulation of transcription is determined by core promoter sequences and competition for region 4.2 of sigma70. Mol. Microbiol. 2008, 67, 1242–1256. [Google Scholar] [CrossRef]
- Neusser, T.; Polen, T.; Geissen, R.; Wagner, R. Depletion of the non-coding regulatory 6S RNA in E. coli causes a surprising reduction in the expression of the translation machinery. BMC Genomics 2010, 11, 165. [Google Scholar] [CrossRef] [Green Version]
- Ando, Y.; Asari, S.; Suzuma, S.; Yamane, K.; Nakamura, K. Expression of a small RNA, bs203 RNA, from the yocI-yocJ intergenic region of Bacillus subtilis genome. FEMS Microbiol. Lett. 2002, 207, 29–33. [Google Scholar] [CrossRef]
- Beckmann, B.M.; Burenina, O.Y.; Hoch, P.G.; Kubareva, E.A.; Sharma, C.M.; Hartmann, R.K. In vivo and in vitro analysis of 6S RNA-templated short transcripts in Bacillus subtilis. RNA Biol. 2011, 8, 839–849. [Google Scholar] [CrossRef] [Green Version]
- Suzuma, S.; Asari, S.; Bunai, K.; Yoshino, K.; Ando, Y.; Kakeshita, H.; Fujita, M.; Nakamura, K.; Yamane, K. Identification and characterization of novel small RNAs in the aspS-yrvM intergenic region of the Bacillus subtilis genome. Microbiology 2002, 148, 2591–2598. [Google Scholar] [CrossRef] [Green Version]
- Cavanagh, A.T.; Wassarman, K.M. 6S-1 RNA function leads to a delay in sporulation in Bacillus subtilis. J. Bacteriol. 2013, 195, 2079–2086. [Google Scholar] [CrossRef] [Green Version]
- Warrier, I.; Hicks, L.D.; Battisti, J.M.; Raghavan, R.; Minnick, M.F. Identification of novel small RNAs and characterization of the 6S RNA of Coxiella burnetii. PLoS ONE 2014, 9, e100147. [Google Scholar]
- Yague, P.; Rodriguez-Garcia, A.; Lopez-Garcia, M.T.; Rioseras, B.; Martin, J.F.; Sanchez, J.; Manteca, A. Transcriptomic analysis of liquid non-sporulating Streptomyces coelicolor cultures demonstrates the existence of a complex differentiation comparable to that occurring in solid sporulating cultures. PLoS ONE 2014, 9, e86296. [Google Scholar] [CrossRef] [Green Version]
- Bibb, M.J.; Molle, V.; Buttner, M.J. Sigma(BldN), an extracytoplasmic function RNA polymerase sigma factor required for aerial mycelium formation in Streptomyces coelicolor A3(2). J. Bacteriol. 2000, 182, 4606–4616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendez, C.; Chater, K.F. Cloning of whiG, a gene critical for sporulation of Streptomyces coelicolor A3(2). J. Bacteriol. 1987, 169, 5715–5720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flardh, K.; Findlay, K.C.; Chater, K.F. Association of early sporulation genes with suggested developmental decision points in Streptomyces coelicolor A3(2). Microbiology 1999, 145 Pt 9, 2229–2243. [Google Scholar] [CrossRef] [Green Version]
- Chater, K.F. Multilevel regulation of Streptomyces differentiation. Trends Genet. 1989, 5, 372–377. [Google Scholar] [CrossRef]
- Ryding, N.J.; Kelemen, G.H.; Whatling, C.A.; Flardh, K.; Buttner, M.J.; Chater, K.F. A developmentally regulated gene encoding a repressor-like protein is essential for sporulation in Streptomyces coelicolor A3(2). Mol. Microbiol. 1998, 29, 343–357. [Google Scholar] [CrossRef]
- Becher, P.G.; Verschut, V.; Bibb, M.J.; Bush, M.J.; Molnar, B.P.; Barane, E.; Al-Bassam, M.M.; Chandra, G.; Song, L.; Challis, G.L.; et al. Developmentally regulated volatiles geosmin and 2-methylisoborneol attract a soil arthropod to Streptomyces bacteria promoting spore dispersal. Nat. Microbiol. 2020, 5, 821–829. [Google Scholar] [CrossRef]
- Guijarro, J.; Santamaria, R.; Schauer, A.; Losick, R. Promoter determining the timing and spatial localization of transcription of a cloned Streptomyces coelicolor gene encoding a spore-associated polypeptide. J. Bacteriol. 1988, 170, 1895–1901. [Google Scholar] [CrossRef] [Green Version]
- Willey, J.; Santamaria, R.; Guijarro, J.; Geistlich, M.; Losick, R. Extracellular complementation of a developmental mutation implicates a small sporulation protein in aerial mycelium formation by S. coelicolor. Cell 1991, 65, 641–650. [Google Scholar] [CrossRef]
- Khokhlov, A.S.; Tovarova, I.I.; Borisova, L.N.; Pliner, S.A.; Shevchenko, L.N.; Kornitskaia, E.; Ivkina, N.S.; Rapoport, I.A. [The a-factor, responsible for streptomycin biosynthesis by mutant strains of actinomyces streptomycini]. Dokl. Akad. Nauk. SSSR 1967, 177, 232–235. [Google Scholar]
- Takano, E. Gamma-butyrolactones: Streptomyces signalling molecules regulating antibiotic production and differentiation. Curr. Opin. Microbiol. 2006, 9, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Kato, J.Y.; Funa, N.; Watanabe, H.; Ohnishi, Y.; Horinouchi, S. Biosynthesis of gamma-butyrolactone autoregulators that switch on secondary metabolism and morphological development in Streptomyces. Proc. Natl. Acad. Sci. USA 2007, 104, 2378–2383. [Google Scholar] [CrossRef] [Green Version]
- Hindra; Pak, P.; Elliot, M.A. Regulation of a novel gene cluster involved in secondary metabolite production in Streptomyces coelicolor. J. Bacteriol. 2010, 192, 4973–4982. [Google Scholar] [CrossRef] [Green Version]
- Park, U.M.; Suh, J.W.; Hong, S.K. Genetic analysis of absR, a new abs locus of Streptomyces coelicolor. J. Microbiol. Biotechnol. 2000, 10, 169–175. [Google Scholar]
- Tenconi, E.; Traxler, M.F.; Hoebreck, C.; van Wezel, G.P.; Rigali, S. Production of prodiginines is part of a programmed cell death process in Streptomyces coelicolor. Front. Microbiol. 2018, 9, 1742. [Google Scholar] [CrossRef]
- Takano, H.; Obitsu, S.; Beppu, T.; Ueda, K. Light-induced carotenogenesis in Streptomyces coelicolor A3(2): Identification of an extracytoplasmic function sigma factor that directs photodependent transcription of the carotenoid biosynthesis gene cluster. J. Bacteriol. 2005, 187, 1825–1832. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Barona-Gomez, F.; Corre, C.; Xiang, L.; Udwary, D.W.; Austin, M.B.; Noel, J.P.; Moore, B.S.; Challis, G.L. Type III polyketide synthase beta-ketoacyl-acp starter unit and ethylmalonyl-coa extender unit selectivity discovered by Streptomyces coelicolor genome mining. J. Am. Chem. Soc. 2006, 128, 14754–14755. [Google Scholar] [CrossRef] [Green Version]
- Aoki, Y.; Matsumoto, D.; Kawaide, H.; Natsume, M. Physiological role of germicidins in spore germination and hyphal elongation in Streptomyces coelicolor A3(2). J. Antibiot. 2011, 64, 607–611. [Google Scholar] [CrossRef]




| Strain or Plasmid | Genotype, Description or Use | Reference or Source |
|---|---|---|
| S. coelicolor | ||
| M145 | SCP1− SCP2− | [18] |
| C6S | scr3559 over-expression: in order to be expressed from the tipA promoter, the scr3559 gene was cloned into the high copy number plasmid pCJW93. | This work |
| C0 | a control strain containing an empty pCJW93 vector | This work |
| E. coli | ||
| DH5α | Plasmid construction and general subcloning | Invitrogen |
| ET12567/pUZ8002 | Generation of methylation-free plasmid DNA and conjugation from E. coli into S. coelicolor | [20] |
| Plasmids/cosmids | ||
| pIJ2925 | General cloning vector | [21] |
| pCJW93 | High-copy-number E. coli/Streptomyces plasmid containing a thiostrepton inducible (tipA) promoter | [22] |
| StH5 | S. coelicolor cosmid containing the scr3559 gene | [23] |
| Gene SCO Number | Average Differential Expression (C6S/C0) | Gene/Cosmid Name | Protein/Gene Cluster | |
|---|---|---|---|---|
| Young Cells (72 h) | Old Cells (144 h) | |||
| 185 | −2.71 | 3.66 | crtB/SCJ1.34 | putative geranyl pyrophosphate synthase/Isorenieratene |
| 186 | −0.15 | 1.85 | crtE/SCJ1.35 | putative phytoene dehydrogenase (putative secreted protein)/Isorenieratene |
| 187 | −0.82 | 2.53 | crtI/SCJ1.36 | putative phytoene synthase/Isorenieratene |
| 188 | −0.41 | 1.52 | crtV/SCJ1.37 | putative methylesterase/Isorenieratene |
| 189 | −0.84 | 1.14 | crtU/SCJ1.38c | putative dehydrogenase/Isorenieratene |
| 190 | 0.02 | 1.20 | crtT/SCJ12.02c | Isorenieratene |
| 191 | −0.76 | 1.11 | crtY/SCJ12.03c | Isorenieratene |
| 216 | −1.27 | 2.72 | narG2 | nitrate reductase alpha chain NarG2 |
| 217 | −1.61 | 2.69 | narH2 | nitrate reductase beta chain NarH2 |
| 379 | 1.49 | 1.05 | katA/EC 1.11.1.6 | catalase KatA |
| 380 | 0.91 | 2.10 | SCF62.06 | conserved hypothetical protein |
| 381 | 2.37 | 2.30 | SCF62.07 | putative glycosyl transferase |
| 382 | 3.14 | 0.82 | SCF62.08 | UDP-glucose/GDP-mannose family dehydrogenase |
| 383 | 2.24 | 1.66 | SCF62.09 | hypothetical protein |
| 384 | 2.01 | 1.58 | SCF62.10 | putative membrane protein |
| 385 | 1.74 | 1.23 | SCF62.11 | putative membrane protein |
| 409 | 1.77 | 1.14 | sapA/SCF51.08c | spore-associated protein precursor |
| 489 | −3.72 | −6.22 | SCF34.08c | conserved hypothetical protein/Siderophore coelichelin |
| 490 | −2.18 | −3.35 | SCF34.09c | putative esterase/Siderophore coelichelin |
| 491 | −1.57 | −3.33 | SCF34.10c | putative ABC transporter transmembrane protein/Siderophore coelichelin |
| 492 | −0.88 | −3.73 | SCF34.11c | putative peptide synthetase/Siderophore coelichelin |
| 493 | −1.66 | −4.03 | SCF34.12c | putative ABC-transporter transmembrane protein/Siderophore coelichelin |
| 494 | −2.85 | −4.16 | SCF34.13c | putative iron-siderophore binding lipoprotein/Siderophore coelichelin |
| 495 | −1.32 | −1.66 | SCF34.14c | putative iron-siderophore ABC-transporter ATP-binding protein/Siderophore coelichelin |
| 496 | 0.61 | −2.29 | SCF34.15c | putative iron-siderophore permease transmembrane protein/Siderophore coelichelin |
| 497 | −0.40 | −2.20 | SCF34.16c | putative iron-siderophore permease transmembrane protein/Siderophore coelichelin |
| 498 | −0.96 | −6.16 | SCF34.17c | putative peptide monooxygenase/Siderophore coelichelin |
| 499 | −2.28 | −4.08 | SCF34.18 | putative formyltransferase/Siderophore coelichelin |
| 895 | −0.57 | −0.25 | hrdC/SCM1.28c | RNA polymerase principal sigma factor HrdC |
| 1849 | −2.84 | −1.28 | cobN | cobalamin biosynthesis protein CobN |
| 2077 | −1.22 | 0.55 | divIVA/SC4A10.10c | DivIVA |
| 2084 | −2.10 | 0.74 | murG/SC4A10.17c | putative UDP-N-acetylglucosamine-N-acetylmuramyl-(pentapeptide) pyrophosphoryl-undecaprenol N-acetylglucosamine transferase MurG |
| 2431 | 1.15 | −0.88 | abfA/SCC24.02c | AbfA |
| 2465 | 0.98 | 0.31 | hrdA/SC7A8.04c | RNA polymerase principal sigma factor HrdA |
| 2779 | 1.86 | 0.99 | acdH/SCC105.10 | acyl-CoA dehydrogenase AcdH |
| 2780 | −1.17 | −2.95 | SCC105.11 | putative secreted protein/Desferrioxamines |
| 2781 | −1.12 | −1.70 | SCC105.12 | hypothetical protein/Desferrioxamines |
| 2782 | −0.07 | −3.94 | SCC105.13 | putative pyridoxal-dependent decarboxylase/Desferrioxamines |
| 2783 | −0.45 | −4.20 | SCC105.14 | putative monooxygenase/Desferrioxamines |
| 2784 | −0.61 | −4.81 | SCC105.15 | putative aceytltranferase/Desferrioxamines |
| 2785 | −1.29 | −4.54 | SCC105.16 | conserved hypothetical protein/Desferrioxamines |
| 3110 | −2.88 | −5.29 | SCE41.19c | putative ABC transport system integral membrane protein |
| 3111 | −4.77 | −5.37 | SCE41.20c | putative ABC transport system ATP-binding protein |
| 3202 | −2.35 | −2.27 | hrdD/SCE22.19c | RNA polymerase principal sigma factor HrdD |
| 3287 | 0.86 | −0.71 | SCE15.04 | putative serine/arginine rich protein AbeA |
| 3288 | 7.22 | −1.63 | SCE15.05 | putative integral membrane protein AbeB |
| 3289 | 5.77 | −1.64 | SCE15.06 | putative large membrane protein AbeC |
| 3290 | 8.07 | −1.80 | SCE15.07 | hypothetical protein AbeD |
| 3291 | 0.39 | 1.53 | SCE15.08 | putative regulatory protein AbeR SARP |
| 3322 | −2.02 | 1.16 | SCE68.20 | putative membrane protein, a SCO3558 homolog |
| 3323 | 3.90 | 2.51 | SCE68.21 | putative RNA polymerase sigma factor BldN |
| 3413 | 3.81 | 1.28 | tipA/SCE9.20 | transcriptional regulator TipA |
| 3558 | −2.38 | 0.90 | SCH5.21 | putative morphological differentiation-associated protein |
| 3559 | −2.45 | −0.08 | SCH5.22c | putative oxidoreductase |
| 3607 | −2.72 | −1.51 | SC66T3.18c | putative secreted protein |
| 3608 | −3.18 | −0.34 | SC66T3.19c | hypothetical protein |
| 4654 | −1.57 | 1.74 | rpoB/SCD82.26 | DNA-directed RNA polymerase beta chain RpoB |
| 4655 | −1.47 | 1.11 | rpoC/SCD82.27 | SCDDNA-directed RNA polymerase beta’ chain RpoC |
| 5069 | 0.40 | 0.40 | SCBAC20F6.12 | putative oxidoreductase/Actinorhodin |
| 5070 | 0.87 | 0.66 | SCBAC20F6.13c | hydroxylacyl-CoA dehydrogenase/Actinorhodin |
| 5071 | 1.92 | 2.55 | SCBAC20F6.14c | hydroxylacyl-CoA dehydrogenase/Actinorhodin |
| 5072 | 1.34 | 1.99 | SCBAC20F6.15 | hydroxylacyl-CoA dehydrogenase/Actinorhodin |
| 5073 | 0.74 | 2.12 | SCBAC20F6.16 | putative oxidoreductase/Actinorhodin |
| 5074 | 1.67 | 1.83 | SCBAC20F6.17 | putative dehydratase/Actinorhodin |
| 5075 | 0.92 | 2.07 | ORF4/SCBAC28G1.01 | putative oxidoreductase/Actinorhodin |
| 5076 | 0.67 | 0.24 | actVA1/SCBAC28G1 | integral membrane protein/Actinorhodin |
| 5077 | −0.09 | 1.19 | actVA2/SCBAC28G1 | hypothetical protein/Actinorhodin |
| 5078 | 1.65 | 0.86 | actVA3/SCBAC28G1 | hypothetical protein/Actinorhodin |
| 5079 | 2.62 | 0.31 | actVA4/SCBAC28G1 | conserved hypothetical protein/Actinorhodin |
| 5080 | 1.25 | −0.41 | actVA5/SCBAC28G1 | putative hydrolase/Actinorhodin |
| 5081 | 0.48 | 2.40 | actVA6/SCBAC28G1 | hypothetical protein/Actinorhodin |
| 5082 | −1.18 | 0.42 | actII-1/SCBAC28G1 | putative transcriptional regulatory protein/Actinorhodin |
| 5083 | −0.12 | −2.64 | actII-2/SCBAC28G1 | putative actinorhodin transporter/Actinorhodin |
| 5084 | 1.31 | −3.45 | actII-3/SCBAC28G1 | putative membrane protein/Actinorhodin |
| 5085 | −0.82 | 5.01 | actII-4/SCBAC28G1 | actinorhodin cluster activator protein/Actinorhodin |
| 5086 | 0.64 | 2.27 | actIII/SCBAC28G1 | ketoacyl reductase/Actinorhodin |
| 5087 | 0.76 | 1.90 | actI-1/SCBAC28G | actinorhodin polyketide beta-ketoacyl synthaselpha subunit/Actinorhodin |
| 5088 | 0.90 | 0.84 | actI-2/SCBAC28G | actinorhodin polyketide beta-ketoacyl synthaseeta subunit/Actinorhodin |
| 5089 | 0.67 | 2.72 | actI-3/SCBAC28G | actinorhodin polyketide synthase acyl carrierprotein/Actinorhodin |
| 5090 | 0.02 | 2.27 | actVII/SCBAC28G1 | actinorhodin polyketide synthase bifunctionalyclase/dehydratase/Actinorhodin |
| 5091 | −0.07 | 1.54 | actIV/SCBAC28G1.1 | cyclase/Actinorhodin |
| 5092 | 0.61 | 0.80 | actVB/SCBAC28G1.1 | actinorhodin polyketide putative dimerase/Actinorhodin |
| 5572 | −3.99 | 2.24 | rnc/SC7A1.16 | ribonuclease III/RNase III |
| 5621 | −1.70 | 3.75 | whiG/SC2E1.38 | RNA polymerase sigma factor WhiG |
| 5819 | 1.33 | 1.96 | whiH | Sporulation transcription factor WhiH |
| 5820 | −0.49 | 1.97 | hrdB/SC5B8.10 | major vegetative sigma factor HrdB |
| 5877 | 1.96 | 3.21 | redD | transcriptional regulator RedD/Prodiginines |
| 5878 | 1.34 | 0.94 | redX | polyketide synthase RedX/Prodiginines |
| 5879 | 0.33 | 0.32 | redW | acyl-coa dehydrogenase RedW/Prodiginines |
| 5880 | 0.09 | 3.34 | redY | RedY protein/Prodiginines |
| 5881 | −1.54 | 2.78 | redZ | response regulator/Prodiginines |
| 5882 | 0.61 | 0.02 | redV/SC3F7.02c | Prodiginines |
| 5883 | 0.79 | 0.87 | redU/SC3F7.03c | Prodiginines |
| 5884 | 1.41 | 0.09 | SC3F7.04c | hypothetical protein/Prodiginines |
| 5885 | 1.21 | −0.66 | SC3F7.05c | hypothetical protein/Prodiginines |
| 5886 | 1.68 | 2.31 | redR/SC3F7.06c | Prodiginines |
| 5887 | 2.60 | 0.20 | redQ/SC3F7.07c | Prodiginines |
| 5888 | 0.94 | −0.50 | redP/SC3F7.08 | 3-oxoacyl-[acyl-carrier-protein] synthase/Prodiginines |
| 5889 | 2.70 | 0.50 | redO/SC3F7.09 | hypothetical protein/Prodiginines |
| 5890 | 1.65 | 0.89 | redN/SC3F7.10 | putative 8-amino-7-oxononanoate synthase/Prodiginines |
| 5891 | 1.02 | −0.79 | redM/St3F7.11 | putative peptide synthase/Prodiginines |
| 5892 | 0.92 | −0.28 | redL/SC3F7.12 | polyketide synthase/Prodiginines |
| 5893 | 0.82 | 0.08 | redK/SC3F7.13 | oxidoreductase/Prodiginines |
| 5894 | 0.69 | 0.02 | redJ/SC3F7.14 | thioesterase/Prodiginines |
| 5895 | 1.24 | −0.04 | redI/SC3F7.15 | putative methyltransferase/Prodiginines |
| 5896 | 1.45 | 0.17 | SC10A5.01 | phosphoenolpyruvate-utilizing enzyme/Prodiginines |
| 5897 | 2.47 | 0.76 | redG/SC10A5.02 | Prodiginines |
| 5898 | 2.38 | 1.57 | redF/SC10A5.03 | Prodiginines |
| 6265 | −0.25 | 0.51 | scbR/SCAH10.30c | Gamma-butyrolactone binding protein |
| 6266 | 1.63 | 1.14 | scbA/SCAH10.31 | SCB1 butanolide synthase, an AfsA homolog/Gamma-butyrolactone |
| 6267 | −0.44 | 1.19 | SCAH10.32 | hypothetical protein, a BprA homolog |
| 6992 | 1.82 | 5.48 | absR1/SC8F11.18c | regulatory protein AbsR1 |
| 6993 | 1.31 | 2.10 | absR2/SC8F11.19 | regulatory protein AbsR2 |
| 7221 | −0.75 | 3.04 | SC2H12.20c | Gcs type III polyketide synthase |

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobek, J.; Mikulová, A.; Šetinová, D.; Elliot, M.; Čihák, M. 6S-Like scr3559 RNA Affects Development and Antibiotic Production in Streptomyces coelicolor. Microorganisms 2021, 9, 2004. https://doi.org/10.3390/microorganisms9102004
Bobek J, Mikulová A, Šetinová D, Elliot M, Čihák M. 6S-Like scr3559 RNA Affects Development and Antibiotic Production in Streptomyces coelicolor. Microorganisms. 2021; 9(10):2004. https://doi.org/10.3390/microorganisms9102004
Chicago/Turabian StyleBobek, Jan, Adéla Mikulová, Dita Šetinová, Marie Elliot, and Matouš Čihák. 2021. "6S-Like scr3559 RNA Affects Development and Antibiotic Production in Streptomyces coelicolor" Microorganisms 9, no. 10: 2004. https://doi.org/10.3390/microorganisms9102004






