Soil Layers Matter: Vertical Stratification of Root-Associated Fungal Assemblages in Temperate Forests Reveals Differences in Habitat Colonization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites Characteristics
2.2. Root and Soil Sampling from Organic and Mineral Soil Layers
2.3. Determination of Soil Chemical Properties
2.4. DNA Extraction and Polymerase Chain Reaction
2.5. Bioinformatics Processing and Analyses
2.6. Data Processing and Statistical Analysis
3. Results
3.1. Differences in Soil Chemistry among Different Biogeographic Regions Are Larger in the Mineral Topsoil Than in the Organic Layer
3.2. Strong Taxonomic Differentiation of Root-Associated Fungal Assemblages between Organic and Mineral Topsoil
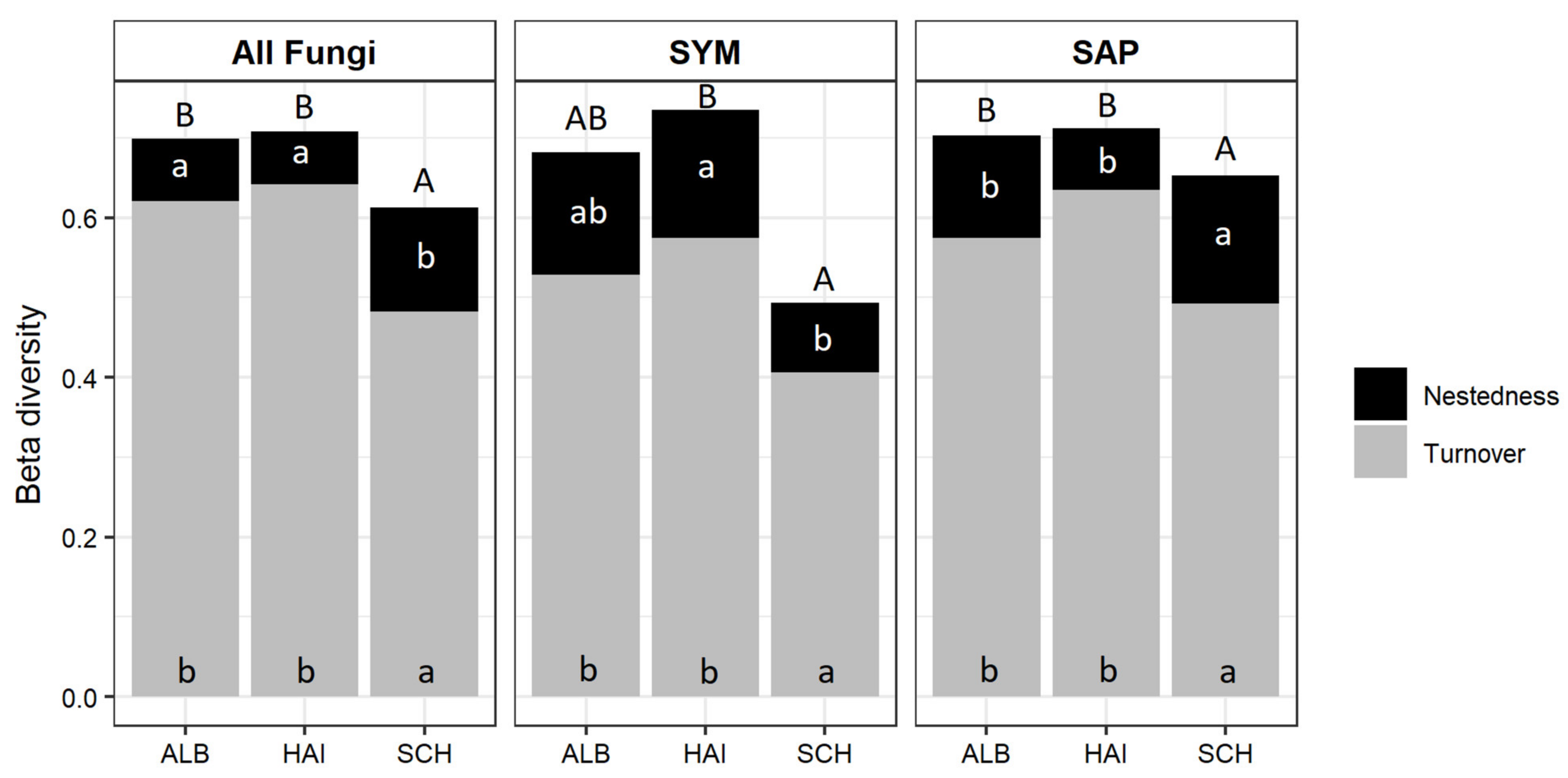
3.3. β-Diversity of Root-Associated Fungal Taxa between Organic and Mineral Soil Shows Regional Differences
3.4. Phylogenetically Related Fungal Groups Show Divergent Responses to Organic Layer and Mineral Soil
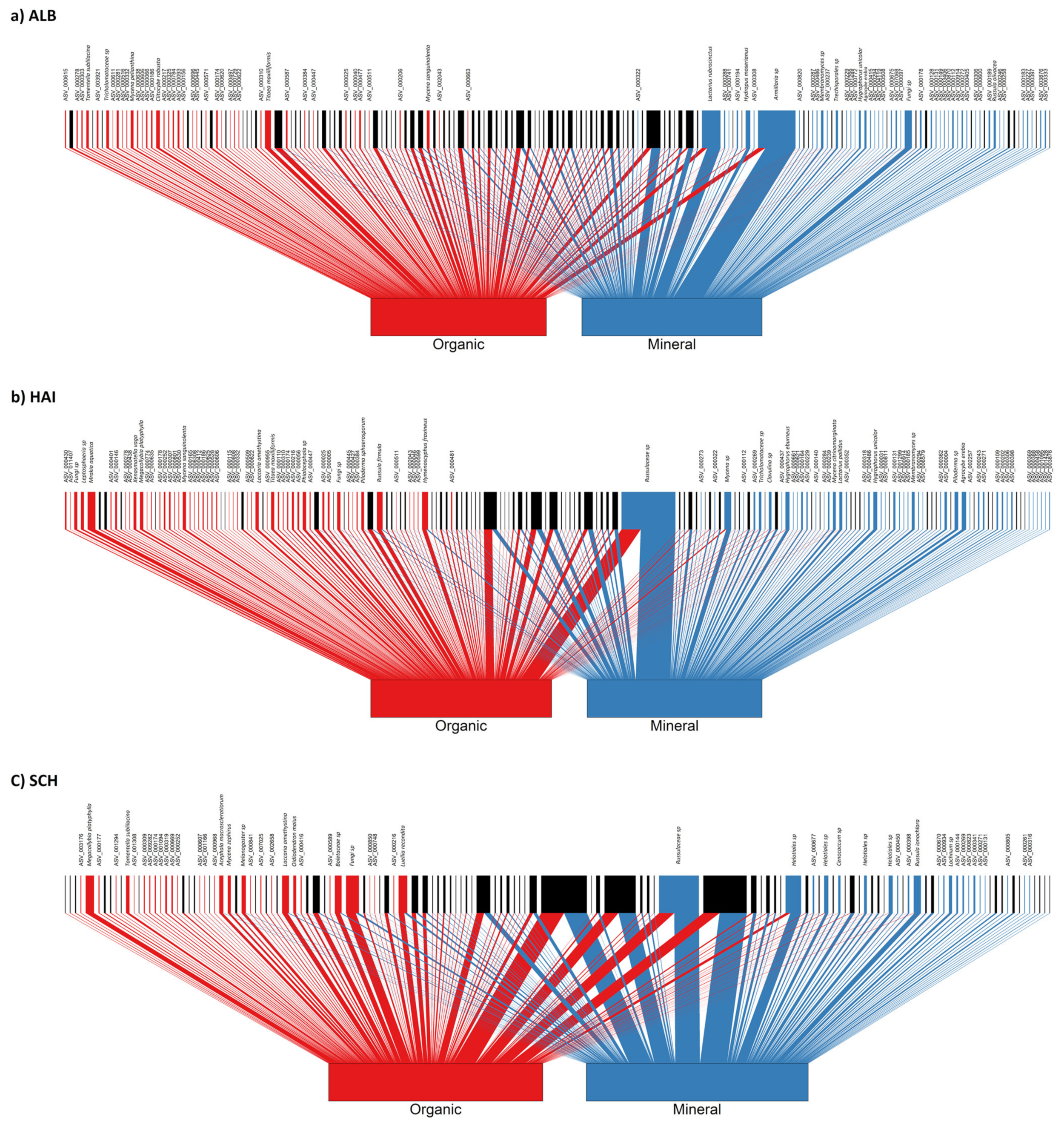
3.5. Fungal Indicator Taxa in Organic Layer and Mineral Soil
4. Discussion
4.1. Stratification of Root-Associated Fungi by Organic Layer and Mineral Topsoil
4.2. Indicator Taxa and Phylogenetic Groups Uncover Different Strategies of Root-Associated Fungi
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heijden, M.G.A.V.D.; Bardgett, R.D.; Straalen, N.M.V. The Unseen Majority: Soil Microbes as Drivers of Plant Diversity and Productivity in Terrestrial Ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Blackwell, M. The Fungi: 1, 2, 3 … 5.1 Million Species? Am. J. Bot. 2011, 98, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P. Microbial Activity and the Dynamics of Ecosystem Processes in Forest Soils. Curr. Opin. Microbiol. 2017, 37, 128–134. [Google Scholar] [CrossRef] [PubMed]
- García-Guzmán, G.; Heil, M. Life Histories of Hosts and Pathogens Predict Patterns in Tropical Fungal Plant Diseases. New Phytol. 2014, 201, 1106–1120. [Google Scholar] [CrossRef]
- Orwin, K.H.; Kirschbaum, M.U.F.; John, M.G.S.; Dickie, I.A. Organic Nutrient Uptake by Mycorrhizal Fungi Enhances Ecosystem Carbon Storage: A Model-Based Assessment. Ecol. Lett. 2011, 14, 493–502. [Google Scholar] [CrossRef]
- Hubbel, S.P. The Unified Neutral Theory of Biodiversity and Biogeography (MPB-32); Princeton University Press: Princeton, NJ, USA, 2001; ISBN 978-0-691-02128-7. [Google Scholar]
- Cline, L.C.; Zak, D.R. Dispersal Limitation Structures Fungal Community Assembly in a Long-Term Glacial Chronosequence. Environ. Microbiol. 2014, 16, 1538–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global Diversity and Geography of Soil Fungi. Science 2014, 346. [Google Scholar] [CrossRef] [Green Version]
- Kraft, N.J.B.; Adler, P.B.; Godoy, O.; James, E.C.; Fuller, S.; Levine, J.M. Community Assembly, Coexistence and the Environmental Filtering Metaphor. Funct. Ecol. 2015, 29, 592–599. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and Function of the Global Topsoil Microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef]
- Birkhofer, K.; Schöning, I.; Alt, F.; Herold, N.; Klarner, B.; Maraun, M.; Marhan, S.; Oelmann, Y.; Wubet, T.; Yurkov, A.; et al. General Relationships between Abiotic Soil Properties and Soil Biota across Spatial Scales and Different Land-Use Types. PLoS ONE 2012, 7, e43292. [Google Scholar] [CrossRef]
- Wubet, T.; Christ, S.; Schöning, I.; Boch, S.; Gawlich, M.; Schnabel, B.; Fischer, M.; Buscot, F. Differences in Soil Fungal Communities between European Beech (Fagus Sylvatica L.) Dominated Forests Are Related to Soil and Understory Vegetation. PLoS ONE 2012, 7, e47500. [Google Scholar] [CrossRef]
- Wang, M.; Shi, S.; Lin, F.; Jiang, P. Response of the Soil Fungal Community to Multi-Factor Environmental Changes in a Temperate Forest. Appl. Soil Ecol. 2014, 81, 45–56. [Google Scholar] [CrossRef]
- Goldmann, K.; Schöning, I.; Buscot, F.; Wubet, T. Forest Management Type Influences Diversity and Community Composition of Soil Fungi across Temperate Forest Ecosystems. Front. Microbiol. 2015, 6, 1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Van, A.L.; Dufresne, A. The Importance of the Microbiome of the Plant Holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Baldauf, S.L.; Leyval, C.; Straczek, J.; Young, J.P.W. Extensive Fungal Diversity in Plant Roots. Science 2002, 295, 2051. [Google Scholar] [CrossRef]
- Goldmann, K.; Schröter, K.; Pena, R.; Schöning, I.; Schrumpf, M.; Buscot, F.; Polle, A.; Wubet, T. Divergent Habitat Filtering of Root and Soil Fungal Communities in Temperate Beech Forests. Sci. Rep. 2016, 6, 31439. [Google Scholar] [CrossRef]
- Schröter, K.; Wemheuer, B.; Pena, R.; Schöning, I.; Ehbrecht, M.; Schall, P.; Ammer, C.; Daniel, R.; Polle, A. Assembly Processes of Trophic Guilds in the Root Mycobiome of Temperate Forests. Mol. Ecol. 2019, 28, 348–364. [Google Scholar] [CrossRef]
- Carteron, A.; Beigas, M.; Joly, S.; Turner, B.L.; Laliberté, E. Temperate Forests Dominated by Arbuscular or Ectomycorrhizal Fungi Are Characterized by Strong Shifts from Saprotrophic to Mycorrhizal Fungi with Increasing Soil Depth. Microb. Ecol. 2021, 82, 377–390. [Google Scholar] [CrossRef]
- Nguyen, D.Q.; Schneider, D.; Brinkmann, N.; Song, B.; Janz, D.; Schöning, I.; Daniel, R.; Pena, R.; Polle, A. Soil and Root Nutrient Chemistry Structure Root-Associated Fungal Assemblages in Temperate Forests. Environ. Microbiol. 2020, 22, 3081–3095. [Google Scholar] [CrossRef] [PubMed]
- Clausing, S.; Likulunga, L.E.; Janz, D.; Feng, H.Y.; Schneider, D.; Daniel, R.; Krüger, J.; Lang, F.; Polle, A. Impact of Nitrogen and Phosphorus Addition on Resident Soil and Root Mycobiomes in Beech Forests. Biol. Fertil. Soils 2021. [Google Scholar] [CrossRef]
- Lindahl, B.D.; Ihrmark, K.; Boberg, J.; Trumbore, S.E.; Högberg, P.; Stenlid, J.; Finlay, R.D. Spatial Separation of Litter Decomposition and Mycorrhizal Nitrogen Uptake in a Boreal Forest. New Phytol. 2007, 173, 611–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemmensen, K.E.; Finlay, R.D.; Dahlberg, A.; Stenlid, J.; Wardle, D.A.; Lindahl, B.D. Carbon Sequestration Is Related to Mycorrhizal Fungal Community Shifts during Long-Term Succession in Boreal Forests. New Phytol. 2015, 205, 1525–1536. [Google Scholar] [CrossRef]
- Toju, H.; Kishida, O.; Katayama, N.; Takagi, K. Networks Depicting the Fine-Scale Co-Occurrences of Fungi in Soil Horizons. PLoS ONE 2016, 11, e0165987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlatter, D.C.; Kahl, K.; Carlson, B.; Huggins, D.R.; Paulitz, T. Fungal Community Composition and Diversity Vary with Soil Depth and Landscape Position in a No-till Wheat-Based Cropping System. FEMS Microbiol. Ecol. 2018, 94, fiy098. [Google Scholar] [CrossRef] [Green Version]
- Asplund, J.; Kauserud, H.; Ohlson, M.; Nybakken, L. Spruce and Beech as Local Determinants of Forest Fungal Community Structure in Litter, Humus and Mineral Soil. FEMS Microbiol. Ecol. 2019, 95, fiy232. [Google Scholar] [CrossRef] [PubMed]
- Jobbágy, E.G.; Jackson, R.B. The Distribution of Soil Nutrients with Depth: Global Patterns and the Imprint of Plants. Biogeochemistry 2001, 53, 51–77. [Google Scholar] [CrossRef]
- Herold, N.; Schöning, I.; Berner, D.; Haslwimmer, H.; Kandeler, E.; Michalzik, B.; Schrumpf, M. Vertical Gradients of Potential Enzyme Activities in Soil Profiles of European Beech, Norway Spruce and Scots Pine Dominated Forest Sites. Pedobiologia 2014, 57, 181–189. [Google Scholar] [CrossRef]
- Rosling, A.; Landeweert, R.; Lindahl, B.D.; Larsson, K.-H.; Kuyper, T.W.; Taylor, A.F.S.; Finlay, R.D. Vertical Distribution of Ectomycorrhizal Fungal Taxa in a Podzol Soil Profile. New Phytol. 2003, 159, 775–783. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.D.; Wardle, D.A.; Lindahl, B.D. Roots and Associated Fungi Drive Long-Term Carbon Sequestration in Boreal Forest. Science 2013, 339, 1615–1618. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.L.; Bruns, T.D. Community Structure of Ectomycorrhizal Fungi in a Pinus Muricata Forest: Minimal Overlap between the Mature Forest and Resistant Propagule Communities. Mol. Ecol. 1999, 8, 1837–1850. [Google Scholar] [CrossRef] [Green Version]
- Dickie, I.A.; Xu, B.; Koide, R.T. Vertical Niche Differentiation of Ectomycorrhizal Hyphae in Soil as Shown by T-RFLP Analysis. New Phytol. 2002, 156, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Genney, D.R.; Anderson, I.C.; Alexander, I.J. Fine-Scale Distribution of Pine Ectomycorrhizas and Their Extramatrical Mycelium. New Phytol. 2006, 170, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Bossdorf, O.; Gockel, S.; Hänsel, F.; Hemp, A.; Hessenmöller, D.; Korte, G.; Nieschulze, J.; Pfeiffer, S.; Prati, D.; et al. Implementing Large-Scale and Long-Term Functional Biodiversity Research: The Biodiversity Exploratories. Basic Appl. Ecol. 2010, 11, 473–485. [Google Scholar] [CrossRef]
- Solly, E.F.; Schöning, I.; Boch, S.; Kandeler, E.; Marhan, S.; Michalzik, B.; Müller, J.; Zscheischler, J.; Trumbore, S.E.; Schrumpf, M. Factors Controlling Decomposition Rates of Fine Root Litter in Temperate Forests and Grasslands. Plant Soil 2014, 382, 203–218. [Google Scholar] [CrossRef] [Green Version]
- Gan, H.Y.; Schöning, I.; Schall, P.; Ammer, C.; Schrumpf, M. Soil Organic Matter Mineralization as Driven by Nutrient Stoichiometry in Soils Under Differently Managed Forest Stands. Front. For. Glob. Chang. 2020, 3. [Google Scholar] [CrossRef]
- Pena, R.; Lang, C.; Lohaus, G.; Boch, S.; Schall, P.; Schöning, I.; Ammer, C.; Fischer, M.; Polle, A. Phylogenetic and Functional Traits of Ectomycorrhizal Assemblages in Top Soil from Different Biogeographic Regions and Forest Types. Mycorrhiza 2017, 27, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Leuschner, C.; Ellenberg, H. Ecology of Central European Forests: Vegetation Ecology of Central Europe; Springer International Publishing: New York, NY, USA, 2017; Volume I, ISBN 978-3-319-43040-9. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of Total, Organic, and Available Forms of Phosphorus in Soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Heinrichs, H.; Brumsack, H.-J.; Loftfield, N.; König, N. Verbessertes Druckaufschlußsystem Für Biologische Und Anorganische Materialien. Z. Für Pflanz. Und Bodenkd. 1986, 149, 350–353. [Google Scholar] [CrossRef]
- Horton, T.R.; Bruns, T.D. The Molecular Revolution in Ectomycorrhizal Ecology: Peeking into the Black-Box. Mol. Ecol. 2001, 10, 1855–1871. [Google Scholar] [CrossRef] [Green Version]
- Toju, H.; Tanabe, A.S.; Yamamoto, S.; Sato, H. High-Coverage ITS Primers for the DNA-Based Identification of Ascomycetes and Basidiomycetes in Environmental Samples. PLoS ONE 2012, 7, e40863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols; Academic Press, Inc.: Cambridge, MA, USA, 1990; pp. 315–322. ISBN 978-0-12-372180-8. [Google Scholar]
- Von Hoyningen-Huene, A.J.E.; Schneider, D.; Fussmann, D.; Reimer, A.; Arp, G.; Daniel, R. Bacterial Succession along a Sediment Porewater Gradient at Lake Neusiedl in Austria. Sci. Data 2019, 6, 163. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A Fast and Accurate Illumina Paired-End ReAd MergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Holmes, S.P. Exact Sequence Variants Should Replace Operational Taxonomic Units in Marker-Gene Data Analysis. ISME J. 2017, 11, 2639–2643. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. Search and Clustering Orders of Magnitude Faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. UNOISE2: Improved Error-Correction for Illumina 16S and ITS Amplicon Sequencing. bioRxiv 2016, 081257. [Google Scholar] [CrossRef] [Green Version]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.S.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a Unified Paradigm for Sequence-Based Identification of Fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef] [Green Version]
- McDonald, D.; Clemente, J.C.; Kuczynski, J.; Rideout, J.R.; Stombaugh, J.; Wendel, D.; Wilke, A.; Huse, S.; Hufnagle, J.; Meyer, F.; et al. The Biological Observation Matrix (BIOM) Format or: How I Learned to Stop Worrying and Love the Ome-Ome. GigaScience 2012, 1, 2047-217X-1-7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Andersen, K.S.; Kirkegaard, R.H.; Karst, S.M.; Albertsen, M. Ampvis2: An R Package to Analyse and Visualise 16S RRNA Amplicon Data. bioRxiv 2018, 299537. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An Open Annotation Tool for Parsing Fungal Community Datasets by Ecological Guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- R Development Core Team. R: The R Project for Statistical Computing: R Foundation for Statistical Computing; R Development Core Team: Vienna, Austria, 2020; Available online: https://www.r-project.org/ (accessed on 13 April 2021).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. 2013. Available online: https://cran.ism.ac.jp/web/packages/vegan/vegan.pdf (accessed on 15 April 2020).
- Ho, N.T.; Li, F.; Wang, S.; Kuhn, L. MetamicrobiomeR: An R Package for Analysis of Microbiome Relative Abundance Data Using Zero-Inflated Beta GAMLSS and Meta-Analysis across Studies Using Random Effects Models. BMC Bioinform. 2019, 20, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baselga, A.; Orme, C.D.L. Betapart: An R Package for the Study of Beta Diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Dormann, C.F.; Fründ, J.; Blüthgen, N.; Gruber, B. Indices, Graphs and Null Models: Analyzing Bipartite Ecological Networks. Open Ecol. J. 2009, 2. [Google Scholar] [CrossRef]
- Cáceres, M.D.; Legendre, P. Associations between Species and Groups of Sites: Indices and Statistical Inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, Use R! 2009; ISBN 978-0-387-98141-3. [Google Scholar]
- Shigyo, N.; Umeki, K.; Hirao, T. Seasonal Dynamics of Soil Fungal and Bacterial Communities in Cool-Temperate Montane Forests. Front. Microbiol. 2019, 10, 1944. [Google Scholar] [CrossRef] [Green Version]
- Voříšková, J.; Brabcová, V.; Cajthaml, T.; Baldrian, P. Seasonal Dynamics of Fungal Communities in a Temperate Oak Forest Soil. New Phytol. 2014, 201, 269–278. [Google Scholar] [CrossRef]
- Brabcová, V.; Nováková, M.; Davidová, A.; Baldrian, P. Dead Fungal Mycelium in Forest Soil Represents a Decomposition Hotspot and a Habitat for a Specific Microbial Community. New Phytol. 2016, 210, 1369–1381. [Google Scholar] [CrossRef] [Green Version]
- Bödeker, I.T.M.; Lindahl, B.D.; Olson, Å.; Clemmensen, K.E. Mycorrhizal and Saprotrophic Fungal Guilds Compete for the Same Organic Substrates but Affect Decomposition Differently. Funct. Ecol. 2016, 30, 1967–1978. [Google Scholar] [CrossRef] [Green Version]
- Santalahti, M.; Sun, H.; Jumpponen, A.; Pennanen, T.; Heinonsalo, J. Vertical and Seasonal Dynamics of Fungal Communities in Boreal Scots Pine Forest Soil. FEMS Microbiol. Ecol. 2016, 92, fiw170. [Google Scholar] [CrossRef] [Green Version]
- Peršoh, D.; Stolle, N.; Brachmann, A.; Begerow, D.; Rambold, G. Fungal Guilds Are Evenly Distributed along a Vertical Spruce Forest Soil Profile While Individual Fungi Show Pronounced Niche Partitioning. Mycol. Prog. 2018, 17, 925–939. [Google Scholar] [CrossRef]
- Tedersoo, L.; Kõljalg, U.; Hallenberg, N.; Larsson, K.-H. Fine Scale Distribution of Ectomycorrhizal Fungi and Roots across Substrate Layers Including Coarse Woody Debris in a Mixed Forest. New Phytol. 2003, 159, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Mrak, T.; Hukić, E.; Štraus, I.; Unuk Nahberger, T.; Kraigher, H. Ectomycorrhizal Community Composition of Organic and Mineral Soil Horizons in Silver Fir (Abies Alba Mill.) Stands. Mycorrhiza 2020, 30, 541–553. [Google Scholar] [CrossRef]
- Grinhut, T.; Hadar, Y.; Chen, Y. Degradation and Transformation of Humic Substances by Saprotrophic Fungi: Processes and Mechanisms. Fungal Biol. Rev. 2007, 21, 179–189. [Google Scholar] [CrossRef]
- Baldrian, P. Chapter 2 Enzymes of saprotrophic basidiomycetes. In Ecology of Saprotrophic Basidiomycetes; Boddy, L., Frankland, J.C., van West, P., Eds.; British Mycological Society Symposia Series; Academic Press: Cambridge, MA, USA, 2008; Volume 28, pp. 19–41. [Google Scholar]
- Colpaert, J.V.; Laere, A.V. A Comparison of the Extracellular Enzyme Activities of Two Ectomycorrhizal and a Leaf-Saprotrophic Basidiomycete Colonizing Beech Leaf Litter. New Phytol. 1996, 134, 133–141. [Google Scholar] [CrossRef]
- Santalahti, M.; Putkinen, A.; Adamczyk, S.; Sun, H.; Heinonsalo, J. Restriction of plant roots in boreal forest organic soils affects the microbial community but does not change the dominance from ectomycorrhizal to saprotrophic fungi. FEMS Microbiol. Ecol. 2019, 95, fiz133. [Google Scholar] [CrossRef]
- Chase, J.M. Drought Mediates the Importance of Stochastic Community Assembly. Proc. Natl. Acad. Sci. USA 2007, 104, 17430–17434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marx, D.H.; Bryan, W.C.; Davey, C.B. Influence of Temperature on Aseptic Synthesis of Ectomycorrhizae by Thelephora Terrestris and Pisolithus Tinctorius on Loblolly Pine. For. Sci. 1970, 16, 424–431. [Google Scholar] [CrossRef]
- Lazarevic, J.; Stojicic, D.; Keca, N. Effects of Temperature, PH and Carbon and Nitrogen Sources on Growth of in Vitro Cultures of Ectomycorrhizal Isolates from Pinus Heldreichii Forest. For. Syst. 2016, 25, 3. [Google Scholar] [CrossRef] [Green Version]
- Leberecht, M.; Tu, J.; Polle, A. Acid and Calcareous Soils Affect Nitrogen Nutrition and Organic Nitrogen Uptake by Beech Seedlings (Fagus Sylvatica L.) under Drought, and Their Ectomycorrhizal Community Structure. Plant Soil 2016, 409, 143–157. [Google Scholar] [CrossRef]
- Mucha, J.; Peay, K.G.; Smith, D.P.; Reich, P.B.; Stefański, A.; Hobbie, S.E. Effect of Simulated Climate Warming on the Ectomycorrhizal Fungal Community of Boreal and Temperate Host Species Growing Near Their Shared Ecotonal Range Limits. Microb. Ecol. 2018, 75, 348–363. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, T.; Kitajima, K.; Douhan, G.W.; Yamanaka, N.; Allen, M.F. A Pulse of Summer Precipitation after the Dry Season Triggers Changes in Ectomycorrhizal Formation, Diversity, and Community Composition in a Mediterranean Forest in California, USA. Mycorrhiza 2018, 28, 665–677. [Google Scholar] [CrossRef] [Green Version]
- Kranabetter, J.M.; Durall, D.M.; MacKenzie, W.H. Diversity and Species Distribution of Ectomycorrhizal Fungi along Productivity Gradients of a Southern Boreal Forest. Mycorrhiza 2009, 19, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.A.; Nara, K.; Hogetsu, T. Host Effects on Ectomycorrhizal Fungal Communities: Insight from Eight Host Species in Mixed Conifer–Broadleaf Forests. New Phytol. 2007, 174, 430–440. [Google Scholar] [CrossRef]
- Lang, C.; Seven, J.; Polle, A. Host Preferences and Differential Contributions of Deciduous Tree Species Shape Mycorrhizal Species Richness in a Mixed Central European Forest. Mycorrhiza 2011, 21, 297–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Linde, S.; Suz, L.M.; Orme, C.D.L.; Cox, F.; Andreae, H.; Asi, E.; Atkinson, B.; Benham, S.; Carroll, C.; Cools, N.; et al. Environment and Host as Large-Scale Controls of Ectomycorrhizal Fungi. Nature 2018, 558, 243–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballauff, J.; Schneider, D.; Edy, N.; Irawan, B.; Daniel, R.; Polle, A. Shifts in Root and Soil Chemistry Drive the Assembly of Belowground Fungal Communities in Tropical Land-Use Systems. Soil Biol. Biochem. 2021, 154, 108140. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Kozak, K.H.; Fine, P.V.A.; Kembel, S.W. The Merging of Community Ecology and Phylogenetic Biology. Ecol. Lett. 2009, 12, 693–715. [Google Scholar] [CrossRef]
- Pausas, J.G.; Verdú, M. The Jungle of Methods for Evaluating Phenotypic and Phylogenetic Structure of Communities. BioScience 2010, 60, 614–625. [Google Scholar] [CrossRef] [Green Version]
- Kohler, A.; Kuo, A.; Nagy, L.G.; Morin, E.; Barry, K.W.; Buscot, F.; Canbäck, B.; Choi, C.; Cichocki, N.; Clum, A.; et al. Convergent Losses of Decay Mechanisms and Rapid Turnover of Symbiosis Genes in Mycorrhizal Mutualists. Nat. Genet. 2015, 47, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Nagy, L.G.; Riley, R.; Tritt, A.; Adam, C.; Daum, C.; Floudas, D.; Sun, H.; Yadav, J.S.; Pangilinan, J.; Larsson, K.-H.; et al. Comparative Genomics of Early-Diverging Mushroom-Forming Fungi Provides Insights into the Origins of Lignocellulose Decay Capabilities. Mol. Biol. Evol. 2016, 33, 959–970. [Google Scholar] [CrossRef] [Green Version]
- Treseder, K.K.; Lennon, J.T. Fungal Traits That Drive Ecosystem Dynamics on Land. Microbiol. Mol. Biol. Rev. 2015, 79, 243–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binder, M.; Justo, A.; Riley, R.; Salamov, A.; Lopez-Giraldez, F.; Sjökvist, E.; Copeland, A.; Foster, B.; Sun, H.; Larsson, E.; et al. Phylogenetic and Phylogenomic Overview of the Polyporales. Mycologia 2013, 105, 1350–1373. [Google Scholar] [CrossRef] [Green Version]
- Žifčáková, L.; Větrovský, T.; Howe, A.; Baldrian, P. Microbial Activity in Forest Soil Reflects the Changes in Ecosystem Properties between Summer and Winter. Environ. Microbiol. 2016, 18, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Cannon, P.F.; Kirk, P.M. Fungal Families of the World; CABI: Wallingford, UK, 2007; ISBN 978-0-85199-827-5. [Google Scholar]
- Badali, H.; Gueidan, C.; Najafzadeh, M.J.; Bonifaz, A.; van den Ende, A.H.G.G.; de Hoog, G.S. Biodiversity of the Genus Cladophialophora. Stud. Mycol. 2008, 61, 175–191. [Google Scholar] [CrossRef]
- Buée, M.; Vairelles, D.; Garbaye, J. Year-Round Monitoring of Diversity and Potential Metabolic Activity of the Ectomycorrhizal Community in a Beech (Fagus Silvatica) Forest Subjected to Two Thinning Regimes. Mycorrhiza 2005, 15, 235–245. [Google Scholar] [CrossRef]
- Agerer, R. Exploration Types of Ectomycorrhizae. Mycorrhiza 2001, 11, 107–114. [Google Scholar] [CrossRef]
- Pena, R.; Tejedor, J.; Zeller, B.; Dannenmann, M.; Polle, A. Interspecific Temporal and Spatial Differences in the Acquisition of Litter-Derived Nitrogen by Ectomycorrhizal Fungal Assemblages. New Phytol. 2013, 199, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Mason, L.M.; Eagar, A.; Patel, P.; Blackwood, C.B.; DeForest, J.L. Potential Microbial Bioindicators of Phosphorus Mining in a Temperate Deciduous Forest. J. Appl. Microbiol. 2021, 130, 109–122. [Google Scholar] [CrossRef]
- Allison, S.D.; Hanson, C.A.; Treseder, K.K. Nitrogen Fertilization Reduces Diversity and Alters Community Structure of Active Fungi in Boreal Ecosystems. Soil Biol. Biochem. 2007, 39, 1878–1887. [Google Scholar] [CrossRef] [Green Version]
- Nicolás, C.; Almeida, J.P.; Ellström, M.; Bahr, A.; Bone, S.E.; Rosenstock, N.P.; Bargar, J.R.; Tunlid, A.; Persson, P.; Wallander, H. Chemical Changes in Organic Matter after Fungal Colonization in a Nitrogen Fertilized and Unfertilized Norway Spruce Forest. Plant Soil 2017, 419, 113–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Looney, B.; Miyauchi, S.; Morin, E.; Drula, E.; Courty, P.E.; Kohler, A.; Kuo, A.; LaButti, K.; Pangilinan, J.; Lipzen, A.; et al. Evolutionary Priming and Transition to the Ectomycorrhizal Habit in an Iconic Lineage of Mushroom-Forming Fungi: Is Preadaptation a Requirement? bioRxiv 2021. [Google Scholar] [CrossRef]
- Baldrian, P.; Kolařík, M.; Štursová, M.; Kopecký, J.; Valášková, V.; Větrovský, T.; Žifčáková, L.; Šnajdr, J.; Rídl, J.; Vlček, Č.; et al. Active and Total Microbial Communities in Forest Soil Are Largely Different and Highly Stratified during Decomposition. ISME J. 2012, 6, 248–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinsabaugh, R.L. Phenol Oxidase, Peroxidase and Organic Matter Dynamics of Soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Agerer, R. Nitrogen Isotopes in Ectomycorrhizal Sporocarps Correspond to Belowground Exploration Types. Plant Soil 2010, 327, 71–83. [Google Scholar] [CrossRef]
- Almeida, J.P.; Rosenstock, N.P.; Forsmark, B.; Bergh, J.; Wallander, H. Ectomycorrhizal Community Composition and Function in a Spruce Forest Transitioning between Nitrogen and Phosphorus Limitation. Fungal. Ecol. 2019, 40, 20–31. [Google Scholar] [CrossRef]
- Mueller, G.M.; History, F.M. Systematics of Laccaria (Agaricales) in the Continental United States and Canada, with Discussions on Extralimital Taxa and Descriptions of Extant Types; Field Museum of Natural History: Chicago, IL, USA, 1992; pp. 1–180. [Google Scholar]
- Martin, F.; Aerts, A.; Ahrén, D.; Brun, A.; Danchin, E.G.J.; Duchaussoy, F.; Gibon, J.; Kohler, A.; Lindquist, E.; Pereda, V.; et al. The Genome of Laccaria Bicolor Provides Insights into Mycorrhizal Symbiosis. Nature 2008, 452, 88–92. [Google Scholar] [CrossRef]
- Imamura, A. Report on Laccaria Amethystina, Newly Confirmed as an Ammonia Fungus. Mycoscience 2001, 42, 623–625. [Google Scholar] [CrossRef]



| Parameters | ALB | HAI | SCH |
|---|---|---|---|
| Location | Southwest Germany | Central Germany | Northeast Germany |
| Size (km2) | 422 | 1300 | 1300 |
| Geology | Calcareous bedrock with karst phenomena | Calcareous bedrock | Young glacial landscape |
| Altitude a.s.l (m) | 460–860 | 285–550 | 3–140 |
| Longitudes east to west (decimal degree) | 9.58024–9.02362 | 10.77917–10.17332 | 14.14796–13.39094 |
| Latitude north to south (decimal degree) | 48.53435–48.34996 | 51.37872–50.93735 | 53.22390–52.79023 |
| Mean annual temperature (°C) | 6.0–7.0 | 6.5–8.0 | 8.0–8.5 |
| Mean sum of annual precipitation (mm) | 700–1000 | 500–800 | 500–600 |
| Plot size (m) | 100 × 100 | 100 × 100 | 100 × 100 |
| Soil types | Cambisol (eutric) and Leptosol | Luvisol | Cambisol (dystric) |
| Mean soil pH | 5.23 ± 0.10 | 4.80 ± 0.12 | 3.55 ± 0.02 |
| Mean sand content (g kg−1) | 59.60 ± 9.84 | 58.00 ± 9.84 | 871 ± 9.84 |
| Mean silt content (g kg−1) | 444 ± 15.70 | 642 ± 15.70 | 84.80 ± 15.70 |
| Mean clay content (g kg−1) | 496 ± 14.40 | 301 ± 14.00 | 44.8 ± 14.40 |
| Main tree species | Beech (Fagus sylvatica) Spruce (Picea abies) | Beech (Fagus sylvatica) Spruce (Picea abies) | Beech (Fagus sylvatica) Pine (Pinus sylvstris) Oak (Quercus sp.) |
| ALB | HAI | SCH | Organic | Mineral | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Organic | Mineral | Organic | Mineral | Organic | Mineral | F | p | F | p | |
| C | 315.47 ± 6.65 (e) | 58.53 ± 1.88 (c) | 358.57 ± 7.80 (f) | 40.86 ± 1.72 (b) | 268.81 ± 8.64 (d) | 22.93 ± 0.71 (a) | 33.62 | <0.001 | 135.97 | <0.001 |
| N | 14.20 ± 0.34 (e) | 4.33 ± 0.13 (c) | 15.94 ± 0.37 (e) | 2.98 ± 0.12 (b) | 11.80 ± 0.27 (d) | 1.21 ± 0.05 (a) | 39.93 | <0.001 | 211.22 | <0.001 |
| C:N | 22.39 ± 0.34 (c) | 13.54 ± 0.14 (a) | 22.62 ± 0.30 (c) | 13.74 ± 0.16 (a) | 22.71 ± 0.43 (c) | 19.48 ± 0.46 (b) | 0.21 | 0.810 | 133.57 | <0.001 |
| Psol | 230.22 ± 11.44 (d) | 87.00 ± 8.06 (ab) | 248.01 ± 11.88 (d) | 58.03 ± 8.40 (a) | 168.49 ± 9.67 (c) | 119.87 ± 6.22 (b) | 14.58 | <0.001 | 16.31 | <0.001 |
| Cations | 506.90 ± 24.95 (d) | 370.14 ± 18.80 (c) | 485.26 ± 14.76 (d) | 334.77 ± 27.18 (c) | 138.68 ± 7.01 (b) | 48.38 ± 2.06 (a) | 144.01 | <0.001 | 85.18 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khokon, A.M.; Schneider, D.; Daniel, R.; Polle, A. Soil Layers Matter: Vertical Stratification of Root-Associated Fungal Assemblages in Temperate Forests Reveals Differences in Habitat Colonization. Microorganisms 2021, 9, 2131. https://doi.org/10.3390/microorganisms9102131
Khokon AM, Schneider D, Daniel R, Polle A. Soil Layers Matter: Vertical Stratification of Root-Associated Fungal Assemblages in Temperate Forests Reveals Differences in Habitat Colonization. Microorganisms. 2021; 9(10):2131. https://doi.org/10.3390/microorganisms9102131
Chicago/Turabian StyleKhokon, Anis Mahmud, Dominik Schneider, Rolf Daniel, and Andrea Polle. 2021. "Soil Layers Matter: Vertical Stratification of Root-Associated Fungal Assemblages in Temperate Forests Reveals Differences in Habitat Colonization" Microorganisms 9, no. 10: 2131. https://doi.org/10.3390/microorganisms9102131






