Homoplasy as an Auxiliary Criterion for Species Delimitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Models Adopted for the Analysis
2.2. Alignment and Sequences Analysis
2.3. HomoDist Algorithm
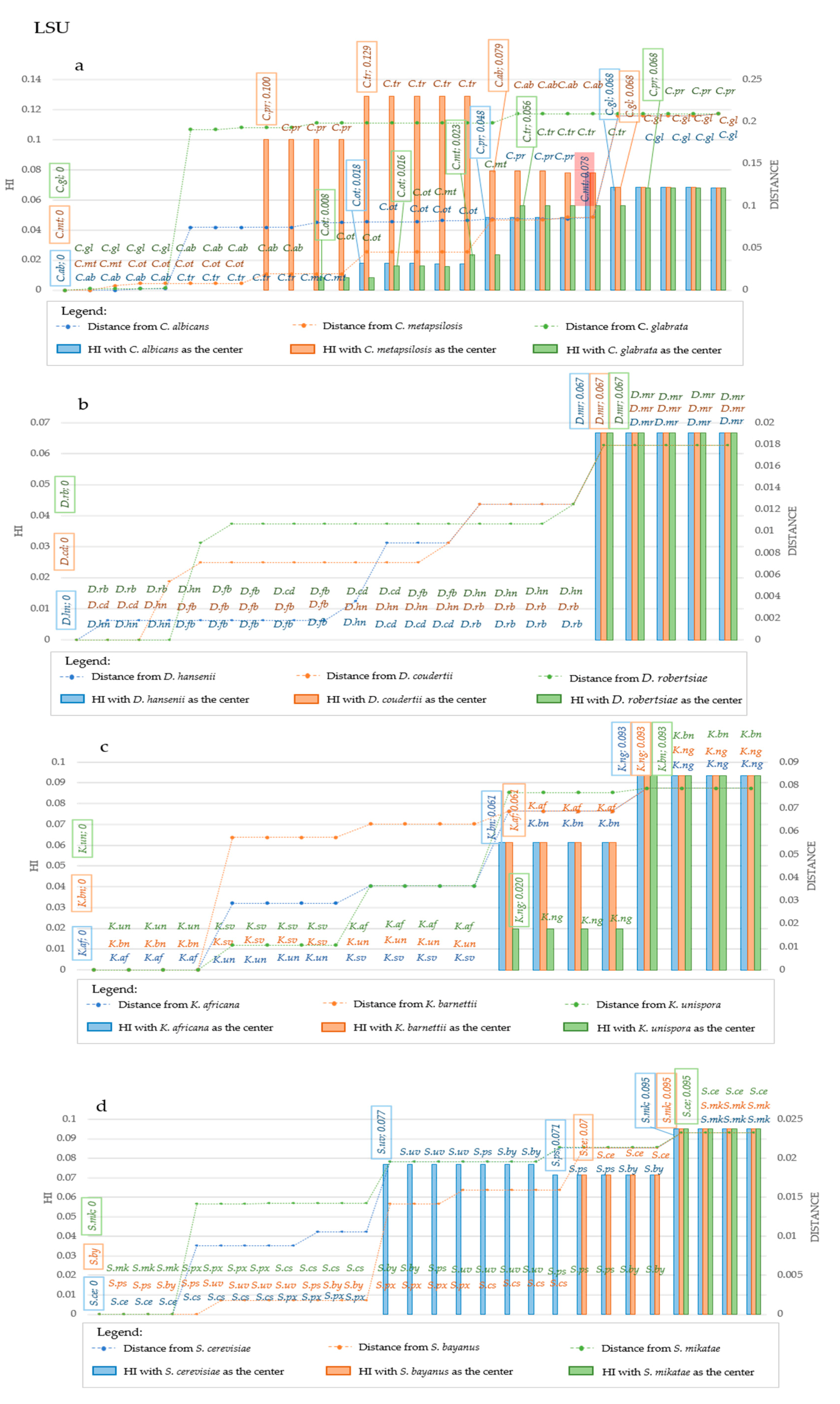
- disCen. refers to the distance of the elements of the series from the central strain.
- Maxd. represents the maximum distance of the sequences in the alignment.
- NJtree. is the tree obtained using the neighbour joining clustering method.
- Utree. is the tree calculated with the algorithm UPGMA.
- CI. is the consistency index, defined as the minimum number of changes divided by the number of changes required on the tree.
- Retention Index. measures the fraction of apparent synapomorphy to actual synapomorphy.
- HI is the Homoplasy index, the complement to 1 of CI.
- SH is an index that correlates homoplasy to distances. It is calculated as
- autCen. can be set as True or False. When True, the algorithm searches for the absolute center of the distribution, which is the object minimizing the distances with all other members of the set. On the contrary, the option False allows the user to choose the center of the series.
- defCen defines the position, in the alignment, of the sequence chosen as center when autCen is set as “False”.
- distmodel. defines the evolutionary model to be used. The default model is “F84” [37]
2.4. Data Analysis
3. Results
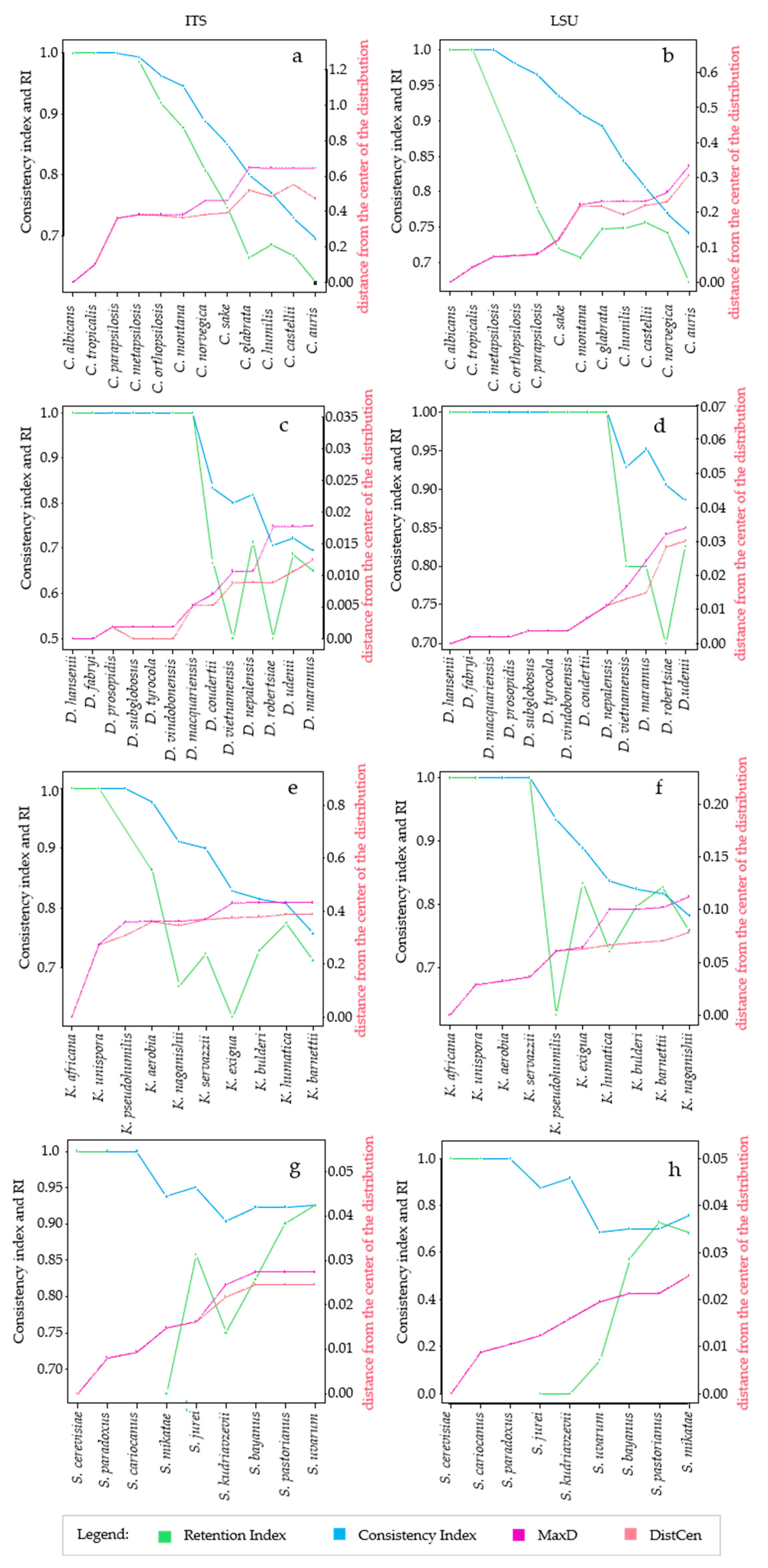
3.1. Consistency Index Decreases with the Increase of the Taxonomic Complexity
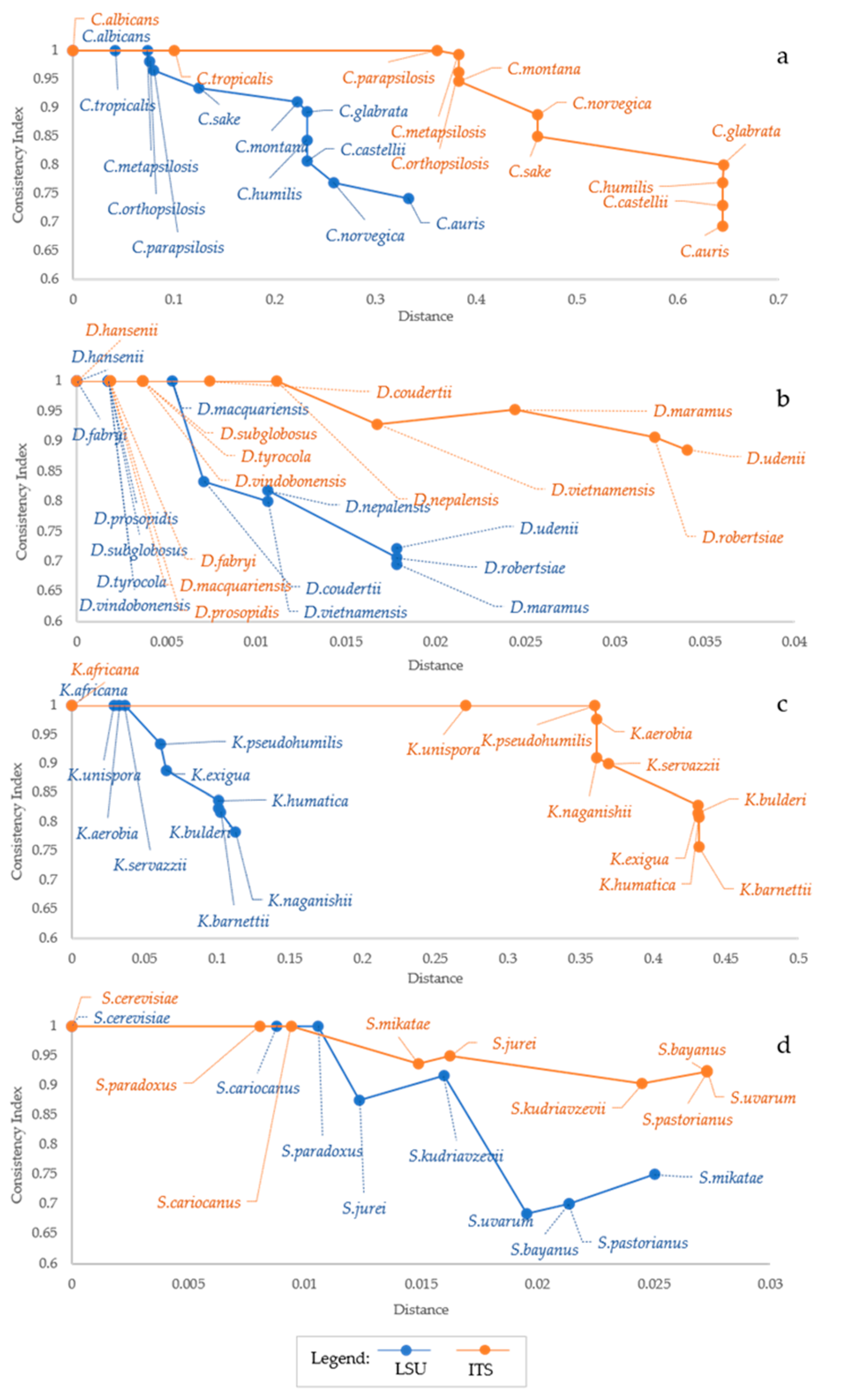
3.2. Consistency Index Decreases Linearly with the Increase of the Taxonomic Distances
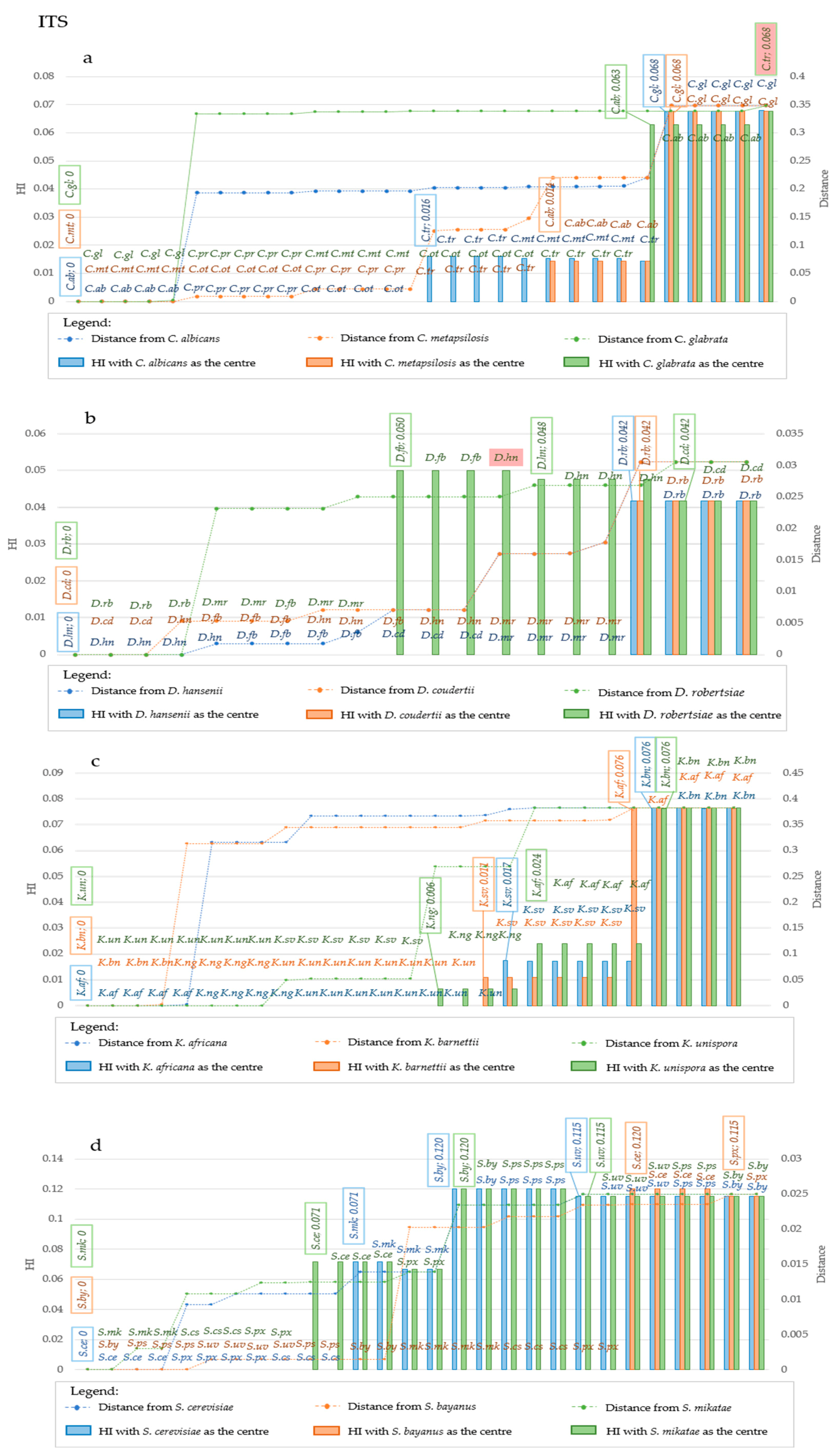
3.3. Consistency Index Changes according to the Combination of Strain Considered
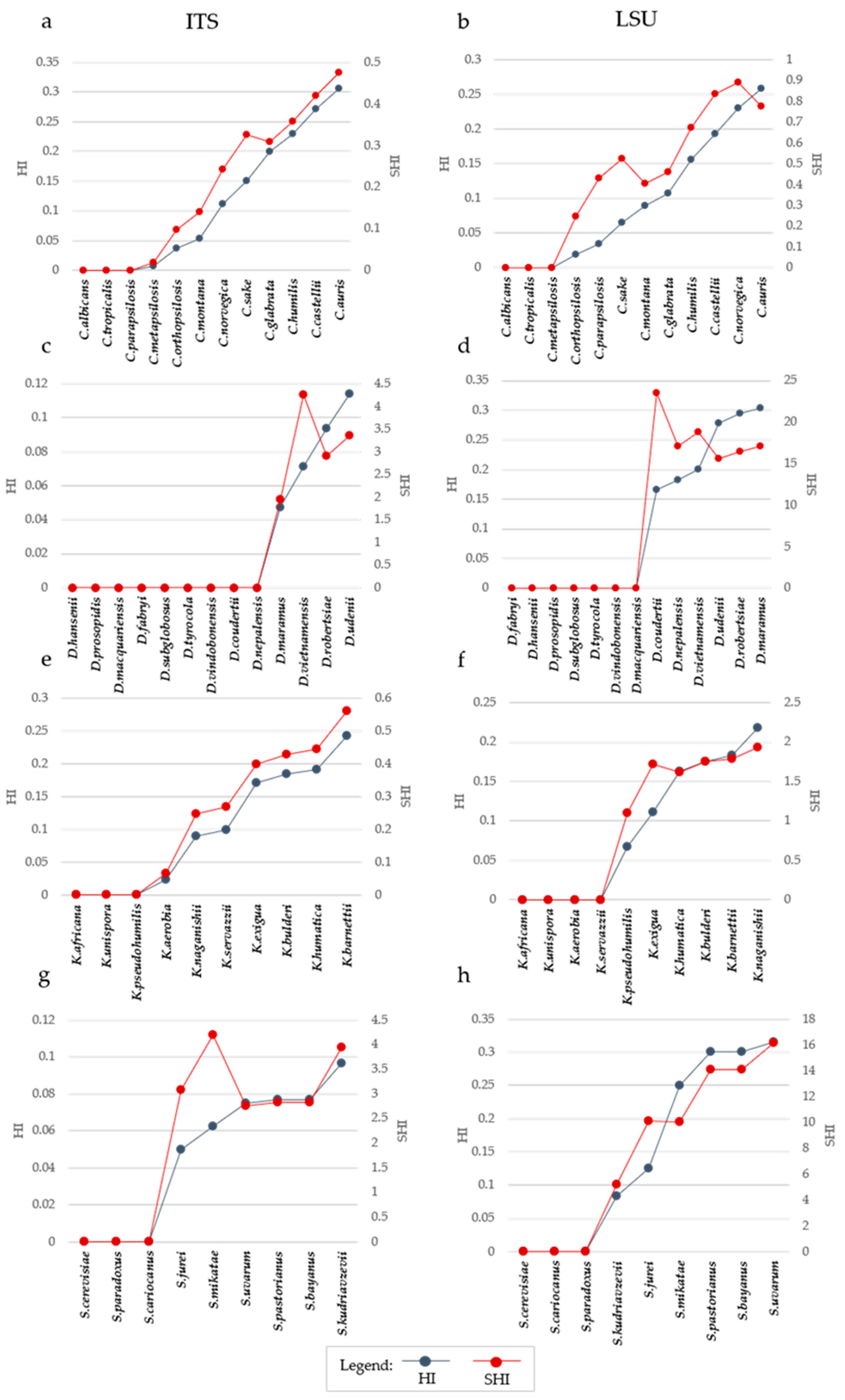
3.4. Homoplasy Variations Using more Strains per Species
3.5. Using homoplasy for Species Delimitation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lankester, E.R. II.—On the use of the term homology in modern zoology, and the distinction between homogenetic and homoplastic agreements. Ann. Mag. Nat. Hist. 1870, 6, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Hennig, W. Phylogenetic systematics. Annu. Rev. Entomol. 1965, 10, 97–116. [Google Scholar] [CrossRef]
- DeSalle, R.; Riley, M. Should Networks Supplant Tree Building? Microorganisms 2020, 8, 1179. [Google Scholar] [CrossRef] [PubMed]
- Wake, D.B.; Specht, M.H.; Chelsea, D. Homoplasy: From detecting pattern to determining process and mechanism of evolution. Science 2011, 331, 1032–1035. [Google Scholar] [CrossRef] [Green Version]
- Fiala, K.L.; Sokal, R.R. Factors determining the accuracy of cladogram estimation: Evaluation using computer simulation. Evolution 1985, 39, 609–622. [Google Scholar] [CrossRef]
- Bobay, L.M.; Ellis, B.S.; Ochman, H. ConSpeciFix: Classifying prokaryotic species based on gene flow. Bioinformatics 2018, 34, 3738–3740. [Google Scholar] [CrossRef]
- Brandley, M.C.; Warren, D.L.; Leache, A.D.; McGuire, J.A. Homoplasy and clade support. Syst. Biol. 2009, 58, 184–198. [Google Scholar] [CrossRef] [Green Version]
- Kälersjö, M.; Albert, V.A.; Farris, J.S. Homoplasy increases phylogenetic structure. Cladistics 1999, 15, 91–93. [Google Scholar]
- Van Veller, M.G.P.; Brooks, D.R.; Zandee, M. Cladistic and phylogenetic biogeography: The art and the science of discovery. J. Biogeogr. 2003, 30, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Kluge, A.G.; Farris, J.S. Quantitative phyletics and the evolution of anurans. Syst. Biol. 1969, 18, 1–32. [Google Scholar] [CrossRef]
- Farris, J.S. The retention index and the rescaled consistency index. Cladistics 1989, 5, 417–419. [Google Scholar] [CrossRef]
- Farris, J.S. The retention index and homoplasy excess. Syst. Zool. 1989, 38, 406–407. [Google Scholar] [CrossRef]
- Archie, J.W. Measures of homoplasy. In Homoplasy: The recurrence of Similarity in Evolution; Sanderson, M.J., Hufford, L., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; pp. 153–188. [Google Scholar]
- Klassen, G.; Mooi, R.; Locke, A. Consistency indices and random data. Syst. Biol. 1991, 40, 446–457. [Google Scholar] [CrossRef]
- Sanderson, M.J.; Donoghue, M.J.J.E. Patterns of variation in levels of homoplasy. J. Evol. 1989, 43, 1781–1795. [Google Scholar] [CrossRef] [PubMed]
- Steenkamp, E.T.; Wingfield, M.J.; McTaggart, A.R.; Wingfield, B.D. Fungal species and their boundaries matter–Definitions, mechanisms and practical implications. Fungal Biol. Rev. 2018, 32, 104–116. [Google Scholar] [CrossRef]
- Mayr, E. Speciation phenomena in birds. Am. Nat. 1940, 74, 249–278. [Google Scholar] [CrossRef]
- Mayr, E. The biological species concept. In Species Concepts Phylogenetic Theory: A Debate; Columbia University Press: New York, NY, USA, 2000; pp. 17–29. [Google Scholar]
- Knop, M. Yeast cell morphology and sexual reproduction–A short overview and some considerations. Comptes Rendus Biol. 2011, 334, 599–606. [Google Scholar] [CrossRef]
- Kurtzman, C.; Fell, J.W.; Boekhout, T. The Yeasts: A Taxonomic Study; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Pigliucci, M. Species as family resemblance concepts: The (dis-) solution of the species problem? BioEssays 2003, 25, 596–602. [Google Scholar] [CrossRef] [Green Version]
- Borneman, A.R.; Pretorius, I.S. Genomic insights into the Saccharomyces sensu stricto complex. Genetics 2015, 199, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Leducq, J.-B.; Nielly-Thibault, L.; Charron, G.; Eberlein, C.; Verta, J.-P.; Samani, P.; Sylvester, K.; Hittinger, C.T.; Bell, G.; Landry, C.R. Speciation driven by hybridization and chromosomal plasticity in a wild yeast. Nat. Microbiol. 2016, 1, 1–10. [Google Scholar] [CrossRef]
- Lucking, R.; Aime, M.C.; Robbertse, B.; Miller, A.N.; Ariyawansa, H.A.; Aoki, T.; Cardinali, G.; Crous, P.W.; Druzhinina, I.S.; Geiser, D.M.; et al. Unambiguous identification of fungi: Where do we stand and how accurate and precise is fungal DNA barcoding? IMA Fungus 2020, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Bruns, T.D.; White, T.J.; Taylor, J.W. Fungal molecular systematics. Annu. Rev. Ecol. Syst. 1991, 22, 525–564. [Google Scholar] [CrossRef]
- Schoch, C.L. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [Green Version]
- Vu, D.; Groenewald, M.; Szöke, S.; Cardinali, G.; Eberhardt, U.; Stielow, B.; de Vries, M.; Verkley, G.J.M.; Crous, P.W.; Boekhout, T.; et al. DNA barcoding analysis of more than 9000 yeast isolates contributes to quantitative thresholds for yeast species and genera delimitation. Stud. Mycol. 2016, 85, 91–105. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Robnett, C.J. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Van Leeuwenhoek 1998, 73, 331–371. [Google Scholar] [CrossRef] [PubMed]
- Letsch, H.O.; Kjer, K.M. Potential pitfalls of modelling ribosomal RNA data in phylogenetic tree reconstruction: Evidence from case studies in the Metazoa. BMC Evol. Biol. 2011, 11, 146. [Google Scholar] [CrossRef]
- Colabella, C.; Corte, L.; Roscini, L.; Bassetti, M.; Tascini, C.; Mellor, J.C.; Meyer, W.; Robert, V.; Vu, D.; Cardinali, G. NGS barcode sequencing in taxonomy and diagnostics, an application in “Candida” pathogenic yeasts with a metagenomic perspectiv. IMA Fungus 2018, 9, 91–105. [Google Scholar] [CrossRef]
- Roscini, L.; Tristezza, M.; Corte, L.; Colabella, C.; Perrotta, C.; Rampino, P.; Robert, V.; Vu, D.; Cardinali, G.; Grieco, F. Early Ongoing Speciation of Ogataea uvarum Sp. Nov. Within the Grape Ecosystem Revealed by the Internal Variability Among the rDNA Operon Repeats. Front. Microbiol. 2018, 9, 1687. [Google Scholar] [CrossRef]
- Krause, D.J.; Whitaker, R.J. Inferring Speciation Processes from Patterns of Natural Variation in Microbial Genomes. Syst. Biol. 2015, 64, 926–935. [Google Scholar] [CrossRef] [Green Version]
- Louis, E.J. Population genomics and speciation in yeasts. Fungal Biol. Rev. 2011, 25, 136–142. [Google Scholar] [CrossRef]
- Wang, Z.; Gudibanda, A.; Ugwuowo, U.; Trail, F.; Townsend, J.P. Using evolutionary genomics, transcriptomics, and systems biology to reveal gene networks underlying fungal development. Fungal Biol. Rev. 2018, 32, 249–264. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonielli, L.; Robert, V.; Corte, L.; Roscini, L.; Ceppitelli, R.; Cardinali, G. Centrality of Objects in a Multidimensional Space and its Effects on Distance-Based Biological Classifications. Open Appl. Inform. J. 2011, 5, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Felsenstein, J. Distance methods for inferring phylogenies: A justification. Evolution 1983, 38, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Bobay, L.M.; Ochman, H. Biological species are universal across Life’s domains. Genome Biol. Evol. 2017, 9, 491–501. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.W. One Fungus = One Name: DNA and fungal nomenclature twenty years after PCR. IMA Fungus 2011, 2, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Del Bove, M.; Lattanzi, M.; Rellini, P.; Pelliccia, C.; Fatichenti, F.; Cardinali, G. Comparison of molecular and metabolomic methods as characterization tools of Debaryomyces hansenii cheese isolates. Food Microbiol. 2009, 26, 453–459. [Google Scholar] [CrossRef]
- Jacques, N.; Mallet, S.; Casaregola, S. Delimitation of the species of the Debaryomyces hansenii complex by intron sequence analysis. Int. J. Syst. Evol. Microbiol. 2009, 59, 1242–1251. [Google Scholar] [CrossRef]
- Suzuki, M.; Prasad, G.S.; Kurtzman, C.P. Debaryomyces Lodder & Kreger-van Rij (1952). In The Yeasts-a taxonomic studies, 5th ed.; Kurtzman, C.P., Fell, J.W., Boeckhout, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 2. [Google Scholar]
- Kurtzman, C.P.; Robnett, C.J. Relationships among genera of the Saccharomycotina (Ascomycota) from multigene phylogenetic analysis of type species. FEMS Yeast Res. 2013, 13, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Schoch, C.L.; Robbertse, B.; Robert, V.; Vu, D.; Cardinali, G.; Irinyi, L.; Meyer, W.; Nilsson, R.H.; Hughes, K.; Miller, A.N.; et al. Finding needles in haystacks: Linking scientific names, reference specimens and molecular data for Fungi. Database 2014, 2014, bau061. [Google Scholar] [CrossRef]
- Archie, J.W. Homoplasy excess ratios: New indices for measuring levels of homoplasy in phylogenetic systematics and a critique of the consistency index. Syst. Zool. 1989, 38, 253–269. [Google Scholar] [CrossRef]
- Dujon, B.A.; Louis, E.J. Genome Diversity and Evolution in the Budding Yeasts (Saccharomycotina). Genetics 2017, 206, 717–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maier, U.G.; Zauner, S.; Woehle, C.; Bolte, K.; Hempel, F.; Allen, J.F.; Martin, W.F. Massively convergent evolution for ribosomal protein gene content in plastid and mitochondrial genomes. Genome Biol. Evol. 2013, 5, 2318–2329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiley, E.O.; Brooks, D.R.; Seigel-Causey, D.; Funk, V.A. The Compleat Cladist: A Primer of Phylogenetic Procedures; Museum of Natural History, University of Kansas: Lawrence, KS, USA, 1991. [Google Scholar]
- Cardinali, G.; Antonielli, L.; Corte, L.; Roscini, L.; Bagnetti, A.; Pelliccia, C.; Puddu, G. Kazachstania ichnusensis a diploid homothallic ascomycetous yeast from Sardinian lentisk rhizosphere. Int. J. Syst. Evol. Microbiol. 2012, 62, 722–727. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Lücking, R.J.T.F.K. Fungal diversity revisited: 2.2 to 3.8 million species. In The Fungal Kingdom; Wiley: Hoboken, NJ, USA, 2017; pp. 79–95. [Google Scholar]
- Koljalg, U.; Nilsson, H.R.; Schigel, D.; Tedersoo, L.; Larsson, K.H.; May, T.W.; Taylor, A.F.S.; Jeppesen, T.S.; Froslev, T.G.; Lindahl, B.D.; et al. The Taxon Hypothesis Paradigm-On the Unambiguous Detection and Communication of Taxa. Microorganisms 2020, 8, 1910. [Google Scholar] [CrossRef]


| Species | ITS Sequences | LSU D1/D2 Sequences | |
|---|---|---|---|
| Candida | C. albicans | AB032172 | U45776 |
| C. auris | AB375772 | AB375773 | |
| C. castelli | AY046196 | U69876 | |
| C. glabrata | AY046165 | U44808 | |
| C. humilis | AY046174 | U69878 | |
| C. metapsilosis | FJ872019 | AY497667 | |
| C. montana | GU246257 | U62305 | |
| C. norvegica | NR_111209 | U62299 | |
| C. orthopsilosis | FJ872018 | FJ746056 | |
| C. parapsilosis | NR_130673 | U45754 | |
| C. sake | AJ549822 | U45728 | |
| C. tropicalis | AF287910 | U45749 | |
| Debaryomyces | D. coudertii | AM992914 | U45846 |
| D. fabryi | AB053098 | U94927 | |
| D. hansenii | AF210327 | U45808 | |
| D. macquariensis | AM992909 | FR799729 | |
| D. maramus | AB053102 | U45838 | |
| D. nepalensis | AB053099 | U45839 | |
| D. prosopidis | NR_077067 | AB054993 | |
| D. robertsiae | AB054019 | U45805 | |
| D. subglobosus | EU816232 | EU816297 | |
| D. tyrocola | EU816237 | EU816302 | |
| D. udenii | NR_077068 | U45844 | |
| D. vietnamensis | AM992908 | AM992907 | |
| D. vindobonensis | FN598876 | FN598875 | |
| Kazachstania | K. aerobia | NR_077087 | AY582127 |
| K. africana | AY046155 | U68550 | |
| K. barnettii | AY046173 | U84231 | |
| K. bulderi | AY046172 | AF398486 | |
| K. exigua | AY046170 | U68553 | |
| K. humatica | AB097397 | AB040999 | |
| K. pseudohumilis | 2-FJ888526 | FJ888526 | |
| K. naganishii | AB097398 | AB088404 | |
| K. servazzii | AY046153 | U68558 | |
| K. unispora | AY046154 | U68554 | |
| Saccharomyces | S. bayanus | AY046152 | U94931 |
| S. cariocanus | AY046147 | AF398478 | |
| S. cerevisiae | AY046146 | U44806 | |
| S. jurei | HG764814 | HG764813 | |
| S. kudriavzevii | AY046150 | AF398480 | |
| S. mikatae | AY046149 | AF398479 | |
| S. paradoxus | AY046148 | U68555 | |
| S. pastorianus | AY046151 | AY048172 | |
| S. uvarum | AY130306 | AY130339 |
| ITS | LSU | |
|---|---|---|
| Candida | −0.944 | −0.910 |
| Saccharomyces | −0.696 | −0.730 |
| Kazachstania | −0.914 | −0.983 |
| Debaryomyces | −0.710 | −0.978 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conti, A.; Casagrande Pierantoni, D.; Robert, V.; Cardinali, G.; Corte, L. Homoplasy as an Auxiliary Criterion for Species Delimitation. Microorganisms 2021, 9, 273. https://doi.org/10.3390/microorganisms9020273
Conti A, Casagrande Pierantoni D, Robert V, Cardinali G, Corte L. Homoplasy as an Auxiliary Criterion for Species Delimitation. Microorganisms. 2021; 9(2):273. https://doi.org/10.3390/microorganisms9020273
Chicago/Turabian StyleConti, Angela, Debora Casagrande Pierantoni, Vincent Robert, Gianluigi Cardinali, and Laura Corte. 2021. "Homoplasy as an Auxiliary Criterion for Species Delimitation" Microorganisms 9, no. 2: 273. https://doi.org/10.3390/microorganisms9020273






