Characterization of Two Unique Cold-Active Lipases Derived from a Novel Deep-Sea Cold Seep Bacterium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Screening of Lipase-Producing Bacteria from Deep-Sea Cold Seep Samples
2.2. Physiological Characterizations of Strain gcc21
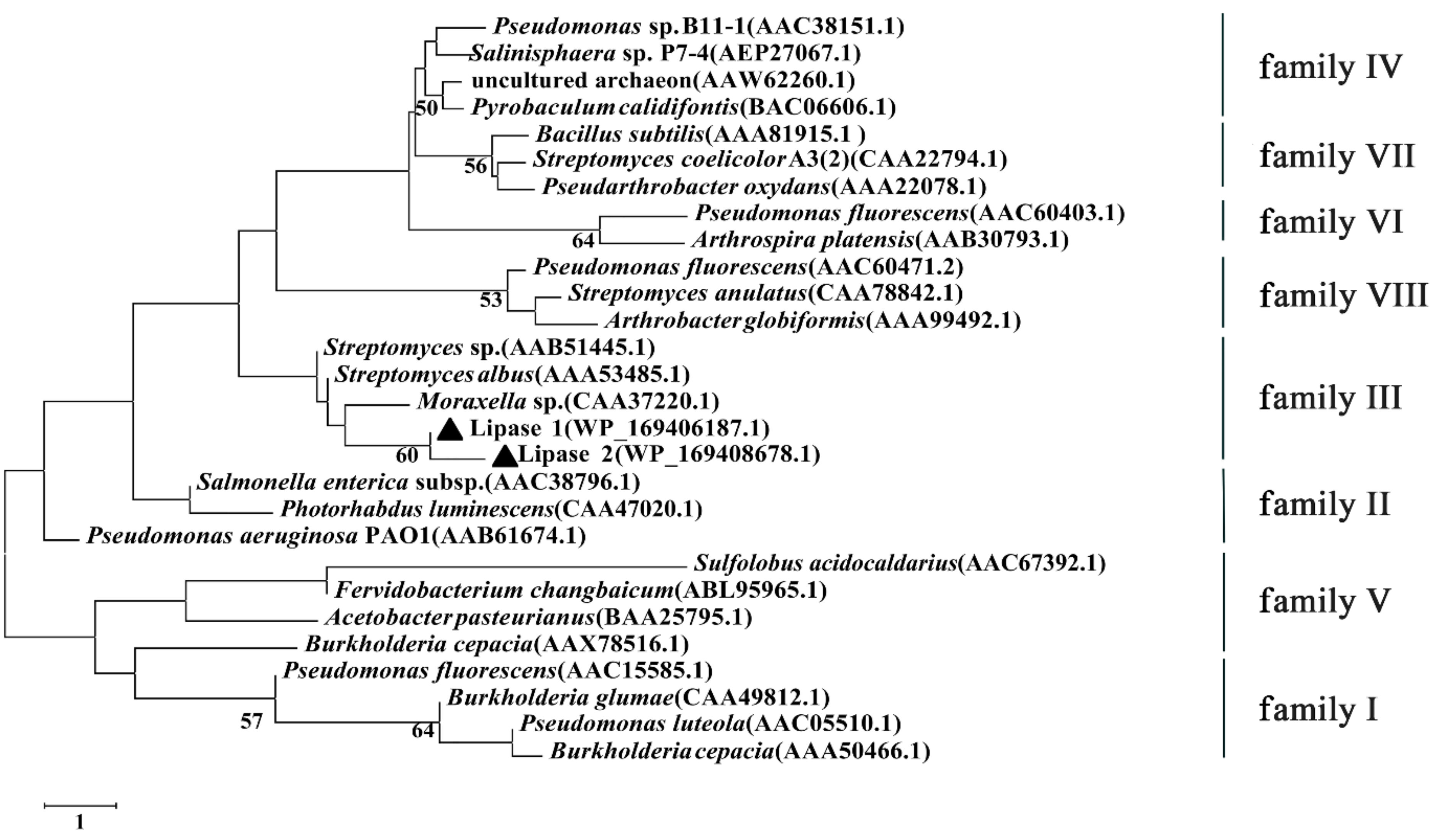
2.3. Phylogenetic Analysis
2.4. Genomic Characterizations of Strain gcc21
2.5. Overexpression, Gene Mutagenesis, and Purification of the Cold-Active Lipases in E. coli
2.6. Sequence Analysis
2.7. Enzyme Assays
2.8. Effects of Temperature and pH on the Lipase Activity and Stability
2.9. Effects of Metal Ions, Inhibitors, Detergents, and Organic Solvents on Lipase Activities
2.10. Statistical Analysis
2.11. Data Availability
3. Results
3.1. Physiological Characterization of Lipase-Producing Strain gcc21 Isolated from the Deep-Sea Cold Seep
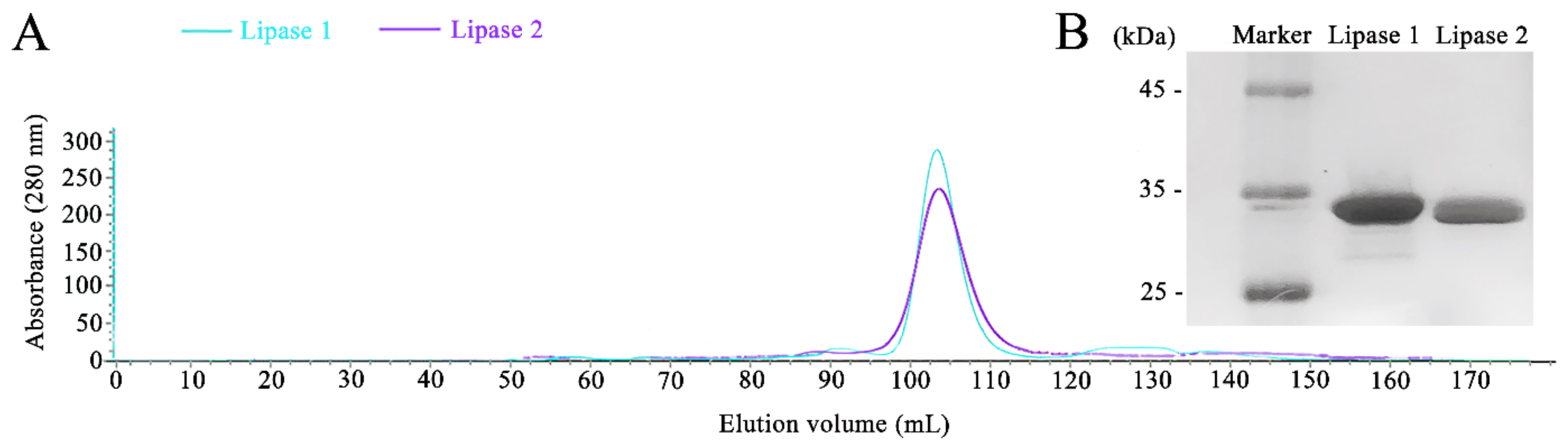
3.2. Overexpression and Purification of the Cold-Active Lipases in E. coli
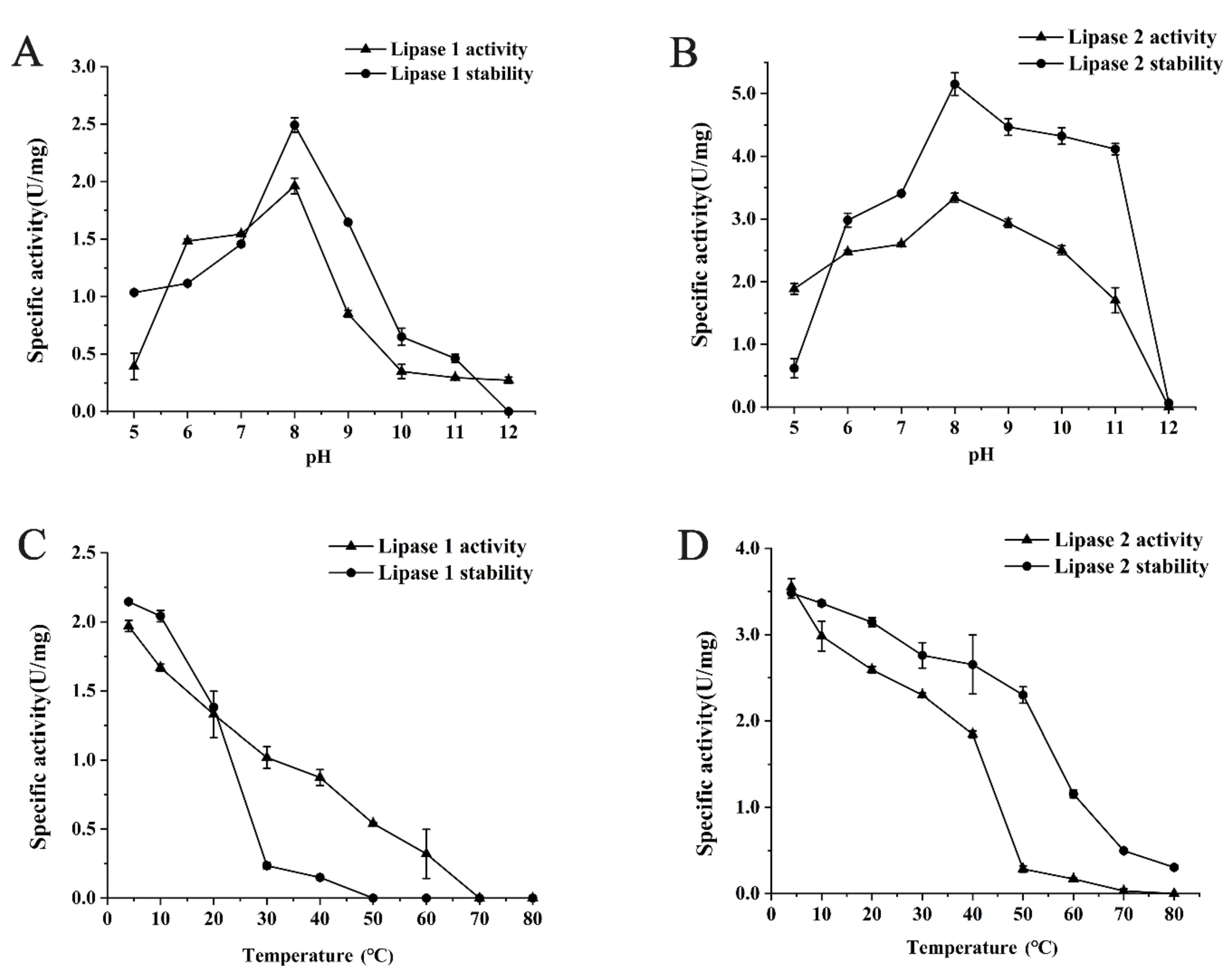
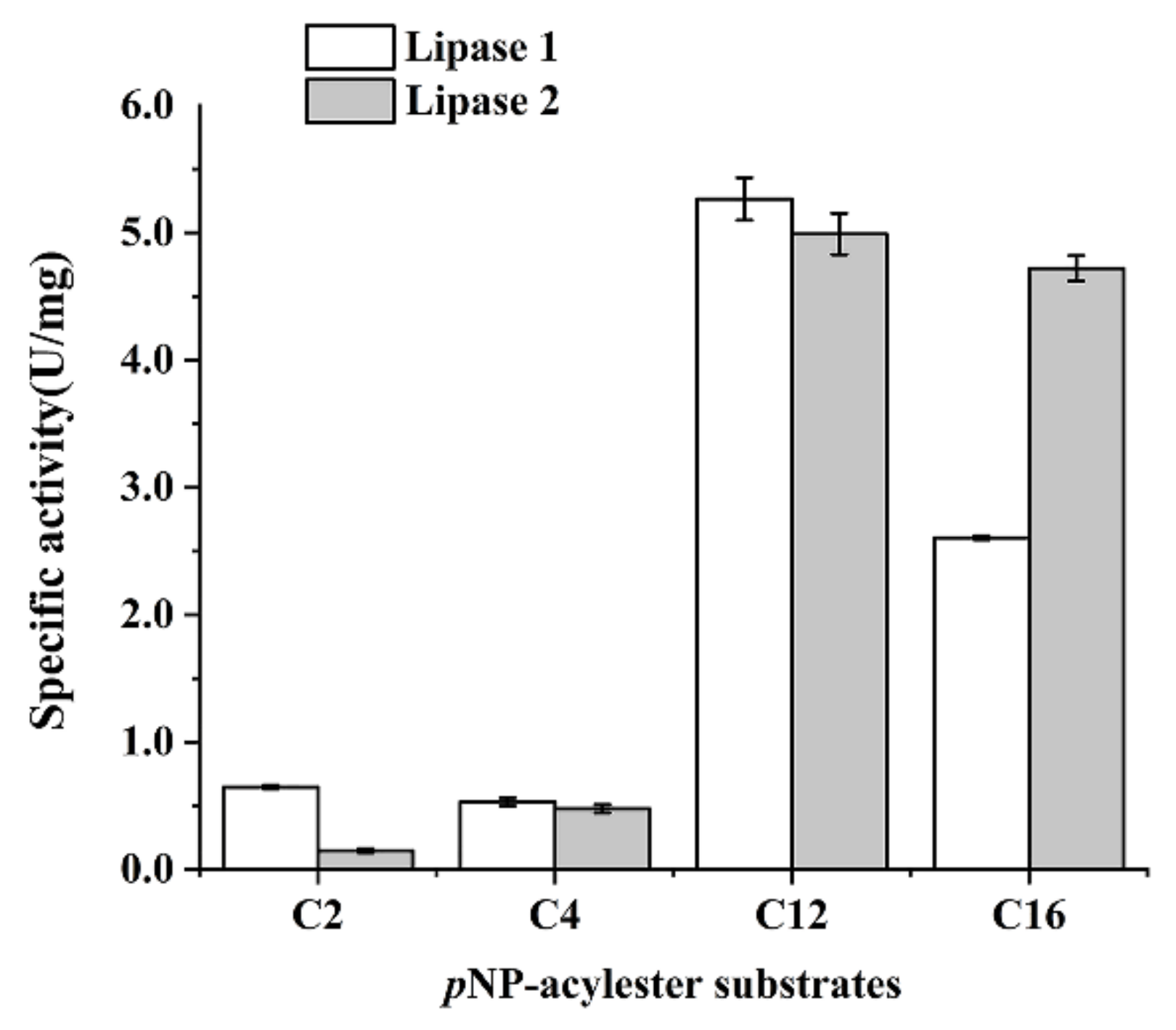
3.3. Characterizations of the Cold-Active Lipases
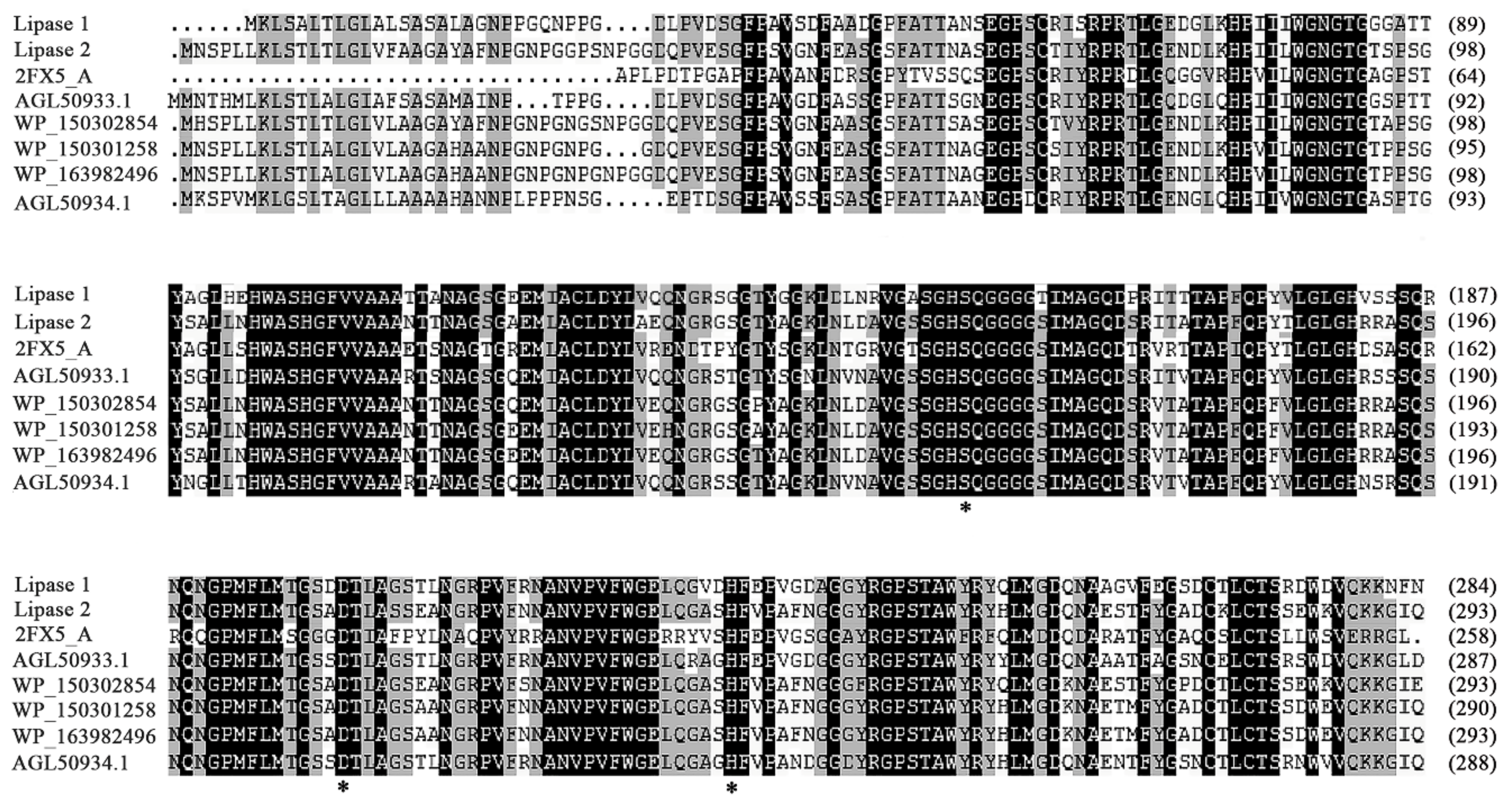
3.4. Functional Verification of Key Amino Acids and Phylogenetic Analysis of Lipase 1 and Lipase 2
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Liu, R.; Xi, S.C.; Cai, R.N.; Zhang, X.; Sun, C.M. A novel bacterial thiosulfate oxidation pathway provides a new clue about the formation of zero-valent sulfur in deep sea. ISME J. 2020, 14, 2261–2274. [Google Scholar] [CrossRef]
- Fujiwara, S. Extremophiles: Developments of their special functions and potential resources. J. Biosci. Bioeng. 2002, 94, 518–525. [Google Scholar] [CrossRef]
- Gerday, C.; Aittaleb, M.; Bentahir, M.; Chessa, J.P.; Claverie, P.; Collins, T.; D’Amico, S.; Dumont, J.; Garsoux, G.; Georlette, D.; et al. Cold-adapted enzymes: From fundamentals to biotechnology. Trends Biotechnol. 2000, 18, 103–107. [Google Scholar] [CrossRef]
- Santiago, M.; Ramirez-Sarmiento, C.A.; Zamora, R.A.; Parra, L.P. Discovery, Molecular Mechanisms, and Industrial Applications of Cold-Active Enzymes. Front. Microbiol. 2016, 7, 1408. [Google Scholar] [CrossRef]
- Siddiqui, K.S.; Cavicchioli, R. Cold-adapted enzymes. Annu. Rev. Biochem. 2006, 75, 403–433. [Google Scholar] [CrossRef] [Green Version]
- Georlette, D.; Blaise, V.; Collins, T.; D’Amico, S.; Gratia, E.; Hoyoux, A.; Marx, J.C.; Sonan, G.; Feller, G.; Gerday, C. Some like it cold: Biocatalysis at low temperatures. FEMS Microbiol. Rev. 2004, 28, 25–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Ruiz, A.; Tovar-Castro, L.; Garcia, H.S.; Saucedo-Castaneda, G.; Favela-Torres, E. Continuous ethyl oleate synthesis by lipases produced by solid-state fermentation by Rhizopus microsporus. Bioresour. Technol. 2018, 265, 52–58. [Google Scholar] [CrossRef]
- Carriere, F.; Thirstrup, K.; Hjorth, S.; Boel, E. Cloning of the Classical Guinea-Pig Pancreatic Lipase and Comparison with the Lipase Related Protein-2. FEBS Lett. 1994, 338, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Fatima, S.; Faryad, A.; Ataa, A.; Joyia, F.A.; Parvaiz, A. Microbial lipase production: A deep insight into the recent advances of lipase production and purification techniques. Biotechnol. Appl. Bioc. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Factories 2020, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, M. Cold active lipases—An update. Front. Life Sci. 2016, 9, 226–238. [Google Scholar] [CrossRef] [Green Version]
- Feller, G.; Narinx, E.; Arpigny, J.L.; Aittaleb, M.; Baise, E.; Genicot, S.; Gerday, C. Enzymes from psychrophilic organisms. FEMS Microbiol. Rev. 1996, 18, 189–202. [Google Scholar] [CrossRef]
- Salwoom, L.; Abd Rahman, R.N.Z.R.; Salleh, A.; Shariff, F.M.; Convey, P.; Pearce, D.; Ali, M.S.M. Isolation, Characterisation, and Lipase Production of a Cold-Adapted Bacterial Strain Pseudomonas sp. LSK25 Isolated from Signy Island, Antarctica. Molecules 2019, 24, 715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.D.; Zheng, R.K.; Liu, G.; Liu, R.; Wu, S.M.; Sun, C.M. Calcium protects bacteria against cadmium stress via reducing nitric oxide production and increasing iron acquisition. Environ. Microbiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.N.; Liu, G.; Zheng, R.K.; Sun, C.M.; Wu, S.M. Structural and Functional Insights into Iturin W, a Novel Lipopeptide Produced by the Deep-Sea Bacterium Bacillus sp. Strain wsm-1. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.W.M.; Latif, H.H.A.; Ali, S.M. Production of Cold-Active Lipase by Free and Immobilized Marine Bacillus cereus HSS: Application in Wastewater Treatment. Front. Microbiol. 2018, 9, 2377. [Google Scholar] [CrossRef] [Green Version]
- Zheng, R.K.; Sun, C.M. Sphingosinithalassobacter tenebrarum sp. nov., isolated from a deep-sea cold seep. Int. J. Syst. Evol. Microbiol. 2020, 70, 5561–5566. [Google Scholar] [CrossRef]
- Hiraishi, A.; Ueda, Y.; Ishihara, J.; Mori, T. Comparative lipoquinone analysis of influent sewage and activated sludge by high-performance liquid chromatography and photodiode array detection. J. Gen. Appl. Microbiol. 1996, 42, 457–469. [Google Scholar] [CrossRef] [Green Version]
- Collins, M.D.; Pirouz, T.; Goodfellow, M.; Minnikin, D.E. Distribution of Menaquinones in Actinomycetes and Corynebacteria. J. Gen. Microbiol. 1977, 100, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Sasser, M. Identification of Bacteria by Gas Chromatography of Cellular Fatty Acids; MIDI Technical Note 101; MIDI Inc.: Newark, DE, USA, 1990; Volume 20, pp. 1–7. [Google Scholar]
- Tindall, B.J.; Sikorski, J.; Smibert, R.A.; Krieg, N.R. Phenotypic Characterization and the Principles of Comparative Systematics. In Methods for General and Molecular Microbiology; ASM Press: Bel Air, MD, USA, 2007; pp. 330–393. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16s Ribosomal DNA Amplification for Phylogenetic Study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [Green Version]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA Sequencing with Chain Terminating Inhibitors. Proc. Natl. Acad. Sci. USA 1978, 74, 5463–5467. [Google Scholar] [CrossRef] [Green Version]
- Saitou, N.; Nei, M. The Neighbor-Joining Method—A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Felsenstein, J. Evolutionary Trees from DNA-Sequences—A Maximum-Likelihood Approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. A stepwise algorithm for finding minimum evolution trees. Mol. Biol. Evol. 1996, 13, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koren, S.; Walenz, B.P.; Berlin, K.; Miller, J.R.; Bergman, N.H.; Phillippy, A.M. Canu: Scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017, 27, 722–736. [Google Scholar] [CrossRef] [Green Version]
- Richter, M.; Rossello-Mora, R.; Glockner, F.O.; Peplies, J. JSpeciesWS: A web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics 2016, 32, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.P.; Goker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. Clustal-W—Improving the Sensitivity of Progressive Multiple Sequence Alignment through Sequence Weighting, Position-Specific Gap Penalties and Weight Matrix Choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [Green Version]
- Felsenstein, J. Confidence-Limits on Phylogenies—An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Lee, Y.P.; Chung, G.H.; Rhee, J.S. Purification and Characterization of Pseudomonas-Fluorescens Sik-W1 Lipase Expressed in Escherichia-Coli. Biochim. Biophys. Acta 1993, 1169, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Bülow, L.; Mosbach, K. The expression in E. coli of a polymeric gene coding for an esterase mimic catalyzing the hydrolysis of p-nitrophenyl esters. FEBS Lett. 1987, 210, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Garrity, G.M.; Bell, J.A.; Lilburn, T. Pseudomonadales Orla-Jensen 1921, 270AL. In Bergey’s Manual® of Systematic Bacteriology: Volume Two the Proteobacteria Part B the Gammaproteobacteria; Brenner, D.J., Krieg, N.R., Staley, J.T., Garrity, G.M., Boone, D.R., De Vos, P., Goodfellow, M., Rainey, F.A., Schleifer, K.-H., Eds.; Springer: Boston, MA, USA, 2005; pp. 323–442. [Google Scholar] [CrossRef]
- Moore, E.B.; Tindall, B.; Martins Dos Santos, V.; Pieper, D.; Ramos, J.-L.; Palleroni, N. Nonmedical: Pseudomonas. Prokaryotes 2006, 6, 646–703. [Google Scholar] [CrossRef]
- Richter, M.; Rossello-Mora, R. Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. USA 2009, 106, 19126–19131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, J.; Oren, A.; Ventosa, A.; Christensen, H.; Arahal, D.R.; da Costa, M.S.; Rooney, A.P.; Yi, H.; Xu, X.W.; De Meyer, S.; et al. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int. J. Syst. Evol. Microbiol. 2018, 68, 461–466. [Google Scholar] [CrossRef]
- Kim, K.H.; Roh, S.W.; Chang, H.W.; Nam, Y.D.; Yoon, J.H.; Jeon, C.O.; Oh, H.M.; Bae, J.W. Pseudomonas sabulinigri sp nov., isolated from black beach sand. Int. J. Syst. Evol. Microbiol. 2009, 59, 38–41. [Google Scholar] [CrossRef]
- Von Neubeck, M.; Huptas, C.; Gluck, C.; Krewinkel, M.; Stoeckel, M.; Stressler, T.; Fischer, L.; Hinrichs, J.; Scherer, S.; Wenning, M. Pseudomonas lactis sp. nov and Pseudomonas paralactis sp. nov., isolated from bovine raw milk. Int. J. Syst. Evol. Microbiol. 2017, 67, 1656–1664. [Google Scholar] [CrossRef]
- Brady, L.; Brzozowski, A.M.; Derewenda, Z.S.; Dodson, E.; Dodson, G.; Tolley, S.; Turkenburg, J.P.; Christiansen, L.; Hugejensen, B.; Norskov, L.; et al. A Serine Protease Triad Forms the Catalytic Center of a Triacylglycerol Lipase. Nature 1990, 343, 767–770. [Google Scholar] [CrossRef]
- Antonian, E. Recent Advances in the Purification, Characterization and Structure Determination of Lipases. Lipids 1988, 23, 1101–1106. [Google Scholar] [CrossRef]
- Ju, H.; Jang, E.; Ryu, B.H.; Kim, T.D. Characterization and preparation of highly stable aggregates of a novel type of hydrolase (BL28) from Bacillus licheniformis. Bioresour. Technol. 2013, 128, 81–86. [Google Scholar] [CrossRef]
- Sun, J.J.; Wang, W.; Ying, Y.; Zhu, X.J.; Liu, J.Z.; Hao, J.H. Pseudomonas profundi sp nov., isolated from deep-sea water. Int. J. Syst. Evol. Microbiol. 2018, 68, 1776–1780. [Google Scholar] [CrossRef] [PubMed]
- Palleroni, N.; Genus, I. Pseudomonas Migula 1894. In Bergey’s Manual of Systematic Bacteriology; Krieg, N.R., Holt, J.G., Eds.; Williams & Wilkins: Baltimore, MD, USA, 1984; Volume 1, pp. 141–199. [Google Scholar]
- Ji, X.L.; Li, S.; Lin, L.B.; Zhang, Q.; Wei, Y.L. Gene cloning, sequence analysis and heterologous expression of a novel cold-active lipase from Pseudomonas sp. PF16. Technol. Health Care 2015, 23, S109–S117. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Pandey, A.; Pasupuleti, M.; Pande, V. Prolonged Production and Aggregation Complexity of Cold-Active Lipase from Pseudomonas proteolytica (GBPI_Hb61) Isolated from Cold Desert Himalaya. Mol. Biotechnol. 2017, 59, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Choo, D.W.; Kurihara, T.; Suzuki, T.; Soda, K.; Esaki, N. A cold-adapted lipase of an Alaskan psychrotroph, Pseudomonas sp. strain B11-1: Gene cloning and enzyme purification and characterization. Appl. Environ. Microbiol. 1998, 64, 486–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T.; Nakayama, T.; Kurihara, T.; Nishino, T.; Esaki, N. Cold-active lipolytic activity of psychrotrophic Acinetobacter sp. strain no. 6. J. Biosci. Bioeng. 2001, 92, 144–148. [Google Scholar] [CrossRef]
- Canak, I.; Berkics, A.; Bajcsi, N.; Kovacs, M.; Belak, A.; Teparic, R.; Maraz, A.; Mrsa, V. Purification and Characterization of a Novel Cold-Active Lipase from the Yeast Candida zeylanoides. J. Mol. Microb. Biotech. 2015, 25, 403–411. [Google Scholar] [CrossRef]
- Li, M.; Yang, L.R.; Xu, G.; Wu, J.P. Screening, purification and characterization of a novel cold-active and organic solvent-tolerant lipase from Stenotrophomonas maltophilia CGMCC 4254. Bioresour. Technol. 2013, 148, 114–120. [Google Scholar] [CrossRef]
- Yan, Q.J.; Duan, X.J.; Liu, Y.; Jiang, Z.Q.; Yang, S.Q. Expression and characterization of a novel 1,3-regioselective cold-adapted lipase from Rhizomucor endophyticus suitable for biodiesel synthesis. Biotechnol. Biofuels 2016, 9, 86. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.J.; Zheng, M.M.; Liu, Y.; Jiang, Z.Q.; Yang, S.Q. High-level expression and biochemical characterization of a novel cold-active lipase from Rhizomucor endophyticus. Biotechnol. Lett. 2016, 38, 2127–2135. [Google Scholar] [CrossRef]
- Ganasen, M.; Yaacob, N.; Abd Rahman, R.N.Z.R.; Leow, A.T.C.; Basri, M.; Salleh, A.; Ali, M.S.M. Cold-adapted organic solvent tolerant alkalophilic family I.3 lipase from an Antarctic Pseudomonas. Int. J. Biol. Macromol. 2016, 92, 1266–1276. [Google Scholar] [CrossRef]
- Novototskaya-Vlasova, K.A.; Petrovskaya, L.E.; Rivkina, E.M.; Dolgikh, D.A.; Kirpichnikov, M.P. Characterization of a cold-active lipase from Psychrobacter cryohalolentis K5(T) and its deletion mutants. Biochemistry 2013, 78, 385–394. [Google Scholar] [CrossRef]
- Li, T.; Zhang, W.; Hao, J.; Sun, M.; Lin, S.-X. Cold-active extracellular lipase: Expression in Sf9 insect cells, purification, and catalysis. Biotechnol. Rep. 2019, 21, e00295. [Google Scholar] [CrossRef]
- Arpigny, J.L.; Jaeger, K.E. Bacterial lipolytic enzymes: Classification and properties. Biochem. J. 1999, 343, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.M.; Fuzi, S.F.M.; Ganasen, M.; Rahman, R.N.Z.R.A.; Basri, M.; Salleh, A.B. Structural Adaptation of Cold-Active RTX Lipase from Pseudomonas sp. Strain AMS8 Revealed via Homology and Molecular Dynamics Simulation Approaches. Biomed. Res. Int. 2013, 2013, 925373. [Google Scholar] [CrossRef] [Green Version]
- Giovanola, M.; D’Antoni, F.; Santacroce, M.; Mari, S.A.; Cherubino, F.; Bossi, E.; Sacchi, V.F.; Castagna, M. Role of a conserved glycine triplet in the NSS amino acid transporter KAAT1. Bba-Biomembranes 2012, 1818, 1737–1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feller, G.; Gerday, C. Psychrophilic enzymes: Molecular basis of cold adaptation. Cell. Mol. Life Sci. 1997, 53, 830–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Name | Sequence (5′–3′) |
|---|---|
| 27-F | AGAGTTTGATCCTGGCTCAG |
| 1492-R | GGTTACCTTGTTACGACTT |
| Lipase 1-F | CGCGAATTCATGAAGCTTTCCGCTCTT |
| Lipase 1-R | CCGCTCGAGGTTGAAATTCTTCTTCTG |
| Lipase 1-S151A-F | GCTCAAGGCGGTGGCGGCACCAT |
| Lipase 1-S151A-R | ATGGCCGGATGCGCCGACACGGT |
| Lipase 1-D201A-F | GCTACGCTGGCAGGCTCGACATT |
| Lipase 1-D201A-R | GTCGCTGCCGGTCATGAGGAAC |
| Lipase 1-H231A-F | GCTTTCGAACCCGTGGGCGA |
| Lipase 1-H231A-R | ATCGACTCCCTGCAGCTCACCC |
| Lipase 2-F | CGCGGATCCATGAATTCACCCCTATTG |
| Lipase 2-R | CCCAAGCTTCTGGATGCCCTTTTTCTG |
| Lipase 2-S160A-F | GCTCAGGGCGGTGGCGGCTC |
| Lipase 2-S160A-R | GTGACCGGAGGAACCAACAGCGTCCAG |
| Lipase 2-D210A-F | GCCACCCTGGCCAGCTCCGA |
| Lipase 2-D210A-R | GGCGCTGCCAGTCATCAGGA |
| Lipase 2-H240A-F | GCCTTTGTTCCGGCCTTCAACGG |
| Lipase 2-H240A-R | GCTGGCCCCCTGCAACTCG |
| Characteristics | 1 | 2 | 3 |
|---|---|---|---|
| Cell morphology and size | rod-shaped 0.8–1.0 µm × 1.1–1.8 µm | short-rod-shapedb 0.7–1.0 µm × 1.5–2.0 µmb | ND ND |
| Growth condition | |||
| Temp (°C) for growth (optimal) | 4–37 (28) | 4–37 (28) | 4–45 (28) |
| pH for growth (optimal) | 5.0–8.5 (7.0) | 5.5–9.0 (6.0) | 4.5–9.0 (6.0) |
| NaCl concentration (%) for growth (optimal) | 0.5–9.0 (1.5) | 0–10.0 (7.0) | 0–7.0 (2.0) |
| Enzyme activity | |||
| Oxidase activity | + | - | + |
| Catalase activity | + | + | + |
| Hydrolysis of | |||
| Starch | - | - | + |
| Tween 20 | + | + | + |
| Tween 80 | + | + | + |
| Sole carbon source utilization | |||
| Ethanol | + | - | + |
| Sorbitol | + | + | - |
| D-mannose | + | + | - |
| L-arabitol | + | - | + |
| Xylitol | + | + | + |
| Acetate | + | + | + |
| Lactate | + | + | + |
| Polar lipids | DPG, PG, PE, PL1-4, APL | ND | ND |
| Major fatty acids | Branched-C16:0, Branched-C17:0 cyclo, Summed Feature 3, Summed Feature 8 | Branched-C12:0, Branched-C16:0, Summed Feature 3, Summed Feature 8 | Branched-C19:0 cyclo ω8 c, Branched-C16:0, Summed Feature 8 |
| DNA G+C content (%) | 58.27 | 58.1 b | 66.6 c |
| Lipase | Km (mM) | Vmax (U/mg• S−1) | Kcat (S−1) | Kcat/Km |
|---|---|---|---|---|
| Lipase 1 | 0.06 | 33.2 | 1.1 | 36.6 |
| Lipase 2 | 0.02 | 154.8 | 2.7 | 140.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Zheng, R.; Cai, R.; Sun, C.; Wu, S. Characterization of Two Unique Cold-Active Lipases Derived from a Novel Deep-Sea Cold Seep Bacterium. Microorganisms 2021, 9, 802. https://doi.org/10.3390/microorganisms9040802
Guo C, Zheng R, Cai R, Sun C, Wu S. Characterization of Two Unique Cold-Active Lipases Derived from a Novel Deep-Sea Cold Seep Bacterium. Microorganisms. 2021; 9(4):802. https://doi.org/10.3390/microorganisms9040802
Chicago/Turabian StyleGuo, Chenchen, Rikuan Zheng, Ruining Cai, Chaomin Sun, and Shimei Wu. 2021. "Characterization of Two Unique Cold-Active Lipases Derived from a Novel Deep-Sea Cold Seep Bacterium" Microorganisms 9, no. 4: 802. https://doi.org/10.3390/microorganisms9040802






