Microbiomes in Suspended Soils of Vascular Epiphytes Differ from Terrestrial Soil Microbiomes and from Each Other
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Studies
2.2. Sample Collection
- (1).
- The bank of the Mixed primary forest in the bank of the Dong Nai River (11°25′55” N, 107°25′48” E). A set of ground soil samples was taken from the upper horizon of a Skeletic Greyzemic Umbrisol (Clayic) soil (FG), and two sets of epiphytic suspended-soil samples were taken from Drynaria quercifolia (FS2) and Vittaria sp. (FS1) specimens, respectively; and
- (2).
- Artificial habitats in a mixed tropical tree plantation (11°24′05” N, 107°22′29” E). Loamy terrestrial soil samples were taken from the top of the AU horizon of 25-year-old grey-humus lithozems (PG). Two suspended soils were taken from D. quercifolia (PS1) and Lemmaphyllum microphyllum (PS2) respectively.
2.3. Routine Soil Analysis
2.4. Metagenomic Analysis
3. Results
3.1. Soil Nutrition Properties
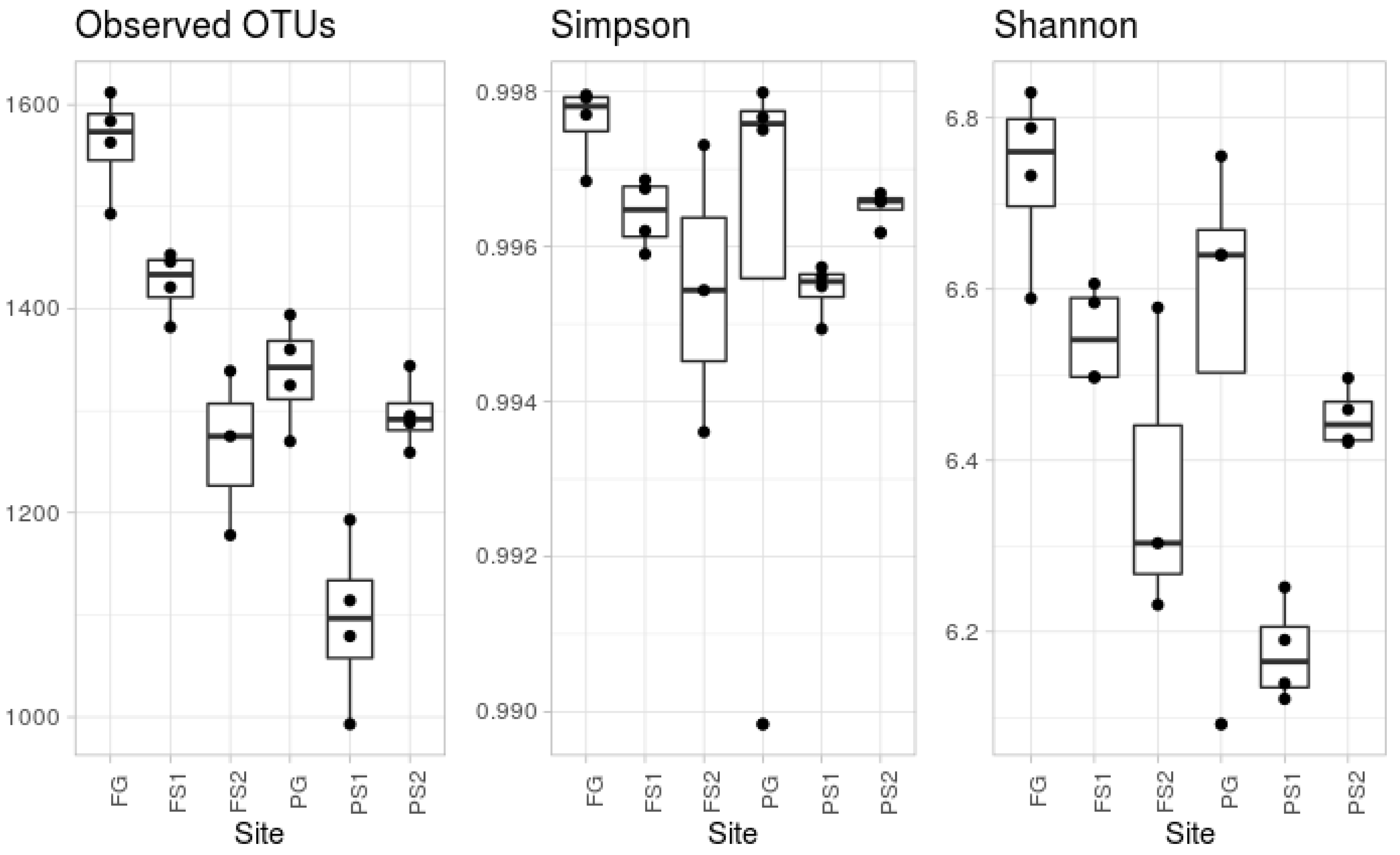
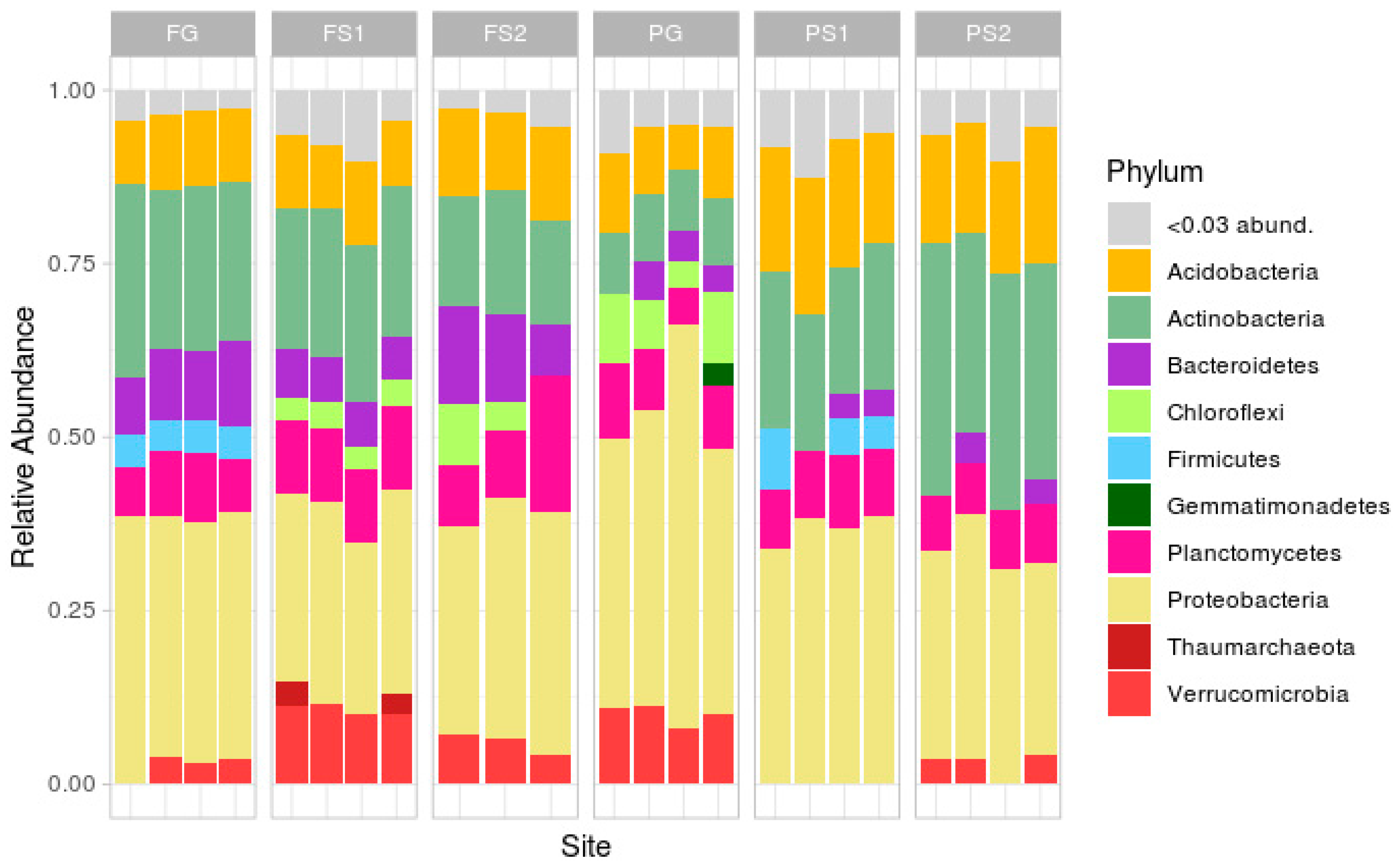
3.2. Terrestrial and Suspended Soil Microbial Diversity
3.3. Significant Variation between Groups
3.4. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benzing, D.H. Vascular Epiphytes: General Biology and Related Biota; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Umana, N.H.N.; Wanek, W. Large canopy exchange fluxes of inorganic and organic nitrogen and preferential retention of nitrogen by epiphytes in a tropical lowland rainforest. Ecosystems 2010, 13, 367–381. [Google Scholar] [CrossRef]
- Hietz, P.; Wanek, W.; Popp, M. Stable isotopic composition of carbon and nitrogen and nitrogen content in vascular epiphytes along an altitudinal transect. Plant Cell Environ. 1999, 22, 1435–1443. [Google Scholar] [CrossRef]
- Hietz, P.; Wanek, W.; Wania, R.; Nadkarni, N.M. Nitrogen-15 natural abundance in a montane cloud forest canopy as an indicator of nitrogen cycling and epiphyte nutrition. Oecologia 2002, 131, 350–355. [Google Scholar] [CrossRef]
- Treseder, K.K.; Davidson, D.W.; Ehleringer, J.R. Absorption of ant provided carbon dioxide and nitrogen by a tropical epiphyte. Nature 1995, 375, 137–139. [Google Scholar] [CrossRef]
- Eskov, A.K.; Onipchenko, V.G.; Prilepsky, N.G.; Abakumov, E.V.; Kolomeitseva, G.L.; Nguyen, V.T.; Tiunov, A.V. Dependence of epiphytic community on autochthonous and allochthonous sources of nitrogen in three forest habitats of southern Vietnam. Plant Soil 2019, 443, 565–574. [Google Scholar] [CrossRef]
- Zotz, G. Plants on Plants—The Biology of Vascular Epiphytes; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Shaw, P. The use of inert pads to study Collembola of suspended soils. Soil Org. 2013, 85, 69–74. [Google Scholar]
- Nadkarni, N.M.; Matelson, T.J. Fine litter dynamics within the tree canopy of a tropical cloud forest. Ecology 1991, 72, 2071–2082. [Google Scholar] [CrossRef]
- Nadkarni, N.M.; Matelson, T.J. Biomass and nutrient dynamics of epiphytic litterfall in a neotropical montane forest, Costa Rica. Biotropica 1992, 24, 24–30. [Google Scholar] [CrossRef]
- Abakumov, E.V.; Rodina, O.A.; Eskov, A.K. Humification and humic acid composition of suspended soil in oligotrophous environments in South Vietnam. Appl. Environ. Soil Sci. 2018, 1–8, 1026237. [Google Scholar] [CrossRef] [Green Version]
- Rodina, O.A.; Abakumov, E.V.; Eskov, A.K.; Prilepskiy, N.G. Humic substance formation as a result of biogenic-abiogenic interactions in epiphytic structures of the South Vietnam Tropical Forest. In Processes and Phenomena on the Boundary between Biogenic and Abiogenic Nature; Lecture Notes in Earth System Sciences; Frank-Kamenetskaya, O., Vlasov, D., Panova, E., Lessovaia, S., Eds.; Springer: Cham, Switzerland, 2020; pp. 417–434. [Google Scholar]
- Zotz, G. The systematic distribution of vascular epiphytes—A critical update. Bot. J. Linn. Soc. 2013, 171, 453–481. [Google Scholar] [CrossRef] [Green Version]
- Nadkarni, N.M. Epiphyte biomass and nutrient capital of a neotropical elfin forest. Biotropica 1984, 16, 249–256. [Google Scholar] [CrossRef]
- Hofstede, R.G.M.; Wolf, J.H.D.; Benzing, D.H. Epiphytic biomass and nutrient status of a Colombian upper montane rain forest. Selbyana 1993, 14, 37–45. [Google Scholar]
- Nakanishi, A.; Sungpalee, W.; Sri-Ngernyuang, K.; Kanzaki, M. Large variations in composition and spatial distribution of epiphyte biomass on large trees in a tropical montane forest of northern Thailand. Plant Ecol. 2016, 217, 1157–1169. [Google Scholar] [CrossRef]
- Lindo, Z.; Winchester, N.N. A comparison of microarthropod assemblages with emphasis on oribatid mites in canopy suspended soils and forest floors associated with ancient western red cedar trees. Pedobiologia 2006, 50, 31–41. [Google Scholar] [CrossRef]
- Lindo, Z.; Winchester, N.N. Oribatid mite communities and foliar litter decomposition in canopy suspended soils and forest floor habitats of western red cedar forests, Vancouver Island, Canada. Soil Biol. Biochem. 2007, 39, 2957–2966. [Google Scholar] [CrossRef]
- Karasawa, S.; Hijii, N. Oribatid Communities (Acari: Oribatida) Associated with Bird’s Nest Ferns (Asplenium Nidus Complex.) in a Subtropical Japanese Forest–A Mini Review; Trends in Acarology; Springer: Dordrecht, The Netherlands, 2010; pp. 149–153. [Google Scholar]
- Sergeeva, T.K.; Kholopova, L.B.; Tyen, N. Pedobionts related to “suspended” soils of a tropical epiphyte Asplenium nidus L. Trop. Zool. 1991, 4, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Bardgett, R.D.; Van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, N.T.; Iwanaga, H.; Charles, S.; Diway, B.; Sabang, J.; Chong, L. Soil bacterial community structure in five tropical forests in Malaysia and one temperate forest in Japan revealed by pyrosequencing analyses of 16Sr RNA gene sequence variation. Genes Genet. Syst. 2013, 88, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Navarrete, A.A.; Tsai, S.M.; Mendes, L.W.; Faust, K.; De Hollander, M.; Cassman, N.A.; Raes, J.; Van Veen, J.A.; Ku-ramae, E.E. Soil microbiome responses to the short-term effects of Amazonian deforestation. Mo.-Lecular Ecol. 2015, 24, 2433–2448. [Google Scholar] [CrossRef]
- Schneider, D.; Engelhaupt, M.; Allen, K.; Kurniawan, S.; Krashevska, V.; Heinemann, M.; Nacke, H.; Wijayanti, M.; Meryandini, A.; Corre, M.D.; et al. Impact of lowland rainforest transformation on diversity and composition of soil prokaryotic communities in Sumatra (Indonesia). Front. Microbiol. 2015, 6, 1339. [Google Scholar] [CrossRef] [Green Version]
- Kroeger, M.E.; Delmont, T.O.; Eren, A.M.; Meyer, K.M.; Guo, J.; Khan, K.; Rodrigues, J.L.M.; Bohannan, B.J.M.; Tringe, S.G.; Borges, C.D.; et al. New biological insights into how deforestation in Amazonia affects soil microbial communities using metagenomics and metagenome-assembled genomes. Front. Microbiol. 2018, 9, 1635. [Google Scholar] [CrossRef]
- Blanc, L.; Maury-Lechon, G.; Pascal, J.P. Structure, floristic composition and natural regeneration in the forests of Cat Tien National Park, Vietnam: An analysis of the successional trends. J. Biogeogr. 2000, 27, 141–157. [Google Scholar] [CrossRef]
- Kuznetsov, A.N.; Kuznetsova, S.P. Forest vegetation: Species composition and stand structure. In Struktura i funktsii pochvennogo naseleniya mussonnogo tropicheskogo lesa natsional’nyi park Kat T’en, Yuzhnyi V’etnam (Structure and Functions of the Soil Fauna in a Tropical Monsoon Forest: Cat Tien National Park, Vietnam); KMK: Moscow, Russia, 2011; pp. 16–43. [Google Scholar]
- Millet, J. Reforestation of dipterocarp forests on denuded areas in Cat Tien National Park in Dong Nai Province, Vietnam. In Proceedings of the 8th Round Table Conference on Dipterocarps, Ho Chi Minh City, Vietnam, 15–17 November 2005. [Google Scholar]
- Eskov, A.K.; Prilepsky, N.G.; Antipina, V.A.; Abakumov, E.V.; Nguyen, V.T. Formation of epiphytic communities in forest plantations of South Vietnam. Russ. J. Ecol. 2020, 51, 208–216. [Google Scholar] [CrossRef]
- Deshcherevskaya, O.A.; Avilov, V.K.; Din, B.Z.; Chan, K.H.; Kurbatova, Y.A. Modern climate of the Cat Tien National Park (southern Vietnam): Climatological data for ecological studies. Izv. Atmos. Ocean. Phys. 2013, 49, 819–838. [Google Scholar] [CrossRef]
- Khokhlova, O.S.; Myakshina, T.N.; Kuznetsov, A.N.; Gubin, S.V. Morphogenetic features of soils in the Cat Tien National Park, southern Vietnam. Eurasian Soil Sci. 2017, 50, 158–175. [Google Scholar] [CrossRef]
- Iuss Working Group WRB. World Reference Base for Soil Resources 2014, update 2015. International Soil Classification System for naming soils and creating legends for soil maps. World Soil Resour. Rep. 2015, 106, 1–192. [Google Scholar]
- Kimble, J.M.; Lal, R.; Follet, R.F. Methods for Assesing Soil C Pools. Methods for Soil Carbon; Lal, R., Kimble, J.M., Follet, R.F., Stewart, B.A., Eds.; Lewis Publishers: Boca Raton, FL, USA, 2001. [Google Scholar]
- Wentworth, C.K. A Scale of Grade and Class Terms for Clastic Sediments. J. Geol. 1922, 30, 377–392. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Powlson, D.S. The effects of biocidal treatment on metabolism in soil. V. A method for measuring soil biomass. Soil Biol. Biochem. 1976, 8, 209–213. [Google Scholar] [CrossRef]
- Abakumov, E.V.; Mukhametova, N. Microbial biomass and basal respiration of selected sub-Antarctic and Antarctic soils in the areas of some Russian polar stations. Solid Earth 2014, 5, 705–712. [Google Scholar] [CrossRef] [Green Version]
- Orlov, D.S. Chemistry of Soils; MSU Publishing: Moscow, Russian, 1981. [Google Scholar]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [Green Version]
- Illumina. 16S Metagenomic Sequencing Library Preparation. Preparing 16S Ribosomal RNA Gene Amplicons for the Illumina MiSeq System; Illumina: San Diego, CA, USA, 2013; Available online: https://support.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf (accessed on 11 May 2021).
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Patiño, J.; González-Mancebo, J.M.; Fernández-Palacios, J.M.; Arévalo, J.R.; Bermúde, A. Short-term effects of clear-cutting on the biomass and richness of epiphytic bryophytes in managed subtropical cloud forests. Ann. For. Sci. 2009, 66, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Nadkarni, N.M.; Schaefer, D.; Matelson, T.J.; Solano, R. Biomass and nutrient pools of canopy and terrestrial components in a primary and a secondary montane cloud forest, Costa Rica. For. Ecol. Manag. 2004, 198, 223–236. [Google Scholar] [CrossRef]
- Matsumoto, T.; Abe, T. The role of termites in an equatorial rain forest ecosystem of west Malaysia. Oecologia 1979, 38, 261–274. [Google Scholar] [CrossRef]
- Brauman, A. Effect of gut transit and mound deposit on soil organic matter transformations in the soil feeding termite: A review. Eur. J. Soil Biol. 2000, 36, 117–125. [Google Scholar] [CrossRef]
- Zytynska, S.E.; Fay, M.F.; Penney, D.; Preziosi, R.F. Genetic variation in a tropical tree species influences the associated epiphytic plant and invertebrate communities in a complex forest ecosystem. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1329–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodgers, D.J.; Kitching, R.L. Vertical stratification of rainforest collembolan (Collembola: Insecta) assemblages: Description of ecological patterns and hypotheses concerning their generation. Ecography 1998, 21, 392–400. [Google Scholar] [CrossRef]
- Osland, M.J.; González, E.; Richardson, C.J. Restoring diversity after cattail expansion: Disturbance, resilience, and seasonality in a tropical dry wetland. Ecol. Appl. 2011, 21, 715–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, R.T.; Robeson, M.S.; Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. A comprehensive survey of soil acidobacterial diversity using pyrosequencing and clone library analyses. ISME J. 2009, 3, 442–453. [Google Scholar] [CrossRef]
- Dedysh, S.N.; Sinninghe Damsté, J.S. Acidobacteria; Els; John Wiley & Sons: Chichester, UK, 2018; pp. 1–10. [Google Scholar]
- Bergkemper, F.; Kublik, S.; Lang, F.; Krüger, J.; Vestergaard, G.; Schloter, M.; Schulz, S. Novel oligonucleotide primers reveal a high diversity of microbes which drive phosphorous turnover in soil. J. Microbiol. Methods 2016, 125, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Catão, E.C.P.; Lopes, F.A.C.; Araújo, J.F.; De Castro, A.P.; Barreto, C.C.; Bustamante, M.M.C.; Quirino, B.F.; Krüger, R.H. Soil acidobacterial 16S rRNA gene sequences reveal subgroup level differences between savanna-like cerrado and Atlantic forest Brazilian biomes. Int. J. Microbiol. 2014, 1–12, 156341. [Google Scholar] [CrossRef]
- Kielak, A.M.; Barreto, C.C.; Kowalchuk, G.A.; van Veen, J.A.; Kuramae, E.E. The ecology of Acidobacteria: Moving beyond genes and genomes. Front. Microbiol. 2016, 7, 744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chernov, T.I.; Zhelezova, A.D.; Tkhakakhova, A.K.; Bgazhba, N.A.; Zverev, A.O. Microbiomes of virgin soils of southern Vietnam tropical forests. Microbiology 2019, 88, 489–498. [Google Scholar] [CrossRef]
- Maxfield, D.J.; Leroy, P.C.; Ellwood, M.F. Epiphytic suspended soils from Borneo and Amazonia differ in their microbial community composition. Acta Oecologica 2020, 106, 103586. [Google Scholar]

| Sample Code | pH, H2O | BR, mg/g/h | Available Forms of Nutrients | N% | C% | C/N | |||
|---|---|---|---|---|---|---|---|---|---|
| P2O5, mg/kg | K2O, mg/kg | N-NH4, mg/kg | N-NO3, mg/kg | ||||||
| FG | 4.99 | 0.11 ± 0.03 | 505 ± 15 | 1161 ± 45 | 115.6 ± 12 | 2.23 ± 0.07 | 2.21 ± 0.12 | 42.0 ± 0.87 | 19.0 |
| FS1 | 5.77 | 0.09 ± 0.02 | 250 ± 12 | 2710 ± 56 | 66.3 ± 4 | 2.24 ± 0.12 | 1.36 ± 0.07 | 18.8 ± 0.12 | 13.8 |
| FS2 | 5.53 | 0.06 ± 0.01 | 57 ± 3 | 668 ± 34 | 83.8 ± 7 | 7.87 ± 0.54 | 9.62 ± 0.05 | 41.4 ± 0.74 | 4.3 |
| PG | 5.99 | 0.03 ± 0.01 | 9 ± 2 | 70 ± 5 | 19.8 ± 2 | <0.05 | 0.06 ± 0.02 | 0.21 ± 0.02 | 3.22 |
| PS1 | 6.14 | 0.06 ± 0.01 | 117 ± 12 | 703 ± 46 | 62.5 ± 6 | 0.07 ± 0.02 | 0.14 ± 0.02 | 1.28 ± 0.07 | 9.04 |
| PS2 | 5.68 | 0.10 ± 0.02 | 238 ± 10 | 2921 ± 76 | 157.5 ± 11 | 16.5 ± 1.20 | 1.68 ± 0.05 | 43.0 ± 0.81 | 25.5 |
| Sample Pair | Variable OTUs | Variable Reads | Common OTUs | Common Reads | % Variable OTUs | % Variable Reads |
|---|---|---|---|---|---|---|
| PS1–PS2 | 557 | 30,458 | 2525 | 18,486 | 18.1 | 62.2 |
| PG–PS1 | 700 | 29,868 | 2443 | 19,076 | 22.3 | 61.0 |
| PG–PS2 | 1002 | 34,063 | 2654 | 14,881 | 27.4 | 69.6 |
| FS1–FS2 | 243 | 14,195 | 3054 | 28,631 | 7.4 | 33.1 |
| FG–FS1 | 757 | 28,685 | 3116 | 20,259 | 19.5 | 58.6 |
| FG–FS2 | 213 | 11,896 | 3300 | 30,930 | 6.1 | 27.8 |
| FG–PG | 857 | 28,359 | 3052 | 20,585 | 21.9 | 57.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eskov, A.K.; Zverev, A.O.; Abakumov, E.V. Microbiomes in Suspended Soils of Vascular Epiphytes Differ from Terrestrial Soil Microbiomes and from Each Other. Microorganisms 2021, 9, 1033. https://doi.org/10.3390/microorganisms9051033
Eskov AK, Zverev AO, Abakumov EV. Microbiomes in Suspended Soils of Vascular Epiphytes Differ from Terrestrial Soil Microbiomes and from Each Other. Microorganisms. 2021; 9(5):1033. https://doi.org/10.3390/microorganisms9051033
Chicago/Turabian StyleEskov, Alen K., Alexei O. Zverev, and Evgeny V. Abakumov. 2021. "Microbiomes in Suspended Soils of Vascular Epiphytes Differ from Terrestrial Soil Microbiomes and from Each Other" Microorganisms 9, no. 5: 1033. https://doi.org/10.3390/microorganisms9051033






