A Small Non-Coding RNA Modulates Expression of Pilus-1 Type in Streptococcus pneumoniae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, Cells Lines, and Growth Conditions
2.2. Transformation of Pneumococcal Cells
2.3. Construction of Strains and Plasmids
2.4. RNA Isolation and Northern Blot
2.5. Quantitative Real-Time PCR
2.6. Rapid Amplification of cDNA Ends (RACE) Experiments
2.7. Negative Staining and Transmission Electron Microscopy
2.8. Adherence to Epithelial Cells
2.9. Total Protein Extraction and Western Blotting
2.10. Flow Cytometry
2.11. Indirect Immunofluorescence (IIF) and Confocal Microscopy
2.12. mRNA Half-Life Studies
3. Results
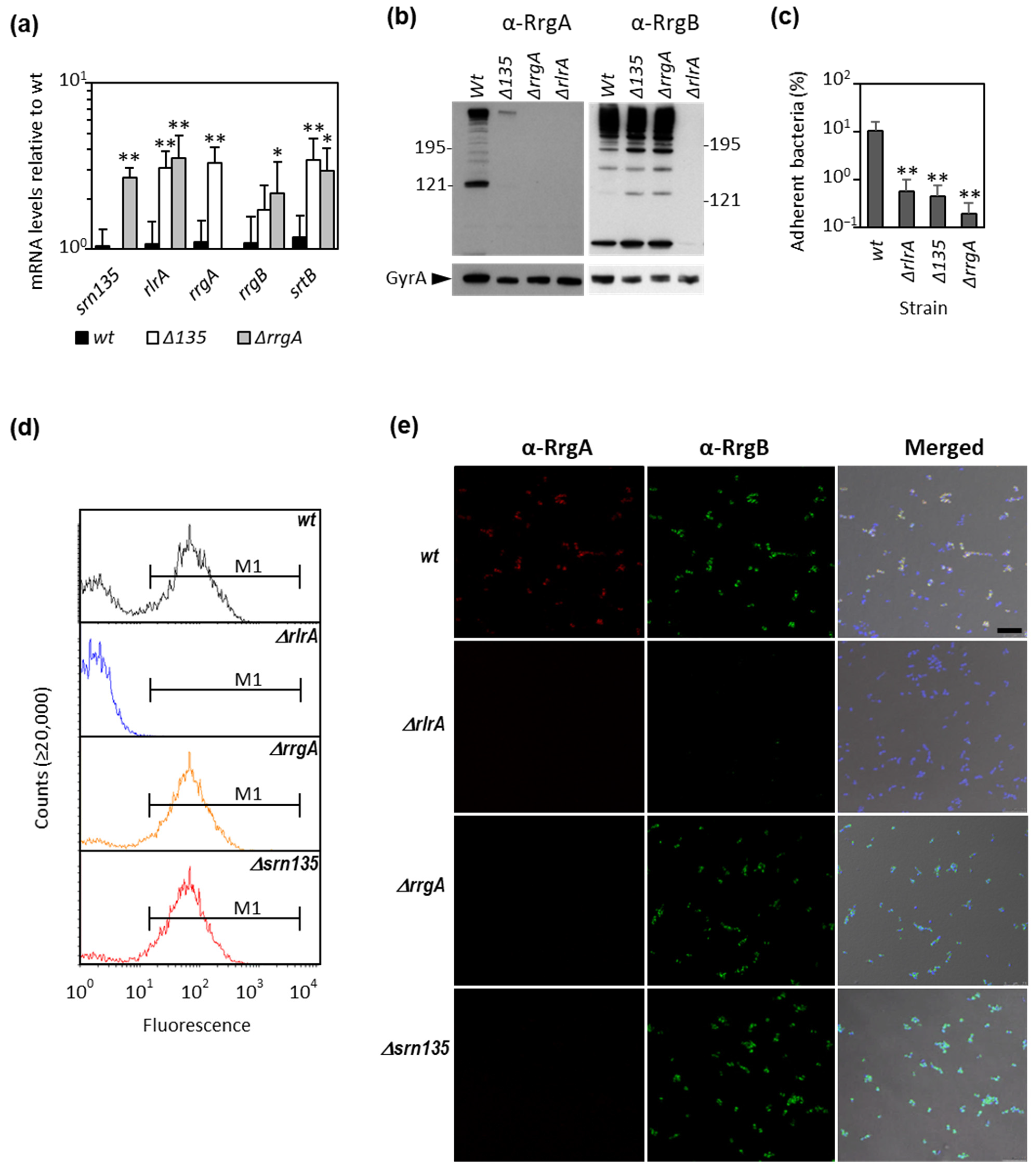
3.1. The Srn135 Coding Sequence Is Necessary for RrgA Translation
3.2. The Srn135 sRNA Is Generated upon Processing of RrgA Transcript
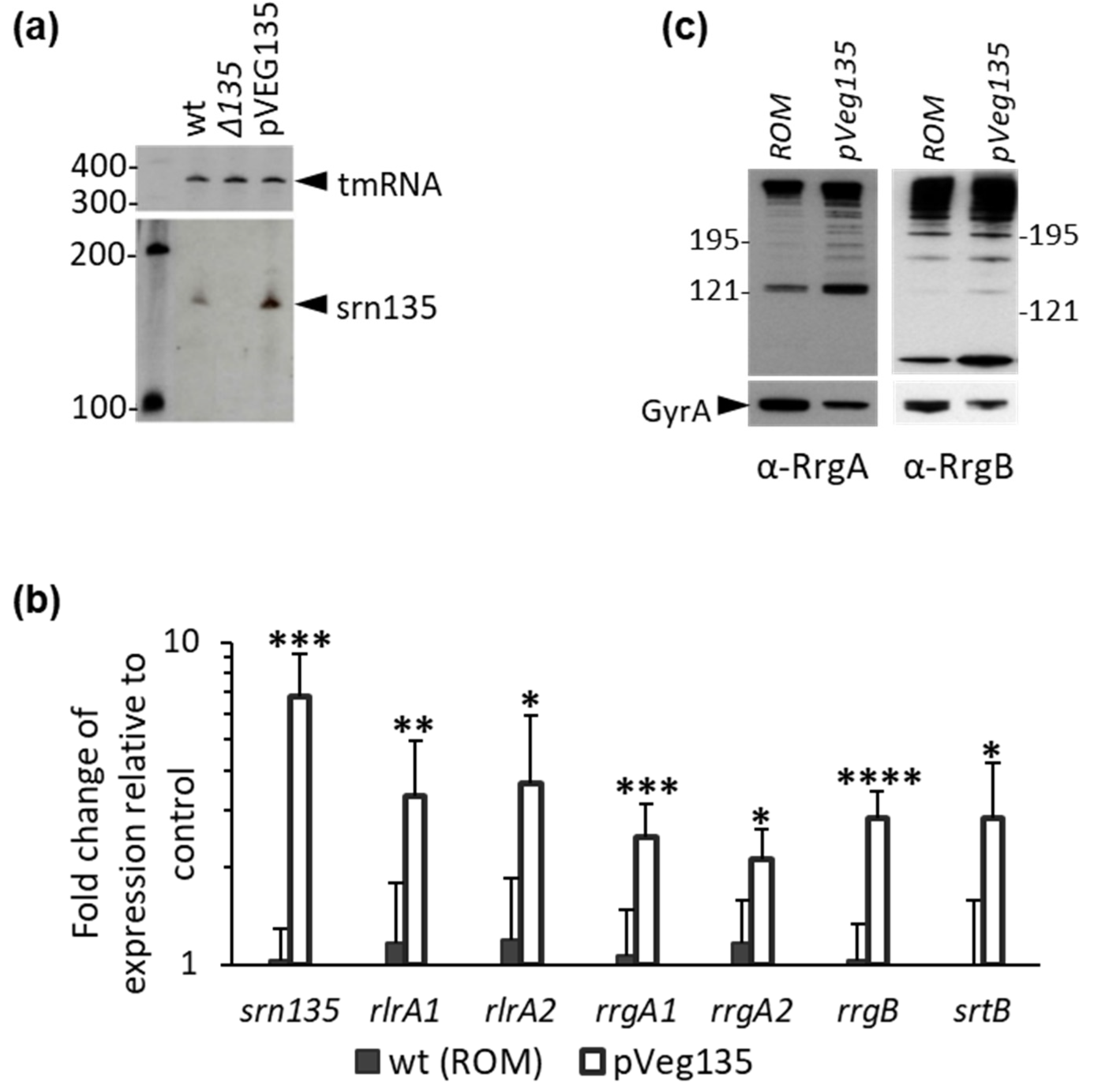
3.3. Overexpression of Srn135 in Trans Increases Pilus Expression
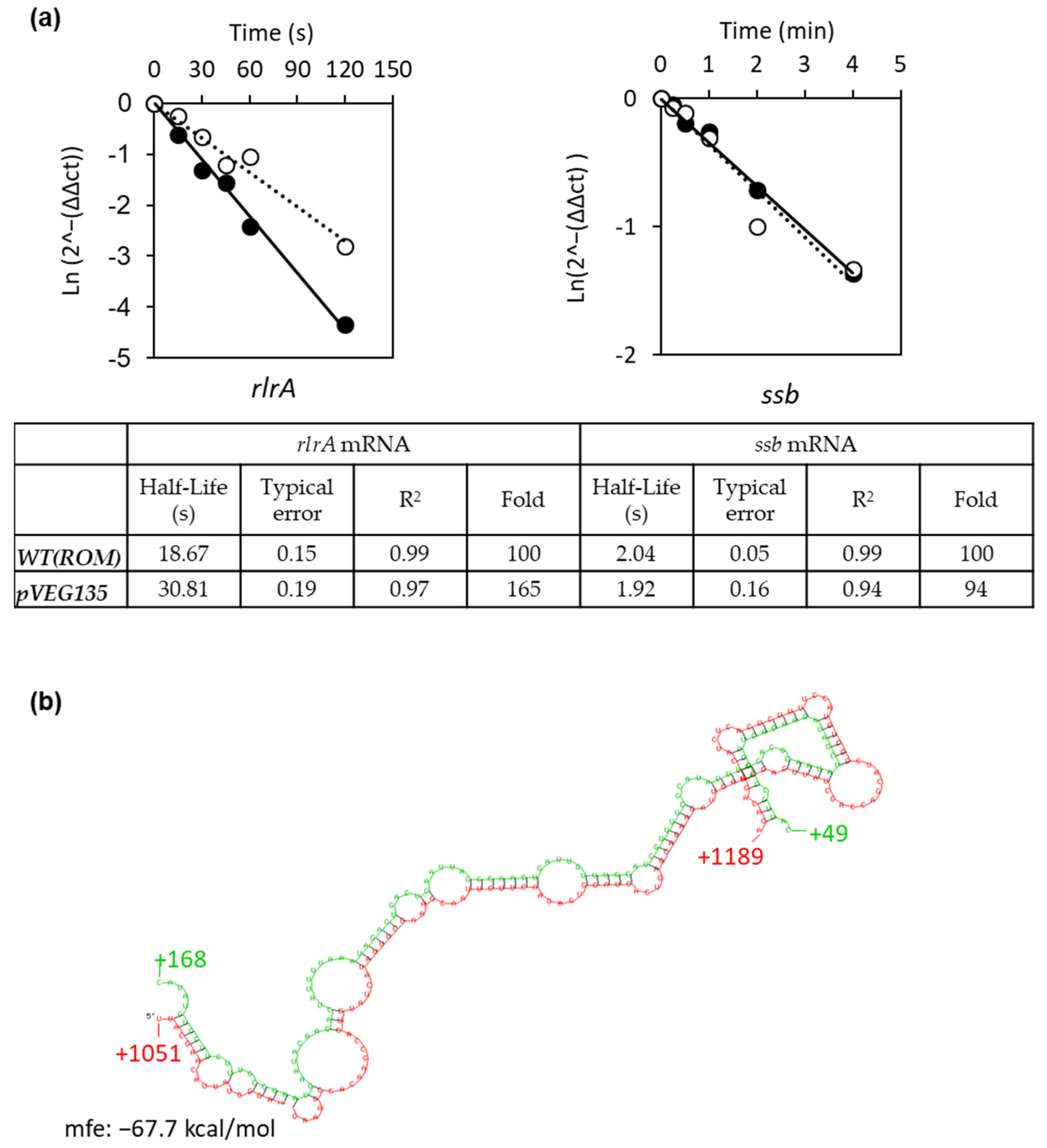
3.4. Srn135 Modulates RlrA Levels by Affecting Its Decay
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Brien, K.L.; Wolfson, L.J.; Watt, J.P.; Henkle, E.; Deloria-Knoll, M.; McCall, N.; Lee, E.; Mulholland, K.; Levine, O.S.; Cherian, T. Burden of Disease Caused by Streptococcus pneumoniae in Children Younger than 5 Years: Global Estimates. Lancet 2009, 374, 893–902. [Google Scholar] [CrossRef]
- Bogaert, D.; de Groot, R.; Hermans, P. Streptococcus pneumoniae Colonisation: The Key to Pneumococcal Disease. Lancet Infect. Dis. 2004, 4, 144–154. [Google Scholar] [CrossRef]
- Jedrzejas, M.J. Pneumococcal Virulence Factors: Structure and Function. Microbiol. Mol. Biol. Rev. 2001, 65, 187–207. [Google Scholar] [CrossRef] [Green Version]
- Barocchi, M.A.; Ries, J.; Zogaj, X.; Hemsley, C.; Albiger, B.; Kanth, A.; Dahlberg, S.; Fernebro, J.; Moschioni, M.; Masignani, V.; et al. A Pneumococcal Pilus Influences Virulence and Host Inflammatory Responses. Proc. Natl. Acad. Sci. USA 2006, 103, 2857–2862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeMieux, J.; Hava, D.L.; Basset, A.; Camilli, A. RrgA and RrgB Are Components of a Multisubunit Pilus Encoded by the Streptococcus pneumoniae rlrA Pathogenicity Islet. Infect. Immun. 2006, 74, 2453–2456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, A.L.; Ries, J.; Bagnoli, F.; Dahlberg, S.; Fälker, S.; Rounioja, S.; Tschöp, J.; Morfeldt, E.; Ferlenghi, I.; Hilleringmann, M.; et al. RrgA Is a Pilus-Associated Adhesin in Streptococcus pneumoniae. Mol. Microbiol. 2007, 66, 329–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilleringmann, M.; Giusti, F.; Baudner, B.C.; Masignani, V.; Covacci, A.; Rappuoli, R.; Barocchi, M.A.; Ferlenghi, I. Pneumococcal Pili Are Composed of Protofilaments Exposing Adhesive Clusters of Rrg A. PLoS Pathog. 2008, 4, e1000026. [Google Scholar] [CrossRef]
- Aguiar, S.I.; Serrano, I.; Pinto, F.R.; Melo-Cristino, J.; Ramirez, M. The Presence of the Pilus Locus Is a Clonal Property among Pneumococcal Invasive Isolates. BMC Microbiol. 2008, 8, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ton-That, H.; Schneewind, O. Assembly of Pili in Gram-Positive Bacteria. Trends Microbiol. 2004, 12, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.R.; Zähner, D. Pili with Strong Attachments: Gram-Positive Bacteria Do It Differently. Mol. Microbiol. 2006, 62, 320–330. [Google Scholar] [CrossRef]
- Hava, D.L.; Hemsley, C.J.; Camilli, A. Transcriptional Regulation in the Streptococcus pneumoniae rlrA Pathogenicity Islet by RlrA. J. Bacteriol. 2003, 185, 413–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagnoli, F.; Moschioni, M.; Donati, C.; Dimitrovska, V.; Ferlenghi, I.; Facciotti, C.; Muzzi, A.; Giusti, F.; Emolo, C.; Sinisi, A.; et al. A Second Pilus Type in Streptococcus pneumoniae Is Prevalent in Emerging Serotypes and Mediates Adhesion to Host Cells. J. Bacteriol. 2008, 190, 5480–5492. [Google Scholar] [CrossRef] [Green Version]
- Ness, S.; Hilleringmann, M. Streptococcus pneumoniae Type 1 Pilus–A Multifunctional Tool for Optimized Host Interaction. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef]
- Moschioni, M.; De Angelis, G.; Melchiorre, S.; Masignani, V.; Leibovitz, E.; Barocchi, M.A.; Dagan, R. Prevalence of Pilus-Encoding Islets among Acute Otitis Media Streptococcus pneumoniae Isolates from Israel. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2010, 16, 1501–1504. [Google Scholar] [CrossRef]
- Kulohoma, B.W.; Gray, K.; Kamng’ona, A.; Cornick, J.; Bentley, S.D.; Heyderman, R.S.; Everett, D.B. Piliation of Invasive Streptococcus pneumoniae Isolates in the Era before Pneumococcal Conjugate Vaccine Introduction in Malawi. Clin. Vaccine Immunol. 2013, 20, 1729–1735. [Google Scholar] [CrossRef] [Green Version]
- Hjálmarsdóttir, M.Á.; Pétursdóttir, B.; Erlendsdóttir, H.; Haraldsson, G.; Kristinsson, K.G. Prevalence of Pilus Genes in Pneumococci Isolated from Healthy Preschool Children in Iceland: Association with Vaccine Serotypes and Antibiotic Resistance. J. Antimicrob. Chemother. 2015, 70, 2203–2208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawaguchiya, M.; Urushibara, N.; Aung, M.S.; Shinagawa, M.; Takahashi, S.; Kobayashi, N. Serotype Distribution, Antimicrobial Resistance and Prevalence of Pilus Islets in Pneumococci Following the Use of Conjugate Vaccines. J. Med. Microbiol. 2017, 66, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Zähner, D.; Gudlavalleti, A.; Stephens, D.S. Increase in Pilus Islet 2–encoded Pili among Streptococcus pneumoniae Isolates, Atlanta, Georgia, USA. Emerg. Infect. Dis. 2010, 16, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Rosch, J.W.; Mann, B.; Thornton, J.; Sublett, J.; Tuomanen, E. Convergence of Regulatory Networks on the Pilus Locus of Streptococcus pneumoniae. Infect. Immun. 2008, 76, 3187–3196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orrskog, S.; Rounioja, S.; Spadafina, T.; Gallotta, M.; Norman, M.; Hentrich, K.; Fälker, S.; Ygberg-Eriksson, S.; Hasenberg, M.; Johansson, B.; et al. Pilus Adhesin RrgA Interacts with Complement Receptor 3, Thereby Affecting Macrophage Function and Systemic Pneumococcal Disease. mBio 2012, 4, e00535-512. [Google Scholar] [CrossRef] [Green Version]
- Iovino, F.; Hammarlöf, D.L.; Garriss, G.; Brovall, S.; Nannapaneni, P.; Henriques-Normark, B. Pneumococcal Meningitis Is Promoted by Single Cocci Expressing Pilus Adhesin RrgA. J. Clin. Investig. 2016, 126, 2821–2826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iovino, F.; Engelen-Lee, J.-Y.; Brouwer, M.; van de Beek, D.; van der Ende, A.; Valls Seron, M.; Mellroth, P.; Muschiol, S.; Bergstrand, J.; Widengren, J.; et al. pIgR and PECAM-1 Bind to Pneumococcal Adhesins RrgA and PspC Mediating Bacterial Brain Invasion. J. Exp. Med. 2017, 214, 1619–1630. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Elías, E.J.; Marcano, J.; Camilli, A. Isolation of Streptococcus pneumoniae Biofilm Mutants and Their Characterization during Nasopharyngeal Colonization. Infect. Immun. 2008, 76, 5049–5061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeMieux, J.; Woody, S.; Camilli, A. Roles of the Sortases of Streptococcus pneumoniae in Assembly of the RlrA Pilus. J. Bacteriol. 2008, 190, 6002–6013. [Google Scholar] [CrossRef] [Green Version]
- Fälker, S.; Nelson, A.L.; Morfeldt, E.; Jonas, K.; Hultenby, K.; Ries, J.; Melefors, O.; Normark, S.; Henriques-Normark, B. Sortase-Mediated Assembly and Surface Topology of Adhesive Pneumococcal Pili. Mol. Microbiol. 2008, 70, 595–607. [Google Scholar] [CrossRef] [Green Version]
- Manzano, C.; Contreras-Martel, C.; El Mortaji, L.; Izoré, T.; Fenel, D.; Vernet, T.; Schoehn, G.; Di Guilmi, A.M.; Dessen, A. Sortase-Mediated Pilus Fiber Biogenesis in Streptococcus pneumoniae. Struct. Lond. Engl. 1993 2008, 16, 1838–1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spraggon, G.; Koesema, E.; Scarselli, M.; Malito, E.; Biagini, M.; Norais, N.; Emolo, C.; Barocchi, M.A.; Giusti, F.; Hilleringmann, M.; et al. Supramolecular Organization of the Repetitive Backbone Unit of the Streptococcus pneumoniae Pilus. PLoS ONE 2010, 5, e10919. [Google Scholar] [CrossRef]
- Hilleringmann, M.; Ringler, P.; Müller, S.A.; De Angelis, G.; Rappuoli, R.; Ferlenghi, I.; Engel, A. Molecular Architecture of Streptococcus pneumoniae TIGR4 Pili. EMBO J. 2009, 28, 3921–3930. [Google Scholar] [CrossRef] [Green Version]
- Hendriksen, W.T.; Silva, N.; Bootsma, H.J.; Blue, C.E.; Paterson, G.K.; Kerr, A.R.; de Jong, A.; Kuipers, O.P.; Hermans, P.W.M.; Mitchell, T.J. Regulation of Gene Expression in Streptococcus pneumoniae by Response Regulator 09 Is Strain Dependent. J. Bacteriol. 2007, 189, 1382–1389. [Google Scholar] [CrossRef] [Green Version]
- Rosch, J.W.; Gao, G.; Ridout, G.; Wang, Y.-D.; Tuomanen, E.I. Role of the Manganese Efflux System mntE for Signalling and Pathogenesis in Streptococcus pneumoniae: Role of the Manganese Efflux System mntE. Mol. Microbiol. 2009, 72, 12–25. [Google Scholar] [CrossRef] [Green Version]
- Song, X.-M.; Connor, W.; Hokamp, K.; Babiuk, L.A.; Potter, A.A. The Growth Phase-Dependent Regulation of the Pilus Locus Genes by Two-Component System TCS08 in Streptococcus pneumoniae. Microb. Pathog. 2009, 46, 28–35. [Google Scholar] [CrossRef]
- Hemsley, C.; Joyce, E.; Hava, D.L.; Kawale, A.; Camilli, A. MgrA, an Orthologue of Mga, Acts as a Transcriptional Repressor of the Genes within the rlrA Pathogenicity Islet in Streptococcus pneumoniae. J. Bacteriol. 2003, 185, 6640–6647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbert, J.A.; Mitchell, A.M.; Mitchell, T.J. A Serine-Threonine Kinase (StkP) Regulates Expression of the Pneumococcal Pilus and Modulates Bacterial Adherence to Human Epithelial and Endothelial Cells In Vitro. PLoS ONE 2015, 10, e0127212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basset, A.; Herd, M.; Daly, R.; Dove, S.L.; Malley, R. The Pneumococcal Type 1 Pilus Genes Are Thermoregulated and Are Repressed by a Member of the Snf2 Protein Family. J. Bacteriol. 2017, 199, e00078-17. [Google Scholar] [CrossRef] [Green Version]
- Basset, A.; Turner, K.H.; Boush, E.; Sayeed, S.; Dove, S.L.; Malley, R. Expression of the Type 1 Pneumococcal Pilus Is Bistable and Negatively Regulated by the Structural Component RrgA. Infect. Immun. 2011, 79, 2974–2983. [Google Scholar] [CrossRef] [Green Version]
- Basset, A.; Turner, K.H.; Boush, E.; Sayeed, S.; Dove, S.L.; Malley, R. An Epigenetic Switch Mediates Bistable Expression of the Type 1 Pilus Genes in Streptococcus pneumoniae. J. Bacteriol. 2012, 194, 1088–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Angelis, G.; Moschioni, M.; Muzzi, A.; Pezzicoli, A.; Censini, S.; Delany, I.; Lo Sapio, M.; Sinisi, A.; Donati, C.; Masignani, V.; et al. The Streptococcus pneumoniae Pilus-1 Displays a Biphasic Expression Pattern. PLoS ONE 2011, 6, e21269. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Romero, M.A.; Casadesús, J. The bacterial epigenome. Nat. Rev. Microbiol. 2020, 18, 7–20. [Google Scholar] [CrossRef]
- Pancotto, L.; De Angelis, G.; Bizzarri, E.; Barocchi, M.A.; Del Giudice, G.; Moschioni, M.; Ruggiero, P. Expression of the Streptococcus pneumoniae Pilus-1 Undergoes on and off Switching during Colonization in Mice. Sci. Rep. 2013, 3, 2040. [Google Scholar] [CrossRef] [Green Version]
- Gottesman, S. Micros for Microbes: Non-Coding Regulatory RNAs in Bacteria. Trends Genet. TIG 2005, 21, 399–404. [Google Scholar] [CrossRef]
- Waters, L.S.; Storz, G. Regulatory RNAs in Bacteria. Cell 2009, 136, 615–628. [Google Scholar] [CrossRef] [Green Version]
- Gottesman, S.; Storz, G. Bacterial Small RNA Regulators: Versatile Roles and Rapidly Evolving Variations. Cold Spring Harb. Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toledo-Arana, A.; Repoila, F.; Cossart, P. Small Noncoding RNAs Controlling Pathogenesis. Curr. Opin. Microbiol. 2007, 10, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Acebo, P.; Martin-Galiano, A.J.; Navarro, S.; Zaballos, A.; Amblar, M. Identification of 88 Regulatory Small RNAs in the TIGR4 Strain of the Human Pathogen Streptococcus pneumoniae. RNA N. Y. N 2012, 18, 530–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halfmann, A.; Kovacs, M.; Hakenbeck, R.; Bruckner, R. Identification of the Genes Directly Controlled by the Response Regulator CiaR in Streptococcus pneumoniae: Five out of 15 Promoters Drive Expression of Small Non-Coding RNAs. Mol. Microbiol 2007, 66, 110–126. [Google Scholar] [CrossRef]
- Kumar, R.; Shah, P.; Swiatlo, E.; Burgess, S.C.; Lawrence, M.L.; Nanduri, B. Identification of Novel Non-Coding Small RNAs from Streptococcus pneumoniae TIGR4 Using High-Resolution Genome Tiling Arrays. BMC Genom. 2010, 11, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsui, H.C.; Mukherjee, D.; Ray, V.A.; Sham, L.T.; Feig, A.L.; Winkler, M.E. Identification and Characterization of Noncoding Small RNAs in Streptococcus pneumoniae Serotype 2 Strain D39. J. Bacteriol 2010, 192, 264–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, B.; van Opijnen, T.; Wang, J.; Obert, C.; Wang, Y.-D.; Carter, R.; McGoldrick, D.J.; Ridout, G.; Camilli, A.; Tuomanen, E.I.; et al. Control of Virulence by Small RNAs in Streptococcus pneumoniae. PLoS Pathog. 2012, 8, e1002788. [Google Scholar] [CrossRef] [PubMed]
- Schnorpfeil, A.; Kranz, M.; Kovács, M.; Kirsch, C.; Gartmann, J.; Brunner, I.; Bittmann, S.; Brückner, R. Target Evaluation of the Non-Coding csRNAs Reveals a Link of the Two-Component Regulatory System CiaRH to Competence Control in Streptococcus pneumoniae R6. Mol. Microbiol. 2013, 89, 334–349. [Google Scholar] [CrossRef]
- Wilton, J.; Acebo, P.; Herranz, C.; Gómez, A.; Amblar, M. Small Regulatory RNAs in Streptococcus pneumoniae: Discovery and Biological Functions. Front. Genet. 2015, 6, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito, L.; Wilton, J.; Ferrándiz, M.J.; Gómez-Sanz, A.; de la Campa, A.G.; Amblar, M. Absence of tmRNA Has a Protective Effect against Fluoroquinolones in Streptococcus pneumoniae. Front. Microbiol. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Tettelin, H.; Nelson, K.E.; Paulsen, I.T.; Eisen, J.A.; Read, T.D.; Peterson, S.; Heidelberg, J.; DeBoy, R.T.; Haft, D.H.; Dodson, R.J.; et al. Complete Genome Sequence of a Virulent Isolate of Streptococcus pneumoniae. Science 2001, 293, 498–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Håvarstein, L.S.; Coomaraswamy, G.; Morrison, D.A. An Unmodified Heptadecapeptide Pheromone Induces Competence for Genetic Transformation in Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA 1995, 92, 11140–11144. [Google Scholar] [CrossRef] [Green Version]
- Lau, G.W.; Haataja, S.; Lonetto, M.; Kensit, S.E.; Marra, A.; Bryant, A.P.; McDevitt, D.; Morrison, D.A.; Holden, D.W. A Functional Genomic Analysis of Type 3 Streptococcus pneumoniae Virulence. Mol. Microbiol. 2001, 40, 555–571. [Google Scholar] [CrossRef]
- Lacks, S.A.; Lopez, P.; Greenberg, B.; Espinosa, M. Identification and Analysis of Genes for Tetracycline Resistance and Replication Functions in the Broad-Host-Range Plasmid pLS1. J. Mol. Biol. 1986, 192, 753–765. [Google Scholar] [CrossRef]
- Weng, L.; Biswas, I.; Morrison, D.A. A Self-Deleting Cre-Lox-ermAM Cassette, Cheshire, for Marker-Less Gene Deletion in Streptococcus pneumoniae. J. Microbiol. Methods 2009, 79, 353–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Boto, D.; Acebo, P.; García-Peña, F.J.; Abad, J.C.; Echeita, M.A.; Amblar, M. Isolation of a Point Mutation Associated with Altered Expression of the CmeABC Efflux Pump in a Multidrug-Resistant Campylobacter jejuni Population of Poultry Origin. J. Glob. Antimicrob. Resist. 2015, 3, 115–122. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods San Diego Calif 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Moreira, R.N.; Domingues, S.; Viegas, S.C.; Amblar, M.; Arraiano, C.M. Synergies between RNA Degradation and Trans-Translation in Streptococcus pneumoniae: Cross Regulation and Co-Transcription of RNase R and SmpB. BMC Microbiol. 2012, 12, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wayne, K.J.; Sham, L.-T.; Tsui, H.-C.T.; Gutu, A.D.; Barendt, S.M.; Keen, S.K.; Winkler, M.E. Localization and Cellular Amounts of the WalRKJ (VicRKX) Two-Component Regulatory System Proteins in Serotype 2 Streptococcus pneumoniae. J. Bacteriol. 2010, 192, 4388–4394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, E.G.; Vogel, J. Approaches to Identify Novel Non-messenger RNAs in Bacteria and to Investigate their Biological Functions: Functional Analysis of Identified Non-mRNAs. In Handbook of RNA Biochemistry; WILEYVCH Verlag GmbH & Co.: Weinheim, Germany, 2005; pp. 614–642. [Google Scholar]
- Reuter, J.S.; Mathews, D.H. RNAstructure: Software for RNA Secondary Structure Prediction and Analysis. BMC Bioinform. 2010, 11, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scharff, L.B.; Childs, L.; Walther, D.; Bock, R. Local Absence of Secondary Structure Permits Translation of mRNAs That Lack Ribosome-Binding Sites. PLoS Genet. 2011, 7, e1002155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caron, M.-P.; Lafontaine, D.A.; Massé, E. Small RNA-Mediated Regulation at the Level of Transcript Stability. RNA Biol. 2010, 7, 140–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storz, G.; Vogel, J.; Wassarman, K.M. Regulation by Small RNAs in Bacteria: Expanding Frontiers. Mol. Cell 2011, 43, 880–891. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Blanco, D.A.; Shell, S.S. Regulation of mRNA Stability During Bacterial Stress Responses. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Rehmsmeier, M. Fast and Effective Prediction of microRNA/target Duplexes. RNA 2004, 10, 1507–1517. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer, V.; Papenfort, K.; Lucchini, S.; Hinton, J.C.D.; Vogel, J. Coding Sequence Targeting by MicC RNA Reveals Bacterial mRNA Silencing Downstream of Translational Initiation. Nat. Struct. Mol. Biol. 2009, 16, 840–846. [Google Scholar] [CrossRef]
- Desnoyers, G.; Morissette, A.; Prévost, K.; Massé, E. Small RNA-Induced Differential Degradation of the Polycistronic mRNA iscRSUA. EMBO J. 2009, 28, 1551–1561. [Google Scholar] [CrossRef] [Green Version]
- Papenfort, K.; Bouvier, M.; Mika, F.; Sharma, C.M.; Vogel, J. Evidence for an Autonomous 5′ Target Recognition Domain in an Hfq-Associated Small RNA. Proc. Natl. Acad. Sci. 2010, 107, 20435–20440. [Google Scholar] [CrossRef] [Green Version]
- Obana, N.; Shirahama, Y.; Abe, K.; Nakamura, K. Stabilization of Clostridium perfringens Collagenase mRNA by VR-RNA-Dependent Cleavage in 5′ Leader Sequence: VR-RNA-Dependent Processing Activates colA. Mol. Microbiol. 2010, 77, 1416–1428. [Google Scholar] [CrossRef]
- Ramirez-Peña, E.; Treviño, J.; Liu, Z.; Perez, N.; Sumby, P. The Group A Streptococcus Small Regulatory RNA FasX Enhances Streptokinase Activity by Increasing the Stability of the Ska mRNA Transcript: FasX-Mediated Regulation of Streptokinase in GAS. Mol. Microbiol. 2010, 78, 1332–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, D.; Frick, J.; Clemmons, K.; Winkler, M.E.; De Lay, N.R. Pivotal Roles for Ribonucleases in Streptococcus pneumoniae Pathogenesis. bioRxiv 2021, 2021.05.04.442624. [Google Scholar] [CrossRef]
- Iovino, F.; Nannapaneni, P.; Henriques-Normark, B.; Normark, S. The Impact of the Ancillary pilus-1 Protein RrgA of Streptococcus pneumoniae on Colonization and Disease. Mol. Microbiol. 2020, 113, 650–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Bacterial Strain | Description | Source |
|---|---|---|
| TIGR4 | Serotype 4 clinical isolate | [52] |
| TIGR4Δ135 | TIGR4 srn135::lox72 | This study |
| TIGR4ΔrlrA | TIGR4 rlrA::lox72 | This study |
| TIGR4Δ rrgA | TIGR4 rrgA::lox72 | This study |
| TIGR4(ROM) | TIGR4 [pROM] | This study |
| TIGR4(pveg135) | TIGR4 [pROM-PvegT-135] | This study |
| R6 | Non-encapsulated strain derived from the capsular type 2 clinical isolate strain D39 | Laboratory collection |
| R6rlrA | R6 bgaA::pTP2-rlrA | This study |
| R6rlrA(ROM) | R6 bgaA::pTP2-rlrA[pROM] | This study |
| R6rlrA(pveg135) | R6 bgaA::pTP2-rlrA[pveg135] | This study |
| Plasmids | ||
| pTP2 | Integrative plasmid catalyzing integration in the loci spr0564 and bgaA of the R6 genome | [49] |
| pTP3 | Integrative plasmid derived from pTP2 | [49] |
| pROM | pLS1ROM lacking PM promoter | [51] |
| pTP3-135 | pTP3 containing a 197 bp fragment of the rrgA 5′-UTR | This study |
| pROM-PvegT-135 | pROM containing the srn135 coding sequence under the control of PvegT promoter derived from pTP3-135 | This study |
| pTP2-rlrA | pTP2 containing rlrA coding sequence | This study |
| Primer | Nucleotide Sequence 5′ to 3′ | Application |
|---|---|---|
| 135-F | TTGCCAGGTTGAGAAGATAGC | RT/Northern blot |
| 135-R | CACGAAGAAACGGATTACTTATGTT | RT/Northern blot |
| rlrA-F1 | TGGAAGTATGGATTGGGTCA | RT/Northern blot |
| rlrA-R1 | TGTTGGCATGTGGCTCTAAG | RT/Northern blot |
| rlrA-F2 | GACAAAGTTGCCTCTGTTACA | RT |
| rlrA-R2 | CTGATAGATGAGACGCTGT | RT |
| rrgA-F1 | CCGCTGGATGTCGTTATCTT | RT |
| rrgA-R1 | CATTCAATCGCTTTCCGTTT | RT |
| rrgA-F2 | CAATGGTCGAACAACCTTAC | RT |
| rrgA-R2 | GTCAACTTACCATCTTCACCT | RT |
| rrgB-F | ATTGCCGGTGTTATGTTCGT | RT |
| rrgB-R | GCTGGTAAATTTGCCGTGTT | RT |
| srtB-F | AAAAGCAACGTTGGATGAGG | RT |
| srtB-R | GTCAATAACGGGGATTTCCA | RT |
| 16s-F | AGCGTTGTCCGGATTTATTG | RT |
| 16S-R | CATTTCACCGCTACACATGG | RT |
| Primer9 | GGGGACGCGTTGGCTTACCGTTCGTATAG | KO construction |
| Primer10 | GGGGCCATGGTCGATACCGTTCGTATAATGT | KO construction/5′-RACE |
| 135KO-Mlu | CGCGACGCGTATTACTATTAACTATCCTAGTATAAATTAAA | KO construction |
| 13KO-Nco | CGCGCCATGGTACCTATGAATCATAGAAGGAT | KO construction |
| rlrAKO-Mlu | CGCGACGCGTCTCATCTATCAGACAA | KO construction/5′-RACE |
| rlrAKO-Nco | CGCGCCATGGAATTTCCGACTTTATT | KO construction |
| rrgAKO-Mlu | CGCGACGCGTGCCTTCTGAAATATCTTTC | KO construction/5′-RACE |
| rrgAKO-Nco | CGCGCCATGGGAGGAGTTCTATTATACAC | KO construction |
| rlrAup-F | ACGTCTGTTATCAAGAATGGTCA | KO construction |
| rrgAdown-R | AACTTGGTGGTTCACAGGTGTAT | KO construction |
| 135up-F | TTTGGACTCAGGGAACTCAAGT | KO construction |
| 135down-R | TTGGTATTGGTTGTAAAACTCGTCT | KO construction |
| 135F-blunt | GTTAATAGTAATACTATACTATACTATATTGTATACAAGT | Cloning of srn135 |
| 135R-Bam | CGCGGATCCTTCTATGATTCATAGGTACTTTC | Cloning of srn135 |
| PvegF-Hind | CGCAAGCTTTGCATGCTTGGACTCC | Cloning of srn135 |
| rrgA-RT | GACTGGTTTCAGGCGTTTCT | 5′-RACE |
| RNA adaptor | GAUAUGCGCGAAUUCCUGUAGAACGAACACUAGAAGAAA | 5′-RACE |
| RACE-5′ | GATATGCGCGAATTCCTGTAG | 5′-RACE |
| RACE-5′-inner | AATTCCTGTAGAACGAACACTAGAA | 5′-RACE |
| RT-RACE | ACAGCACAGTCCTGCAACTG | 5′-RACE |
| wt-inner | ACAGTCCTGCAACTGCCTTC | 5′-RACE |
| rlrA-Sph | GCGCGGCATGCGATATTTTTATCACATATTTTTTTATAGAACGAC | R6rlrA construction |
| rlrA-Bam | CGCGGATCCTTATGGGACTTTTTTGATACTC | R6rlrA construction |
| SP6 | ATTTAGGTGACACTATAG | Sequencing |
| T7 | TAATACGACTCACTATAGGG | Sequencing |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acebo, P.; Herranz, C.; Espenberger, L.B.; Gómez-Sanz, A.; Terrón, M.C.; Luque, D.; Amblar, M. A Small Non-Coding RNA Modulates Expression of Pilus-1 Type in Streptococcus pneumoniae. Microorganisms 2021, 9, 1883. https://doi.org/10.3390/microorganisms9091883
Acebo P, Herranz C, Espenberger LB, Gómez-Sanz A, Terrón MC, Luque D, Amblar M. A Small Non-Coding RNA Modulates Expression of Pilus-1 Type in Streptococcus pneumoniae. Microorganisms. 2021; 9(9):1883. https://doi.org/10.3390/microorganisms9091883
Chicago/Turabian StyleAcebo, Paloma, Cristina Herranz, Lucas Bernal Espenberger, Alicia Gómez-Sanz, María Carmen Terrón, Daniel Luque, and Mónica Amblar. 2021. "A Small Non-Coding RNA Modulates Expression of Pilus-1 Type in Streptococcus pneumoniae" Microorganisms 9, no. 9: 1883. https://doi.org/10.3390/microorganisms9091883






