ssc-miR-451 Regulates Porcine Primary Adipocyte Differentiation by Targeting ACACA
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Porcine Primary Adipocyte Culture and Cell Transfection
2.3. RNA Extraction and Quantitative Real-Time PCR
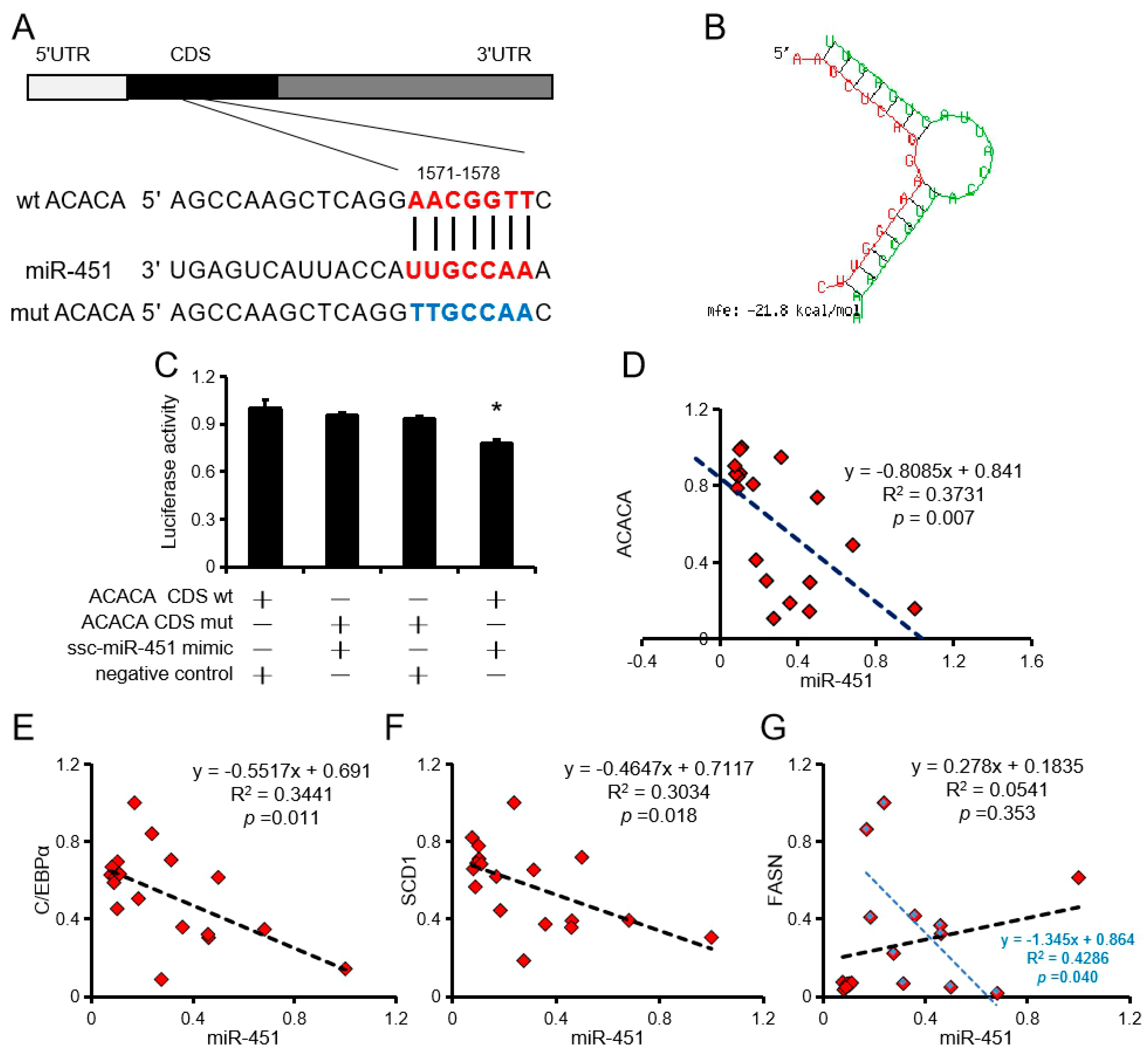
2.4. Prediction of the Binding Site of miR-451 and ACACA
2.5. Luciferase Reporter Assay
2.6. Oil Red O Staining
2.7. Measurement of Meat Quality
2.8. Chemical Composition
2.9. Analysis of Fatty Acids
2.10. Statistical Analysis
3. Results
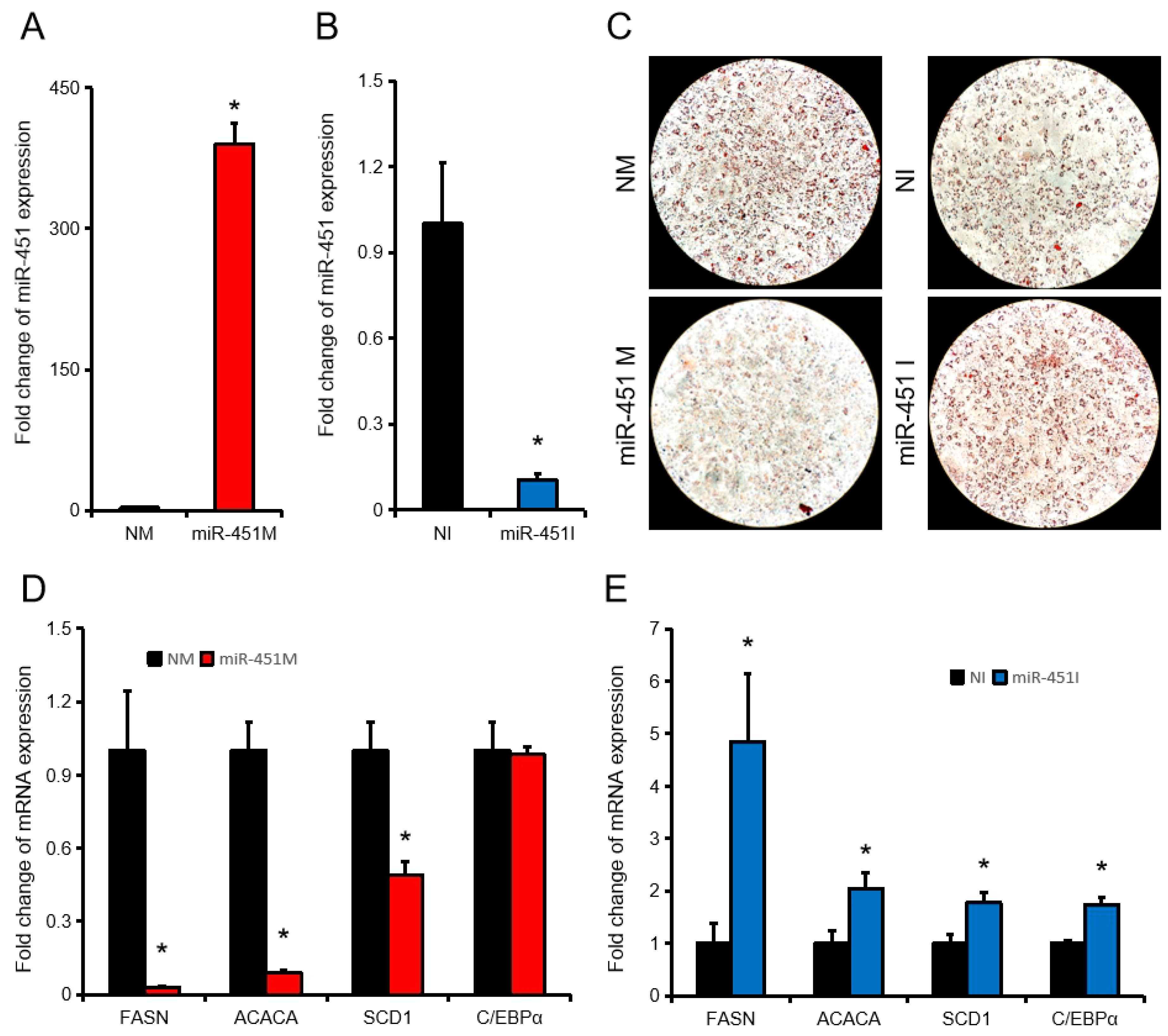
3.1. Downregulation of ssc-miR-451 during Porcine Primary Adipocyte Differentiation
3.2. ssc-miR-451 Inhibits Porcine Pre-Adipocyte Differentiation
3.3. ssc-miR-451 Tragets ACACA in Porcine
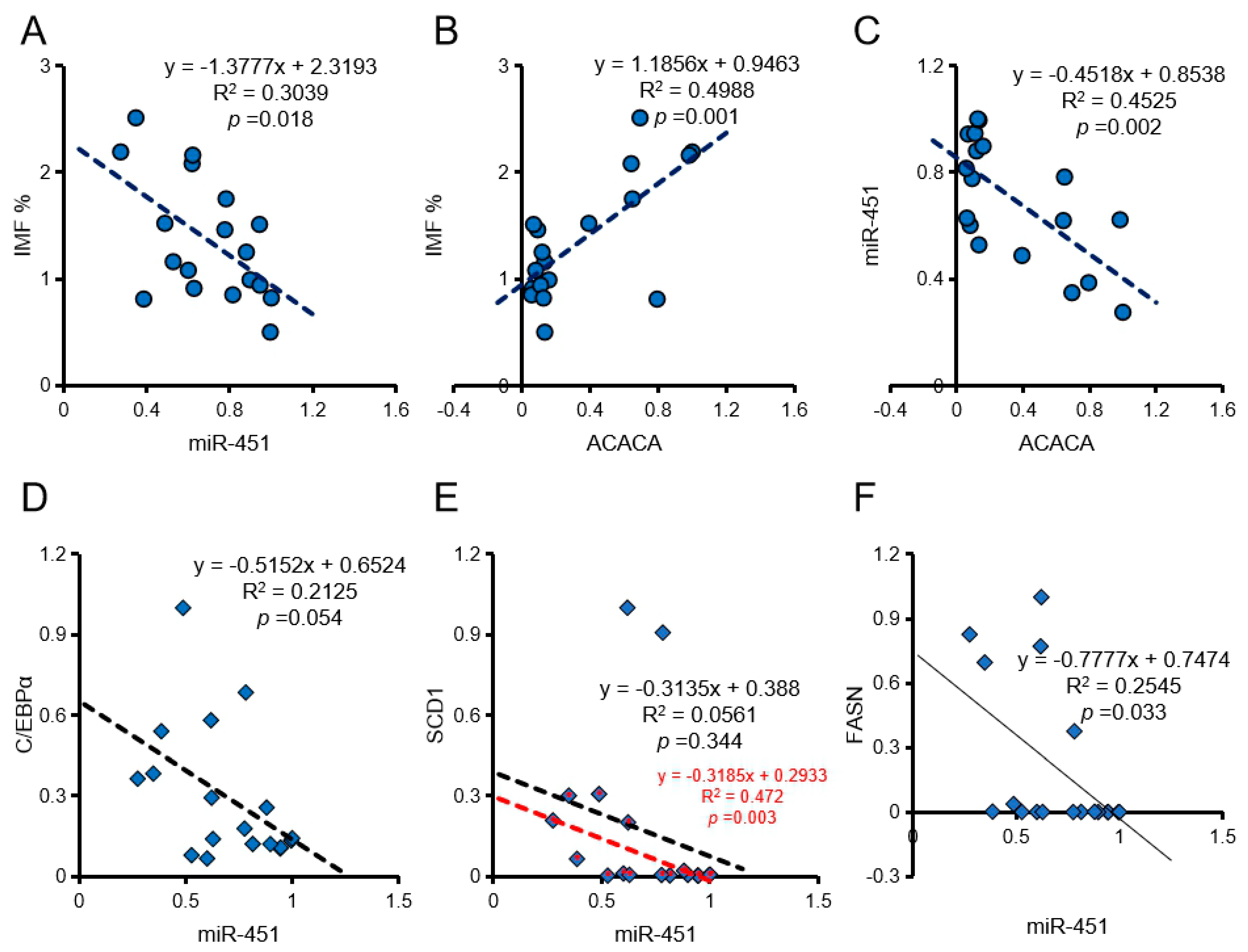
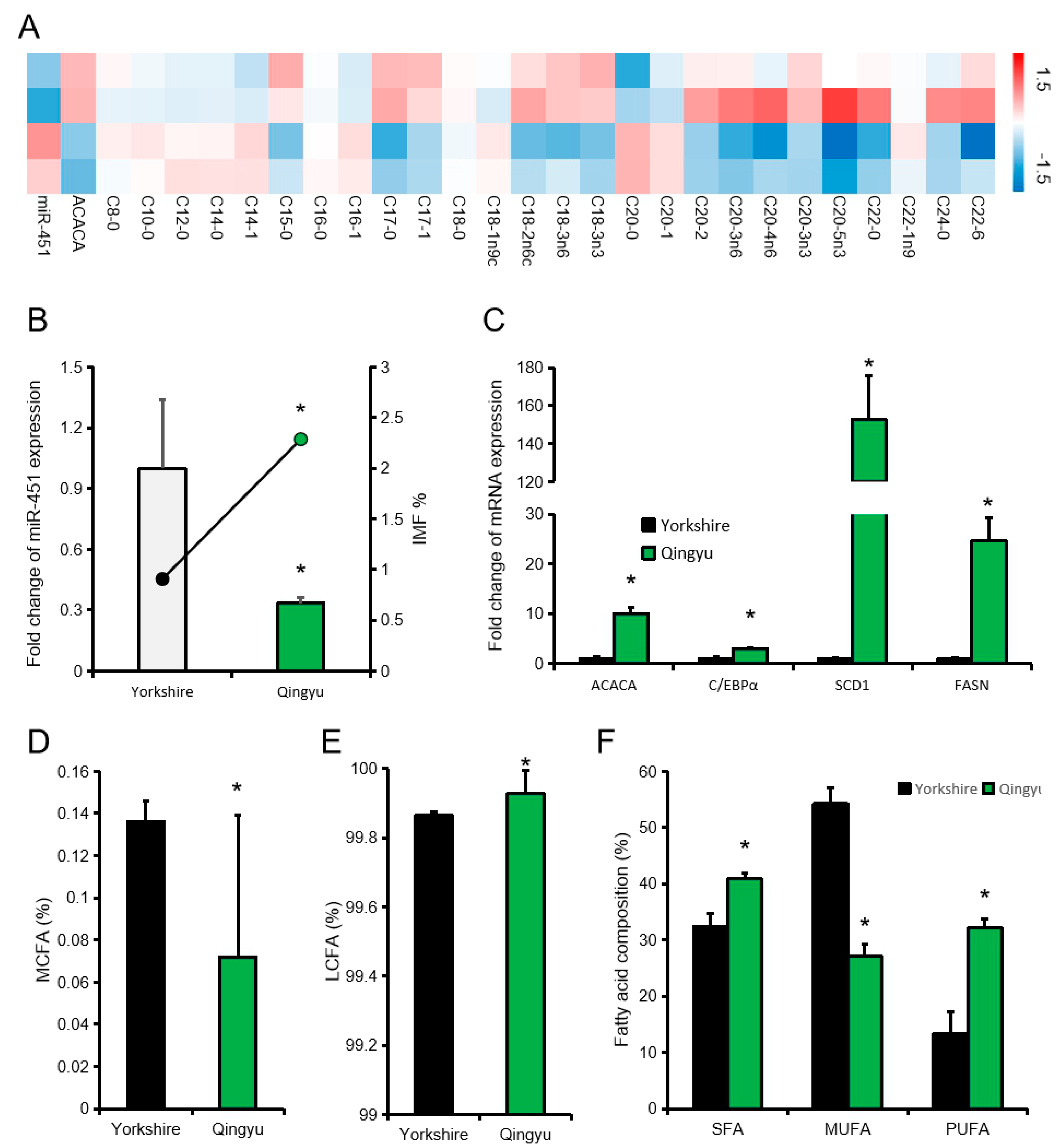
3.4. Correlation of ssc-miR-451 and ACACA with Pork Quality Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Meier, U.; Gressner, A.M. Endocrine Regulation of Energy Metabolism: Review of Pathobiochemical and Clinical Chemical Aspects of Leptin, Ghrelin, Adiponectin, and Resistin. Clin. Chem. 2004, 50, 1511–1525. [Google Scholar] [CrossRef]
- Switonski, M.; Stachowiak, M.; Cieslak, J.; Bartz, M.; Grzes, M. Genetics of fat tissue accumulation in pigs: A comparative approach. J. Appl. Genet. 2010, 51, 153–168. [Google Scholar] [CrossRef]
- Rosen, E.; Hsu, C.; Wang, X.; Sakai, S.; Freeman, M.; Gonzalez, F.; Spiegelman, B. C/ebpalpha induces adipogenesis through ppargamma: A unified pathway. Genes Dev. 2002, 16, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Piórkowska, K.; Małopolska, M.; Ropka-Molik, K.; Szyndler-Nędza, M.; Wiechniak, A.; Żukowski, K.; Lambert, B.D.; Tyra, M. Evaluation of SCD, ACACA and FASN Mutations: Effects on Pork Quality and Other Production Traits in Pigs Selected Based on RNA-Seq Results. Animals 2020, 10, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corominas, J.; Ramayo-Caldas, Y.; Puig-Oliveras, A.; Estellé, J.; Castelló, A.; Alves, E.; Pena, R.N.; Ballester, M.; Folch, J.M. Analysis of porcine adipose tissue transcriptome reveals differences in de novo fatty acid synthesis in pigs with divergent muscle fatty acid composition. BMC Genom. 2013, 14, 843. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Fernández-Hernando, C. miRNA regulation of white and brown adipose tissue differentiation and function. Biochim. Biophys. Acta 2016, 1861, 2104–2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurizi, G.; Babini, L.; Della Guardia, L. Potential role of microRNAs in the regulation of adipocytes liposecretion and adipose tissue physiology. J. Cell. Physiol. 2018, 233, 9077–9086. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, L.; Wang, Y.; Ding, Y.; Chen, T.; Wang, Y.; Wang, H.; Li, Y.; Duan, K.; Chen, S.; et al. Involvement of miR-451 in resistance to paclitaxel by regulating YWHAZ in breast cancer. Cell Death Dis. 2017, 8, e3071. [Google Scholar] [CrossRef] [Green Version]
- Yin, P.; Peng, R.; Peng, H.; Yao, L.; Sun, Y.; Wen, L.; Wu, T.; Zhou, J.; Zhang, Z. MiR-451 Suppresses Cell Proliferation and Metastasis in A549 Lung Cancer Cells. Mol. Biotechnol. 2014, 57, 1–11. [Google Scholar] [CrossRef]
- Li, L.; Gao, R.; Yu, Y.; Kaul, Z.; Wang, J.; Kalra, R.S.; Zhang, Z.; Kaul, S.C.; Wadhwa, R. Tumor suppressor activity of miR-451: Identification of CARF as a new target. Sci. Rep. 2018, 8, 375. [Google Scholar] [CrossRef] [Green Version]
- Su, Z.; Zhao, J.; Rong, Z.; Geng, W.; Wang, Z. MiR-451, a potential prognostic biomarker and tumor suppressor for gastric cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 9154–9160. [Google Scholar] [PubMed]
- Xing, K.; Zhao, X.; Ao, H.; Chen, S.; Yang, T.; Tan, Z.; Wang, Y.; Zhang, F.; Liu, Y.; Ni, H.; et al. Transcriptome analysis of miRNA and mRNA in the livers of pigs with highly diverged backfat thickness. Sci. Rep. 2019, 9, 16740. [Google Scholar] [CrossRef] [PubMed]
- Gan, M.; Shen, L.; Fan, Y.; Guo, Z.; Liu, B.; Chen, L.; Tang, G.; Jiang, Y.; Li, X.; Zhang, S.; et al. High Altitude Adaptability and Meat Quality in Tibetan Pigs: A Reference for Local Pork Processing and Genetic Improvement. Animals 2019, 9, 1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.; Gan, M.; Fan, Y.; Li, L.; Zhong, Z.; Li, X.; Bai, L.; Zhao, Y.; Niu, L.; Shang, Y.; et al. miR-10b-5p regulates 3T3-L1 cells differentiation by targeting Apol6. Gene 2019, 687, 39–46. [Google Scholar] [CrossRef]
- Gan, M.; Du, J.; Shen, L.; Yang, D.; Jiang, A.; Li, Q.; Jiang, Y.; Tang, G.; Li, M.; Wang, J.; et al. miR-152 regulates the proliferation and differentiation of C2C12 myoblasts by targeting E2F3. In Vitro Cell. Dev. Biol. Anim. 2018, 54, 304–310. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gan, M.; Zheng, T.; Shen, L.; Tan, Y.; Fan, Y.; Shuai, S.; Bai, L.; Li, X.; Wang, J.; Zhang, S.; et al. Genistein reverses isoproterenol-induced cardiac hypertrophy by regulating mir-451/timp2. Biomed. Pharmacother. 2019, 112, 108618. [Google Scholar] [CrossRef]
- Luo, J.; Shen, Y.L.; Lei, G.H.; Zhu, P.K.; Jiang, Z.Y.; Bai, L.; Li, Z.M.; Tang, Q.G.; Li, W.X.; Zhang, H.S.; et al. Correlation between three glycometabolic-related hormones and muscle glycolysis, as well as meat quality, in three pig breeds. J. Sci. Food Agric. 2016, 97, 2706–2713. [Google Scholar] [CrossRef]
- Shen, L.; Lei, H.; Zhang, S.; Li, X.; Li, M.; Jiang, X.; Zhu, K.; Zhu, L. The comparison of energy metabolism and meat quality among three pig breeds. Anim. Sci. J. 2014, 85, 770–779. [Google Scholar] [CrossRef]
- Peng, J.; Liu, Z.; Zhou, Y.; Wei, H.; Zhang, X.; Xia, M.; Deng, Z.; Zou, Y.; Jiang, S.; Peng, J. Effect of oregano essential oil supplementation to a reduced-protein, amino acid-supplemented diet on meat quality, fatty acid composition, and oxidative stability of Longissimus thoracis muscle in growing-finishing pigs. Meat Sci. 2017, 133, 103–109. [Google Scholar] [CrossRef]
- Sheng, X.; Wang, L.; Ni, H.; Wang, L.; Qi, X.; Xing, S.; Guo, Y. Comparative analyses between skeletal muscle miRNAomes from large white and min pigs revealed MicroRNAs associated with postnatal muscle hypertrophy. PLoS ONE 2016, 11, e0156780. [Google Scholar] [CrossRef] [PubMed]
- Hüttenhofer, A.; Brosius, J.; Bachellerie, J.-P. RNomics: Identification and function of small, non-messenger RNAs. Curr. Opin. Chem. Biol. 2002, 6, 835–843. [Google Scholar] [CrossRef]
- Pu, M.; Chen, J.; Tao, Z.; Miao, L.; Qi, X.; Wang, Y.; Ren, J. Regulatory network of miRNA on its target: Coordination between transcriptional and post-transcriptional regulation of gene expression. Cell. Mol. Life Sci. 2018, 76, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Feng, L.; Yang, M.; Chen, Q.; Wang, H.; Wang, X.; Hou, J. Prognostic value of microRNA-451 in various cancers: A meta-analysis. Pathol. Res. Pract. 2019, 215, 152726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henriquez-Rodriguez, E.; Bosch, L.; Tor, M.; Pena, R.N.; Estany, J. The effect of SCD and LEPR genetic polymorphisms on fat content and composition is maintained throughout fattening in Duroc pigs. Meat Sci. 2016, 121, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-H. Regulation of mammalian acetyl-coenzyme a carboxylase. Annu. Rev. Nutr. 1997, 17, 77–99. [Google Scholar] [CrossRef]
- Benítez, R.; Núñez, Y.; Fernández, A.I.; Isabel, B.; Rodríguez, M.D.C.; Barragán, C.; Palomino, P.M.; Lopez-Bote, C.; Silió, L.; Óvilo, C. Effects of dietary fat saturation on fatty acid composition and gene transcription in different tissues of Iberian pigs. Meat Sci. 2015, 102, 59–68. [Google Scholar] [CrossRef]
- Tan, B.; Yin, J.; Liu, Z.; Tang, W.; Xu, H.; Kong, X.; Li, X.; Yao, K.; Gu, W.; Smith, S.B.; et al. Dietary l-arginine supplementation differentially regulates expression of lipid-metabolic genes in porcine adipose tissue and skeletal muscle. J. Nutr. Biochem. 2011, 22, 441–445. [Google Scholar] [CrossRef]
- Sobol, M.; Krawczyńska, A.; Skiba, G.; Raj, S.; Weremko, D.; Herman, A.P. The effect of breed and feeding level on carcass composition, fatty acid profile and expression of genes encoding enzymes involved in fat metabolism in two muscles of pigs fed a diet enriched in n-3 fatty acids. A preliminary study. J. Anim. Feed. Sci. 2015, 24, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Ponsuksili, S.; Murani, E.; Brand, B.; Schwerin, M.; Wimmers, K. Integrating expression profiling and whole-genome association for dissection of fat traits in a porcine model. J. Lipid Res. 2011, 52, 668–678. [Google Scholar] [CrossRef] [Green Version]
- Ropka-Molik, K.; Pawlina-Tyszko, K.; Żukowski, K.; Tyra, M.; Derebecka, N.; Wesoły, J.; Szmatoła, T.; Piórkowska, K. Identification of Molecular Mechanisms Related to Pig Fatness at the Transcriptome and miRNAome Levels. Genes 2020, 11, 600. [Google Scholar] [CrossRef] [PubMed]
- Girousse, A.; Gil-Ortega, M.; Bourlier, V.; Bergeaud, C.; Sastourné-Arrey, Q.; Moro, C.; Barreau, C.; Guissard, C.; Vion, J.; Arnaud, E.; et al. The Release of Adipose Stromal Cells from Subcutaneous Adipose Tissue Regulates Ectopic Intramuscular Adipocyte Deposition. Cell Rep. 2019, 27, 323–333.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Liu, S.-Y.; He, Y.-B.; Huang, R.-L.; Deng, S.-Y.; Ni, G.-X.; Yu, B. MiR-451 suppresses proliferation, migration and promotes apoptosis of the human osteosarcoma by targeting macrophage migration inhibitory factor. Biomed. Pharmacother. 2017, 87, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-Y.; Cui, J.-Y.; Yuan, J.; Wang, X. MiR-451a suppressed cell migration and invasion in non-small cell lung cancer through targeting ATF2. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5554–5561. [Google Scholar]
- Kim, E.-S.; Ros-Freixedes, R.; Pena, R.N.; Baas, T.J.; Estany, J.; Rothschild, M.F. Identification of signatures of selection for intramuscular fat and backfat thickness in two Duroc populations. J. Anim. Sci. 2015, 93, 3292–3302. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, R.; Estany, J.; Tor, M.; Doran, O. Hepatic lipogenic enzyme expression in pigs is affected by selection for decreased backfat thickness at constant intramuscular fat content. Meat Sci. 2013, 93, 746–751. [Google Scholar] [CrossRef]

| Gene | Primer Sequence (5′–3′) | TM/°C | Efficiency |
|---|---|---|---|
| C/EBPα | F-CAAGAACAGCAACGAGTACCG | 59 | 0.94 |
| R-GTCACTGGTCAACTCCAGCAC | |||
| FASN | F-TCGTGGGCTACAGCATGATA | 60.7 | 0.93 |
| R-TTAGGCTTCAGCAGGACGTT | |||
| SCD1 | F-ACACTTGGGAGCCCTGTATG | 60 | 0.91 |
| R-GGGCAGTCGAGCTTTGTAAG | |||
| ACACA | F-ACCTCTGGAGTTGAACCAGC | 60 | 0.96 |
| R-GTGTAAGGCCAAGCCATCCT | |||
| β-actin | F-AAGGACCTCTACGCCAACAC | 60 | 0.95 |
| R-CTGGCTGATCCACATCTGCT | |||
| ssc-miR-451 | F-AAACCGTTACCATTACTGAGTT | 60 | 0.92 |
| R-Uni-miR qPCR Primer, included in kit (miRNA Universal Downstream Primer, TaKaRa) | |||
| U6 | F-Uni-miR qPCR Primer, included in kit (TaKaRa) | 60 | 0.93 |
| R-Uni-miR qPCR Primer, included in kit (TaKaRa) |
| gene Name | L | a | b | Shear Force | Drip Loss | Cooking Loss | Marbling Score | Crude Protein | IMF 1 |
|---|---|---|---|---|---|---|---|---|---|
| miR-451 | 0.304 | −0.122 | 0.522 * | −0.033 | 0.123 | −0.518 * | −0.311 | −0.466 | −0.551 * |
| ACACA | −0.193 | 0.411 | −0.565 | −0.102 | 0.001 | 0.105 | 0.638 * | 0.409 | 0.706 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, M.; Shen, L.; Fan, Y.; Tan, Y.; Liu, L.; Chen, L.; Zhao, Y.; Niu, L.; Tang, G.; Li, Q.; et al. ssc-miR-451 Regulates Porcine Primary Adipocyte Differentiation by Targeting ACACA. Animals 2020, 10, 1891. https://doi.org/10.3390/ani10101891
Gan M, Shen L, Fan Y, Tan Y, Liu L, Chen L, Zhao Y, Niu L, Tang G, Li Q, et al. ssc-miR-451 Regulates Porcine Primary Adipocyte Differentiation by Targeting ACACA. Animals. 2020; 10(10):1891. https://doi.org/10.3390/ani10101891
Chicago/Turabian StyleGan, Mailin, Linyuan Shen, Yuan Fan, Ya Tan, Lin Liu, Lei Chen, Ye Zhao, Lili Niu, Guoqing Tang, Qiang Li, and et al. 2020. "ssc-miR-451 Regulates Porcine Primary Adipocyte Differentiation by Targeting ACACA" Animals 10, no. 10: 1891. https://doi.org/10.3390/ani10101891





