Fallow Deer (Dama dama) as a Reservoir of Shiga Toxin-Producing Escherichia coli (STEC)
Abstract
:Simple Summary
Abstract
1. Introduction
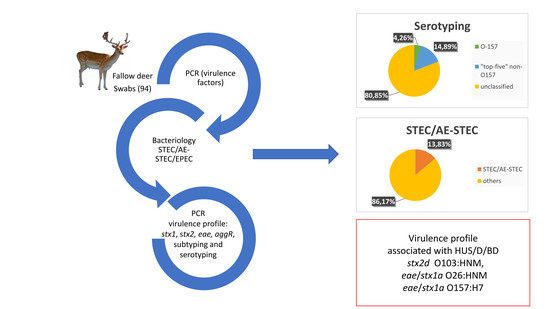
2. Materials and Methods
2.1. Sampling
2.2. Detection of STEC Strains and stx1 and stx2 Subtypes
2.3. Serotyping by Polymerase Chain Reaction (PCR)
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO/WHO. Shiga toxin-producing Escherichia coli (STEC) and food: Attribution, characterization, and monitoring. Microbiological Risk Assessment Series, No. 31, Food and Agriculture Organization of the United Nations and World Health Organization. 2018. Available online: https://apps.who.int/iris/handle/10665/272871 (accessed on 20 September 2019).
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17. [Google Scholar] [CrossRef] [Green Version]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Mellmann, A.; Zhang, W.; Koeck, R.; Fruth, A.; Bauwens, A.; Peters, G.; Karch, H. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: A microbiological study. Lancet Infect. Dis. 2011, 11, 671–676. [Google Scholar] [CrossRef] [Green Version]
- Scheutz, F.; Teel, L.D.; Beutin, L.; Pierard, D.; Buvens, G.; Karch, H.; Mellmann, A.; Caprioli, A.; Tozzoli, R.; Morabito, S.; et al. Multicenter Evaluation of a Sequence-Based Protocol for Subtyping Shiga Toxins and Standardizing Stx Nomenclature. J. Clin. Microbiol. 2012, 50, 2951–2963. [Google Scholar] [CrossRef] [Green Version]
- Asakura, H.; Boisen, N.; Chinen, I.; Cook, R.; Dallman, T.; Devleesschauwer, B.; Feng, P.; Franz, E.; Fratamico, P.; Gill, A.; et al. Hazard Identification and Characterization: Criteria for Categorizing Shiga Toxin-Producing Escherichia coli on a Risk Basis. J. Food Prot. 2019, 82, 7–21. [Google Scholar]
- Haddad, N.; Johnson, N.; Kathariou, S.; Metris, A.; Phister, T.; Pielaat, A.; Tassou, C.; Wells-Bennikh, M.H.J.; Zwietering, M.H. Next generation microbiological risk assessment-Potential of omics data for hazard characterisation. Int. J. Food Microbiol. 2018, 287, 28–39. [Google Scholar] [CrossRef]
- Caprioli, A.; Morabito, S.; Brugere, H.; Oswald, E. Enterohaemorrhagic Escherichia coli: Emerging issues on virulence and modes of transmission. Vet. Res. 2005, 36, 289–311. [Google Scholar] [CrossRef] [Green Version]
- Dias, D.; Caetano, T.; Torres, R.T.; Fonseca, C.; Mendo, S. Shiga toxin-producing Escherichia coli in wild ungulates. Sci. Total Environ. 2019, 651, 203–209. [Google Scholar] [CrossRef]
- Diaz-Sanchez, S.; Sanchez, S.; Herrera-Leon, S.; Porrero, C.; Blanco, J.; Dahbi, G.; Blanco, J.E.; Mora, A.; Mateo, R.; Hanning, I.; et al. Prevalence of Shiga toxin-producing Escherichia coli, Salmonella spp. and Campylobacter spp. in large game animals intended for consumption: Relationship with management practices and livestock influence. Vet. Microbiol. 2013, 163, 274–281. [Google Scholar] [CrossRef]
- Frank, E.; Bonke, R.; Drees, N.; Heurich, M.; Martlbauer, E.; Gareis, M. Shiga toxin-producing Escherichia coli (STEC) shedding in a wild roe deer population. Vet. Microbiol. 2019, 239, 8. [Google Scholar] [CrossRef]
- Garcia-Sanchez, A.; Sanchez, S.; Rubio, R.; Pereira, G.; Alonso, J.M.; de Mendoza, J.H.; Rey, J. Presence of Shiga toxin-producing E-coli O157: H7 in a survey of wild artiodactyls. Vet. Microbiol. 2007, 121, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.; Garcia-Sanchez, A.; Martinez, R.; Blanco, J.; Blanco, J.E.; Blanco, M.; Dahbi, G.; Mora, A.; de Mendoza, J.H.; Alonso, J.M.; et al. Detection and characterisation of Shiga toxin-producing Escherichia coli other than Escherichia coli O157:H7 in wild ruminants. Vet. J. 2009, 180, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.; Martinez, R.; Garcia, A.; Vidal, D.; Blanco, J.; Blanco, M.; Blanco, J.E.; Mora, A.; Herrera-Leon, S.; Echeita, A.; et al. Detection and characterisation of O157:H7 and non-O157 Shiga toxin-producing Escherichia coli in wild boars. Vet. Microbiol. 2010, 143, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Szczerba-Turek, A.; Siemionek, J.; Socha, P.; Bancerz-Kisiel, A.; Platt-Samoraj, A.; Lipczynska-Ilczuk, K.; Szweda, W. Shiga toxin-producing Escherichia coli isolates from red deer (Cervus elaphus), roe deer (Capreolus capreolus) and fallow deer (Dama dama) in Poland. Food Microbiol. 2020, 86, 7. [Google Scholar] [CrossRef]
- Szczerba-Turek, A.; Socha, P.; Bancerz-Kisiel, A.; Platt-Samoraj, A.; Lipczynska-Ilczuk, K.; Siemionek, J.; Konczyk, K.; Terech-Majewska, E.; Szweda, W. Pathogenic potential to humans of Shiga toxin-producing Escherichia coli isolated from wild boars in Poland. Int. J. Food Microbiol. 2019, 300, 8–13. [Google Scholar] [CrossRef]
- Regulation of the Minister of the Environment of 16 March 2005 on the definition of hunting periods for wildlife, Polish Law Gazette no. 48. 459, 2005. Internet System of Legal Documents. Available online: http://prawo.sejm.gov.pl/isap.nsf/download.xsp/WDU20050480459/O/D20050459.pdf (accessed on 30 April 2020). (In Polish)
- Regulation of the Minister of the Environment of 1 August 2017 amending the Regulation of the Minister of the Environment of 16 March 2005 on the definition of hunting periods for wildlife. Polish Law Gazette 1487, 2017. Internet System of Legal Documents. Available online: http://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20170001487/O/D20171487.pdf (accessed on 30 April 2020). (In Polish)
- European Union Reference Laboratory for E. coli. Identification and characterisation of Verotoxin-producing Escherichia coli (VTEC) by PCR amplification of the main virulence genes. 2013a. Available online: http://old.iss.it/binary/vtec/cont/EU_RL_VTEC_Method_01_Rev_0.pdf (accessed on 20 June 2017).
- Paton, A.W.; Paton, J.C. Detection and characterization of shiga toxigenic Escherichia coli by using multiplex PCR assays for stx(1), stx(2), eaeA, enterohemorrhagic E-coli hlyA, rfb(O111), and rfb(O157). J. Clin. Microbiol. 1998, 36, 598–602. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, H.; Scheef, J.; Morabito, S.; Caprioli, A.; Wieler, L.H.; Karch, H. A new Shiga toxin 2 variant (Stx2f) from Escherichia coli isolated from pigeons. Appl. Environ. Microbiol. 2000, 66, 1205–1208. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, H.; Plaschke, B.; Franke, S.; Russmann, H.; Schwarzkopf, A.; Heesemann, J.; Karch, H. Differentiation in virulenece patternes of Escherichia coli possessing eae genes. Med. Microbiol. Immun. 1994, 183, 23–31. [Google Scholar] [CrossRef]
- European Union Reference Laboratory for E. coli. Identification of VTEC serogroups mainly associated with human infections by conventional PCR amplification of O-associated genes. 2013b. Available online: http://old.iss.it/binary/vtec/cont/EU_RL_VTEC_Method_03_Rev_1.pdf (accessed on 20 June 2017).
- European Union Reference Laboratory for E. coli. Identification of the subtypes of Verocytotoxin encoding genes (vtx) of Escherichia coli by conventional PCR. 2013c. Available online: http://old.iss.it/binary/vtec/cont/EU_RL_VTEC_Method_06_Rev_1.pdf (accessed on 20 June 2017).
- Monday, S.R.; Beisaw, A.; Feng, P.C.H. Identification of Shiga toxigenic Escherichia coli seropathotypes A and B by multiplex PCR. Mol. Cell. Probe. 2007, 21, 308–311. [Google Scholar] [CrossRef]
- Durso, L.M.; Bono, J.L.; Keen, J.E. Molecular serotyping of Escherichia coli O26:H11. Appl. Environ. Microbiol. 2005, 71, 4941–4944. [Google Scholar] [CrossRef] [Green Version]
- Gannon, V.P.J.; Dsouza, S.; Graham, T.; King, R.K.; Rahn, K.; Read, S. Use of the flagellar H7 gene as a target in multiplex PCR assays and improved specificity in identification of enterohemorrhagic Escherichia coli strains. J. Clin. Microbiol. 1997, 35, 656–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora, A.; Lopez, C.; Dhabi, G.; Lopez-Beceiro, A.M.; Fidalgo, L.E.; Diaz, E.A.; Martinez-Carrasco, C.; Mamani, R.; Herrera, A.; Blanco, J.E.; et al. Seropathotypes, Phylogroups, Stx Subtypes, and Intimin Types of Wildlife-Carried, Shiga Toxin-Producing Escherichia coli Strains with the Same Characteristics as Human-Pathogenic Isolates. App. Environ. Microbiol. 2012, 78, 2578–2585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, L.D.; Cai, T.T.; DasGupta, A.; Agresti, A.; Coull, B.A.; Casella, G.; Corcoran, C.; Mehta, C.; Ghosh, M.; Santner, T.J. Interval estimation for a binomial proportion—Comment—Rejoinder. Stat. Sci. 2001, 16, 101–133. [Google Scholar]
- Gibbs, E.P.J. The evolution of One Health: A decade of progress and challenges for the future. Vet. Record 2014, 174, 85–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruegg, S.R.; McMahon, B.J.; Hasler, B.; Esposito, R.; Nielsen, L.R.; Speranza, C.I.; Ehlinger, T.; Peyre, M.; Aragrande, M.; Zinsstag, J.; et al. A Blueprint to evaluate One Health. Front. Public Health 2017, 5, 20. [Google Scholar] [CrossRef]
- Zinsstag, J.; Schelling, E.; Wyss, K.; Mahamat, M.B. Potential of cooperation between human and animal health to strengthen health systems. Lancet 2005, 366, 2142–2145. [Google Scholar] [CrossRef]
- Diaz-Sanchez, S.; Sanchez, S.; Sanchez, M.; Herrera-Leon, S.; Hanning, I.; Vidal, D. Detection and characterization of Shiga toxin-producing Escherichia coli in game meat and ready-to-eat meat products. Int. J. Food Microbiol. 2012, 160, 179–182. [Google Scholar] [CrossRef]
- Espinosa, L.; Gray, A.; Duffy, G.; Fanning, S.; McMahon, B.J. A scoping review on the prevalence of Shiga-toxigenic Escherichia coli in wild animal species. Zoonoses Public Health 2018, 65, 911–920. [Google Scholar] [CrossRef] [Green Version]
- Langholz, J.A.; Jay-Russell, M.T. Potential role of wildlife in pathogenic contamination of fresh produce. Hum-Wildl Interact. 2013, 7, 140–157. [Google Scholar]
- Rounds, J.M.; Rigdon, C.E.; Muhl, L.J.; Forstner, M.; Danzeisen, G.T.; Koziol, B.S.; Taylor, C.; Shaw, B.T.; Short, G.L.; Smith, K.E. Non-O157 Shiga Toxin-producing Escherichia coli Associated with Venison. Emerg. Infect. Dis. 2012, 18, 279–282. [Google Scholar] [CrossRef]
- Sanno, A.; Jacobson, M.; Sterner, S.; Thisted-Lambertz, S.; Aspan, A. The development of a screening protocol for Salmonella spp. and enteropathogenic Yersinia spp. in samples from wild boar (Sus scrofa) also generating MLVA-data for Y. enterocolitica and Y. pseudotuberculosis. J. Microbiol. Meth. 2018, 150, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Sauvala, M.; Laaksonen, S.; Laukkanen-Ninios, R.; Jalava, K.; Stephan, R.; Fredriksson-Ahomaa, M. Microbial contamination of moose (Alces alces) and white-tailed deer (Odocoileus virginianus) carcasses harvested by hunters. Food microbiol. 2019, 78, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Smith-Palmeri, A.; Hawkinsi, G.; Browningi, L.; Allison, L.; Hanson, M.; Bruce, R.; McElhiney, J.; Horne, J.; Incident Management, T. Outbreak of Escherichia coli O157 Phage Type 32 linked to the consumption of venison products. Epidemiol. Infect. 2018, 146, 1922–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daszkiewicz, T.; Hnatyk, N.; Dabrowski, D.; Janiszewski, P.; Gugolek, A.; Kubiak, D.; Smiecinska, K.; Winarski, R.; Koba-Kowalczyk, M. A comparison of the quality of the Longissimus lumborum muscle from wild and farm-raised fallow deer (Dama dama L.). Small Ruminant Res. 2015, 129, 77–83. [Google Scholar] [CrossRef]
- Hoffman, L.C.; Wiklund, E. Game and venison—meat for the modern consumer. Meat Sci. 2006, 74, 197–208. [Google Scholar] [CrossRef]
- Zochowska-Kujawska, J.; Kotowicz, M.; Sobczak, M.; Lachowicz, K.; Wojcik, J. Age-related changes in the carcass composition and meat quality of fallow deer (Dama Dama). Meat Sci. 2019, 147, 37–43. [Google Scholar] [CrossRef]
- Bures, D.; Barton, L.; Kotrba, R.; Hakl, J. Quality attributes and composition of meat from red deer (Cervus elaphus), fallow deer (Dama dama) and Aberdeen Angus and Holstein cattle (Bos taurus). J. Sci. Food Agric. 2015, 95, 2299–2306. [Google Scholar] [CrossRef]
- Kudrnacova, E.; Barton, L.; Bures, D.; Hoffman, L.C. Carcass and meat characteristics from farm-raised and wild fallow deer (Dama dama) and red deer (Cervus elaphus): A review. Meat Sci. 2018, 141, 9–27. [Google Scholar] [CrossRef]
- Kwiecinska, K.; Kosicka-Gebska, M.; Gebski, J.; Gutkowska, K. Prediction of the conditions for the consumption of game by Polish consumers. Meat Sci. 2017, 131, 28–33. [Google Scholar] [CrossRef]
- Singh, P.; Sha, Q.; Lacher, D.W.; Del Valle, J.; Mosci, R.E.; Moore, J.A.; Scribner, K.T.; Manning, S.D. Characterization of enteropathogenic and Shiga toxin-producing Escherichia coli in cattle and deer in a shared agroecosystem. Front. Cell. Infect. Microbiol. 2015, 5, 29. [Google Scholar] [CrossRef] [Green Version]
- Regulation (EC) No. 853/2004, 2004. of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin. Official Journal of the European Union, L 139/55. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32004R0853&from=EN (accessed on 1 February 2019).
- Regulation (EC) No. 854/2004, 2004. of the European Parliament and of the Council of 29 April 2004 laying down specific rules for the organisation of official controls on products of animal origin intended for human consumption. Official Journal of the European Union, L 155/206. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32004R0854&from=en (accessed on 1 February 2019).
| Virulence Genes | Number of Samples | Number of Strains | stx1 Subtype (Number) | stx2 Subtype (Number) | Serogroup (Number) | Time of Collection Year/Month |
|---|---|---|---|---|---|---|
| STEC stx1 | 0 | -- | -- | -- | -- | |
| STEC stx2 | 4 | 2 | stx2a (1) stx2d (1) | ONT:HNM (1) O103:HNM (1) | 2019/Jan. 2018/Nov | |
| STEC stx1 stx2 | 2 | 2 | stx1a (1) stx1NS (1) | stx2NS (1) stx2b (1) | ONT:H7 (1) ONT:HNM (1) | 2019/Jan. 2018/Dec. |
| AE-STEC stx1 eae | 8 | 3 | stx1a (2) stx1NS (1) | O26:HNM (1), O157:H7 (1) O157:H7 (1) | 2018/Oct., 2018/Nov. 2018/Nov. | |
| AE-STEC stx2 eae | 11 | 4 | stx2b (2) stxNS (2) | O103:HNM (2) O103:HNM (1), O26:HNM (1) | 2017/Nov., 2018/Oct. 2018/Feb., 2018/Dec. | |
| AE-STEC stx1 stx2 eae | 4 | 2 | stx1NS (1) stx1NS (1) | stx2b (1) stxNS (1) | O103:HNM (1) O26:HNM (1) | 2019/Jan. 2018/Oct. |
| EPEC eae | 34 | 8 | O26:HNM (1), O103:HNM (2), O145:H7 (3), O157:H7 (1), O157:HNM (1) | 2018/Dec., 2018/Oct., 2018/Oct. 2018/Jan. (2), 2018/Feb., 2019/Jan. 2018/Nov. | ||
| Total | 63 | 21 | 7 | 10 | 21 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczerba-Turek, A.; Kordas, B. Fallow Deer (Dama dama) as a Reservoir of Shiga Toxin-Producing Escherichia coli (STEC). Animals 2020, 10, 881. https://doi.org/10.3390/ani10050881
Szczerba-Turek A, Kordas B. Fallow Deer (Dama dama) as a Reservoir of Shiga Toxin-Producing Escherichia coli (STEC). Animals. 2020; 10(5):881. https://doi.org/10.3390/ani10050881
Chicago/Turabian StyleSzczerba-Turek, Anna, and Bernard Kordas. 2020. "Fallow Deer (Dama dama) as a Reservoir of Shiga Toxin-Producing Escherichia coli (STEC)" Animals 10, no. 5: 881. https://doi.org/10.3390/ani10050881