Metabolic Flexibility in Canine Mammary Tumors: Implications of the Carnitine System
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Mammary Tissue Samples
2.3. Sample Preparation, Protein Extraction and Western Blot Analysis
2.4. Immunohistochemistry
Scoring of Immunoreactivity
2.5. Statistical Analysis
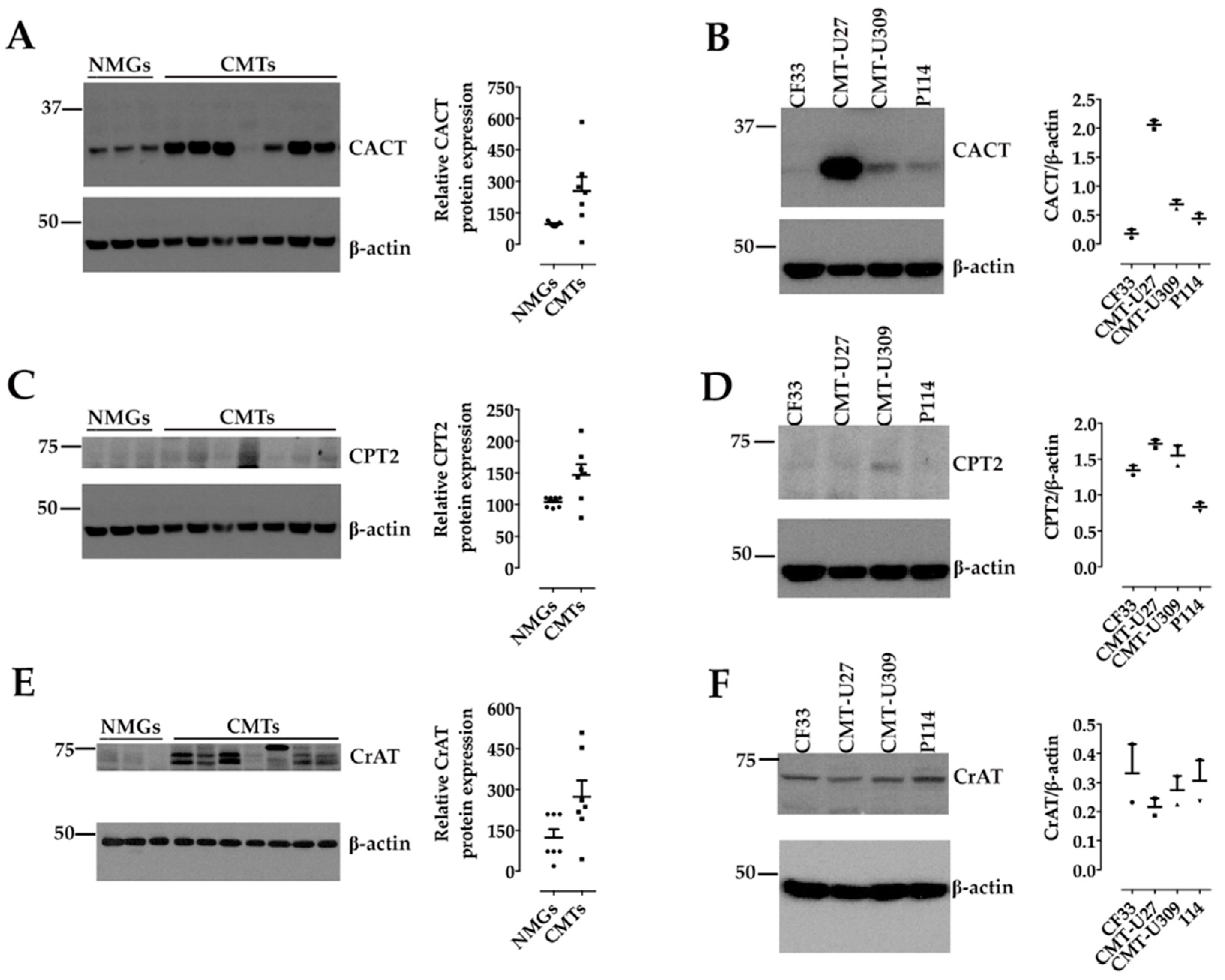
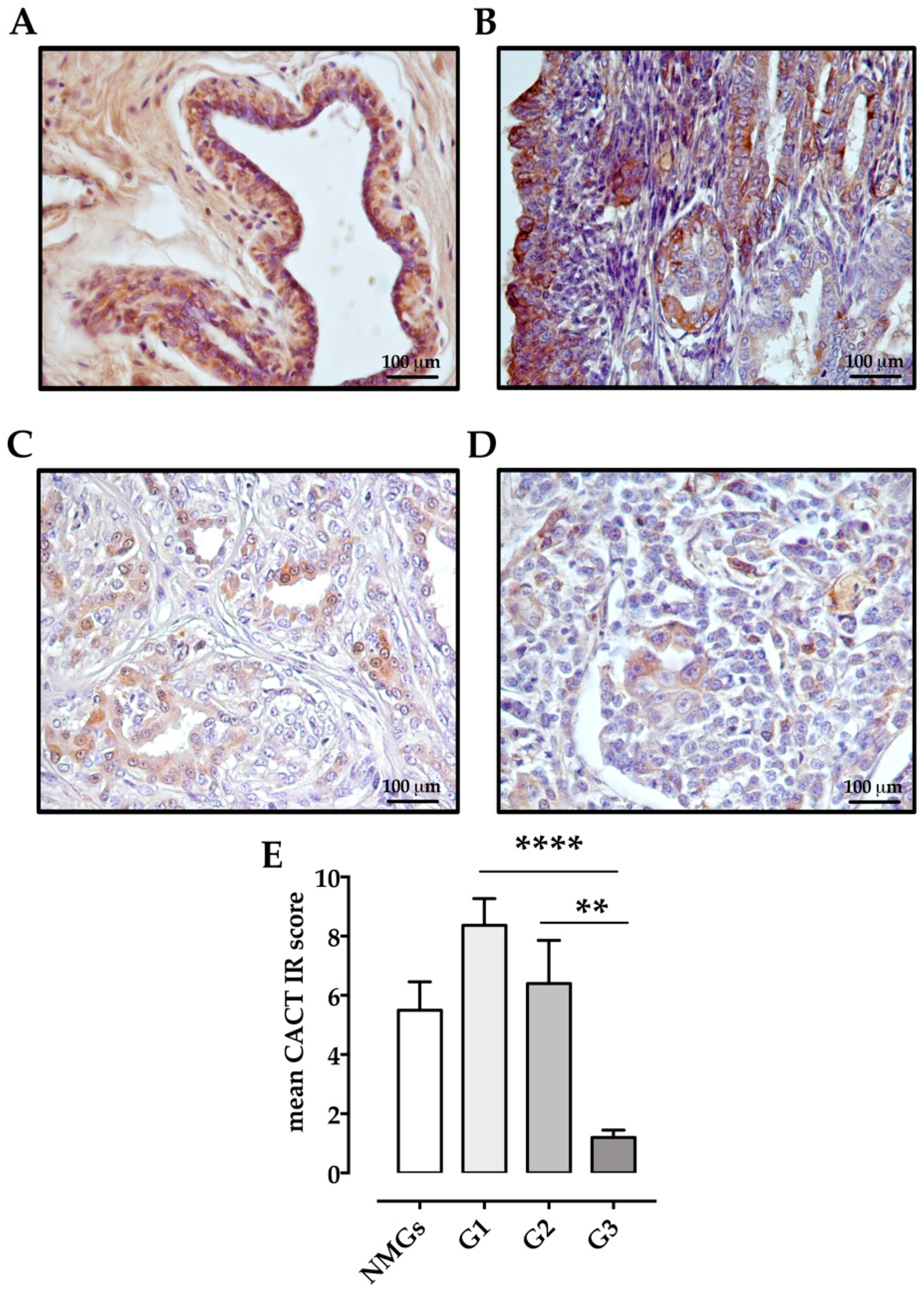
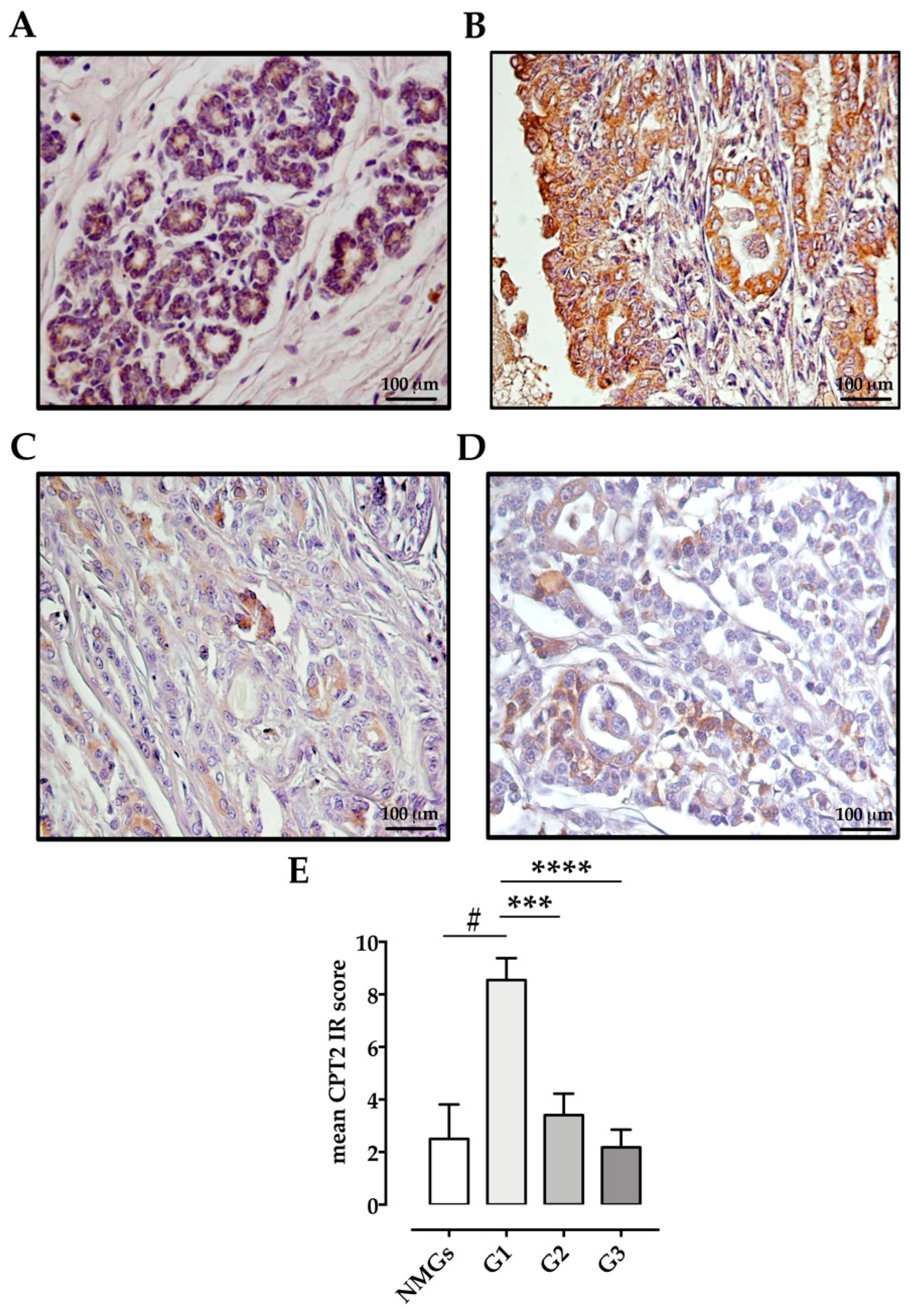
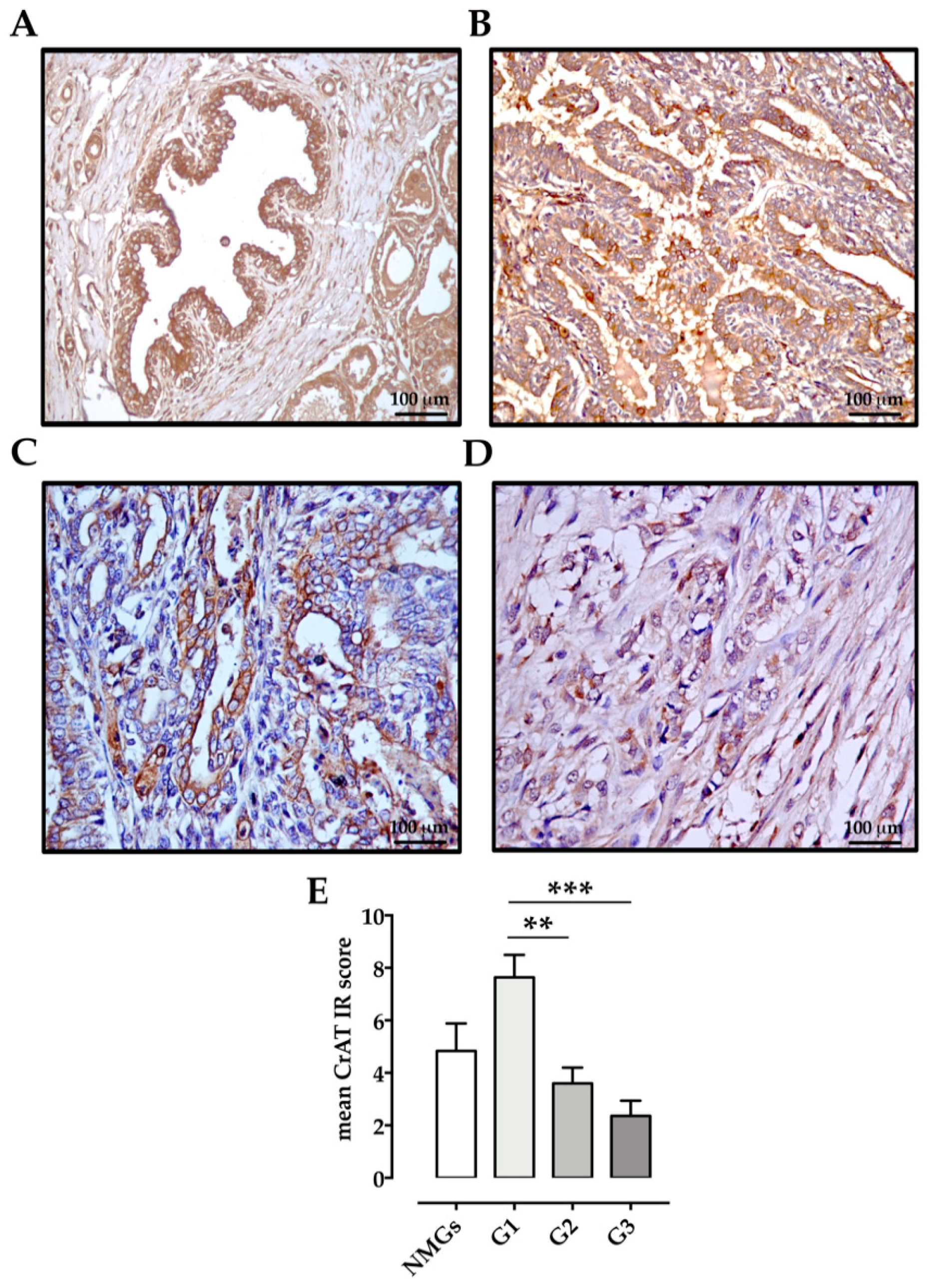
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melone, M.A.B.; Valentino, A.; Margarucci, S.; Galderisi, U.; Giordano, A.; Peluso, G. The carnitine system and cancer metabolic plasticity. Cell Death Dis. 2018, 9, 228. [Google Scholar] [CrossRef] [Green Version]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Voelker, H.U.; Kapp, M.; Krockenberger, M.; Dietl, J.; Kammerer, U. Glycolytic phenotype in breast cancer: Activation of Akt, up-regulation of GLUT1, TKTL1 and down-regulation of M2PK. J. Cancer Res. Clin. Oncol. 2010, 136, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Nomura, D.K.; Long, J.Z.; Niessen, S.; Hoover, H.S.; Ng, S.W.; Cravatt, B.F. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell 2010, 140, 49–61. [Google Scholar] [CrossRef] [Green Version]
- Carracedo, A.; Cantley, L.C.; Pandolfi, P.P. Cancer metabolism: Fatty acid oxidation in the limelight. Nat. Rev. Cancer 2013, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef] [Green Version]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [Green Version]
- Qu, Q.; Zeng, F.; Liu, X.; Wang, Q.J.; Deng, F. Fatty acid oxidation and carnitine palmitoyltransferase I: Emerging therapeutic targets in cancer. Cell Death Dis. 2016, 7, e2226. [Google Scholar] [CrossRef] [PubMed]
- Rufer, A.C.; Thoma, R.; Hennig, M. Structural insight into function and regulation of carnitine palmitoyltransferase. Cell Mol. Life Sci. 2009, 66, 2489–2501. [Google Scholar] [CrossRef]
- Violante, S.; Ijlst, L.; Te Brinke, H.; Koster, J.; Tavares de Almeida, I.; Wanders, R.J.; Ventura, F.V.; Houten, S.M. Peroxisomes contribute to the acylcarnitine production when the carnitine shuttle is deficient. Biochim. Biophys. Acta 2013, 1831, 1467–1474. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camarda, R.; Zhou, A.Y.; Kohnz, R.A.; Balakrishnan, S.; Mahieu, C.; Anderton, B.; Eyob, H.; Kajimura, S.; Tward, A.; Krings, G.; et al. Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat. Med. 2016, 22, 427–432. [Google Scholar] [CrossRef]
- Caro, P.; Kishan, A.U.; Norberg, E.; Stanley, I.A.; Chapuy, B.; Ficarro, S.B.; Polak, K.; Tondera, D.; Gounarides, J.; Yin, H.; et al. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer Cell 2012, 22, 547–560. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis. 2006, 9, 230–234. [Google Scholar] [CrossRef] [Green Version]
- Padanad, M.S.; Konstantinidou, G.; Venkateswaran, N.; Melegari, M.; Rindhe, S.; Mitsche, M.; Yang, C.; Batten, K.; Huffman, K.E.; Liu, J.; et al. Fatty Acid Oxidation Mediated by Acyl-CoA Synthetase Long Chain 3 Is Required for Mutant KRAS Lung Tumorigenesis. Cell Rep. 2016, 16, 1614–1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salas, Y.; Marquez, A.; Diaz, D.; Romero, L. Epidemiological Study of Mammary Tumors in Female Dogs Diagnosed during the Period 2002-2012: A Growing Animal Health Problem. PLoS ONE 2015, 10, e0127381. [Google Scholar] [CrossRef] [Green Version]
- Queiroga, F.L.; Raposo, T.; Carvalho, M.I.; Prada, J.; Pires, I. Canine mammary tumours as a model to study human breast cancer: Most recent findings. Vivo 2011, 25, 455–465. [Google Scholar]
- Abdelmegeed, S.M.; Mohammed, S. Canine mammary tumors as a model for human disease. Oncol. Lett. 2018, 15, 8195–8205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera, P.; von Euler, H. Molecular biological aspects on canine and human mammary tumors. Vet. Pathol. 2011, 48, 132–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visan, S.; Balacescu, O.; Berindan-Neagoe, I.; Catoi, C. In vitro comparative models for canine and human breast cancers. Clujul Med. 2016, 89, 38–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cacciola, N.A.; Sgadari, M.; Petillo, O.; Margarucci, S.; Martano, M.; Cocchia, N.; Maiolino, P.; Restucci, B. Carnitine palmitoyltransferase 1 A expression profile in canine mammary tumors. Vet. J. 2020, 257, 105453. [Google Scholar] [CrossRef]
- Zappulli, V.; Pena, L.; Rasotto, R.; Goldschmidt, M.H.; Gama, A.; Scruggs, J.L.; Kiupel, M. Volume 2: Mammary Tumors. In Surgical Pathology of Tumors of Domestic Animals; Kiupel, M., Ed.; Davis-Thompson DVM Foundation: Washington, DC, USA, 2019; pp. 1–195. [Google Scholar]
- Pena, L.; De Andres, P.J.; Clemente, M.; Cuesta, P.; Perez-Alenza, M.D. Prognostic value of histological grading in noninflammatory canine mammary carcinomas in a prospective study with two-year follow-up: Relationship with clinical and histological characteristics. Vet. Pathol. 2013, 50, 94–105. [Google Scholar] [CrossRef]
- Restucci, B.; Martano, M.; Maiolino, P. Expression of endothelin-1 and endothelin-1 receptor A in canine mammary tumours. Res. Vet. Sci. 2015, 100, 182–188. [Google Scholar] [CrossRef]
- Burrai, G.P.; Tanca, A.; Cubeddu, T.; Abbondio, M.; Polinas, M.; Addis, M.F.; Antuofermo, E. A first immunohistochemistry study of transketolase and transketolase-like 1 expression in canine hyperplastic and neoplastic mammary lesions. BMC Vet. Res. 2017, 13, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gugiatti, E.; Tenca, C.; Ravera, S.; Fabbi, M.; Ghiotto, F.; Mazzarello, A.N.; Bagnara, D.; Reverberi, D.; Zarcone, D.; Cutrona, G.; et al. A reversible carnitine palmitoyltransferase (CPT1) inhibitor offsets the proliferation of chronic lymphocytic leukemia cells. Haematologica 2018, 103, e531–e536. [Google Scholar] [CrossRef] [PubMed]
- Pacilli, A.; Calienni, M.; Margarucci, S.; D’Apolito, M.; Petillo, O.; Rocchi, L.; Pasquinelli, G.; Nicolai, R.; Koverech, A.; Calvani, M.; et al. Carnitine-acyltransferase system inhibition, cancer cell death, and prevention of myc-induced lymphomagenesis. J. Natl. Cancer Inst. 2013, 105, 489–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricciardi, M.R.; Mirabilii, S.; Allegretti, M.; Licchetta, R.; Calarco, A.; Torrisi, M.R.; Foa, R.; Nicolai, R.; Peluso, G.; Tafuri, A. Targeting the leukemia cell metabolism by the CPT1a inhibition: Functional preclinical effects in leukemias. Blood 2015, 126, 1925–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Temkin, S.M.; Hawkridge, A.M.; Guo, C.; Wang, W.; Wang, X.Y.; Fang, X. Fatty acid oxidation: An emerging facet of metabolic transformation in cancer. Cancer Lett. 2018, 435, 92–100. [Google Scholar] [CrossRef]
- Balaban, S.; Nassar, Z.D.; Zhang, A.Y.; Hosseini-Beheshti, E.; Centenera, M.M.; Schreuder, M.; Lin, H.M.; Aishah, A.; Varney, B.; Liu-Fu, F.; et al. Extracellular Fatty Acids Are the Major Contributor to Lipid Synthesis in Prostate Cancer. Mol. Cancer Res. 2019, 17, 949–962. [Google Scholar] [CrossRef]
- Balaban, S.; Lee, L.S.; Varney, B.; Aishah, A.; Gao, Q.; Shearer, R.F.; Saunders, D.N.; Grewal, T.; Hoy, A.J. Heterogeneity of fatty acid metabolism in breast cancer cells underlies differential sensitivity to palmitate-induced apoptosis. Mol. Oncol. 2018, 12, 1623–1638. [Google Scholar] [CrossRef]
- Yuan, P.; Mu, J.; Wang, Z.; Ma, S.; Da, X.; Song, J.; Zhang, H.; Yang, L.; Li, J.; Yang, J. Down-regulation of SLC25A20 promotes hepatocellular carcinoma growth and metastasis through suppression of fatty-acid oxidation. Cell Death Dis. 2021, 12, 361. [Google Scholar] [CrossRef]
- Kim, W.T.; Yun, S.J.; Yan, C.; Jeong, P.; Kim, Y.H.; Lee, I.S.; Kang, H.W.; Park, S.; Moon, S.K.; Choi, Y.H.; et al. Metabolic Pathway Signatures Associated with Urinary Metabolite Biomarkers Differentiate Bladder Cancer Patients from Healthy Controls. Yonsei Med. J. 2016, 57, 865–871. [Google Scholar] [CrossRef]
- Guo, H.; Zeng, W.; Feng, L.; Yu, X.; Li, P.; Zhang, K.; Zhou, Z.; Cheng, S. Integrated transcriptomic analysis of distance-related field cancerization in rectal cancer patients. Oncotarget 2017, 8, 61107–61117. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.; Lv, D.; Zheng, Y.; Wu, M.; Xu, C.; Zhang, Q.; Wu, L. Downregulation of CPT2 promotes tumorigenesis and chemoresistance to cisplatin in hepatocellular carcinoma. OncoTargets Ther. 2018, 11, 3101–3110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, Z.; Liu, S.; Li, J.; Wu, L.; Lv, X.; Xu, J.; Chen, B.; Zhao, S.; Yang, H. CPT2 down-regulation promotes tumor growth and metastasis through inducing ROS/NFkappaB pathway in ovarian cancer. Transl. Oncol. 2021, 14, 101023. [Google Scholar] [CrossRef]
- Fendt, S.M.; Frezza, C.; Erez, A. Targeting Metabolic Plasticity and Flexibility Dynamics for Cancer Therapy. Cancer Discov. 2020, 10, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.G.; Park, J.T.; Lee, H.M.; Lee, H.W.; Jeon, T.J.; Han, K.; Lee, S.A.; Dong, S.M.; Ryu, Y.H.; Son, E.J.; et al. Standardized uptake value of (1)(8)F-fluorodeoxyglucose positron emission tomography for prediction of tumor recurrence in breast cancer beyond tumor burden. Breast Cancer Res. 2014, 16, 502. [Google Scholar] [CrossRef] [Green Version]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Normal Mammary Glands | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case Number | Breed | Age (Years) | Histotype | CACT | CPT2 | CrAT | ||||||
| IS Cells (%) | Staining Intensity | IR Score | IS Cells (%) | Staining Intensity | IR Score | IS Cells (%) | Staining Intensity | IR Score | ||||
| 1 | Epagneul Breton | 10 | 5 | 1 | 1 | 4 | 1 | 1 | 11 | 2 | 4 | |
| 2 | Mixed Breed | 13 | 74.2 | 2 | 8 | 3 | 1 | 1 | 38.3 | 1 | 3 | |
| 3 | Yorkshire Terrier | 8 | 38 | 2 | 6 | 12 | 1 | 2 | 18.4 | 2 | 4 | |
| 4 | Mixed Breed | 10 | 31.3 | 2 | 6 | 36 | 3 | 9 | 64.8 | 2 | 8 | |
| 5 | Mixed Breed | 9 | 49.4 | 2 | 6 | 9 | 1 | 1 | 11.9 | 1 | 2 | |
| 6 | Cocker | 7 | 59.4 | 2 | 6 | 9 | 1 | 1 | 61.9 | 2 | 8 | |
| Well Differentiated G1 Carcinomas | ||||||||||||
| 1 | Mixed breed | 14 | Tubular carcinoma | 31.4 | 3 | 9 | 49 | 3 | 9 | 45.9 | 3 | 9 |
| 2 | Mixed breed | 11 | Tubular carcinoma | 37.9 | 3 | 9 | 18.2 | 3 | 6 | 13.9 | 3 | 6 |
| 3 | Yorkshire terrier | 13 | Complex carcinoma | 11.9 | 2 | 4 | 17.6 | 3 | 6 | 48.7 | 3 | 9 |
| 4 | Yorkshire terrier | 8 | Complex carcinoma | 38.1 | 2 | 6 | 24.5 | 2 | 4 | 22.7 | 2 | 4 |
| 5 | Mixed breed | 7 | Tubulopapillary carcinoma | 87.1 | 3 | 12 | 67 | 3 | 12 | 84.5 | 3 | 12 |
| 6 | Maltese | 7 | Mixed carcinoma | 13.4 | 2 | 4 | 38.6 | 3 | 9 | 29.4 | 1 | 2 |
| 7 | Mixed breed | 13 | Tubular carcinoma | 24 | 3 | 6 | 60 | 2 | 8 | 38.7 | 3 | 9 |
| 8 | Cocker | 10 | Tubular carcinoma | 56.4 | 3 | 9 | 54.6 | 3 | 9 | 55 | 3 | 9 |
| 9 | Cocker | 9 | Tubulopapillary carcinomay | 74 | 3 | 12 | 75.2 | 3 | 12 | 72 | 2 | 6 |
| 10 | Mixed breed | 9 | Tubular carcinoma | 70.1 | 3 | 12 | 53.9 | 3 | 9 | 66.5 | 3 | 9 |
| 11 | Beagle | 6 | Tubular carcinoma | 57.1 | 3 | 9 | 78.6 | 3 | 12 | 65.3 | 3 | 9 |
| Moderately differentiated G2 carcinomas | ||||||||||||
| 12 | Shih tzu | 11 | Tubular carcinoma | 6.2 | 1 | 1 | 2.4 | 1 | 1 | 59 | 2 | 6 |
| 13 | Epagneul breton | 10 | Tubulopapillary carcinoma | 39 | 1 | 3 | 2.8 | 1 | 1 | 53.8 | 2 | 6 |
| 14 | Cocker spaniel | 10 | Tubular carcinoma | 58.3 | 3 | 9 | 64.1 | 2 | 8 | 24.7 | 2 | 4 |
| 15 | Mixed breed | 9 | Solid carcinoma | 78.4 | 3 | 12 | 1.3 | 1 | 1 | 9.3 | 2 | 2 |
| 16 | Mixed breed | 13 | Tubular carcinoma | 97.3 | 3 | 12 | 23 | 2 | 4 | 12.7 | 1 | 2 |
| 17 | Mixed breed | 9 | Tubulopapillary carcinoma | 3.7 | 1 | 1 | 3.2 | 1 | 1 | 1.2 | 1 | 1 |
| 18 | Mixed breed | 10 | Tubulopapillary carcinoma | 13.5 | 3 | 6 | 33.9 | 2 | 6 | 23.4 | 2 | 4 |
| 19 | German sheperd | 8 | Tubulopapillary carcinoma | 77 | 3 | 12 | 40.5 | 2 | 6 | 59.4 | 2 | 6 |
| 20 | Mixed breed | 8 | Tubulopapillary carcinoma | 14 | 1 | 2 | 26.2 | 1 | 2 | 33.4 | 1 | 3 |
| 21 | Mixed breed | 7 | Mixed carcinoma | 35.2 | 2 | 6 | 83 | 1 | 4 | 24.7 | 1 | 2 |
| Poorly differentiated G3 carcinomas | ||||||||||||
| 22 | Greyhound | 8 | Tubular carcinoma | 19.3 | 2 | 2 | 19.2 | 1 | 2 | 52.3 | 2 | 6 |
| 23 | Mixed breed | 10 | Tubular carcinoma | 3 | 1 | 1 | 2.5 | 1 | 1 | 4.5 | 1 | 1 |
| 24 | Shih tzu | 9 | Tubular carcinoma | 1.4 | 1 | 1 | 3.3 | 1 | 1 | 1.5 | 1 | 1 |
| 25 | Greyhound | 9 | Tubular carcinoma | 19.3 | 2 | 2 | 19.6 | 1 | 2 | 19.3 | 2 | 4 |
| 26 | Mixed breed | 9 | Solid carcinoma | 0.6 | 1 | 1 | 0 | 0 | 0 | 24.3 | 2 | 4 |
| 27 | Mixed breed | 13 | Complex carcinoma | 0 | 0 | 0 | 0 | 0 | 0 | 14.2 | 1 | 2 |
| 28 | Mixed breed | 13 | Mixed carcinoma | 1.4 | 2 | 2 | 32.8 | 2 | 6 | 0 | 0 | 0 |
| 29 | Mixed breed | 6 | Complex carcinoma | 4 | 2 | 2 | 53.3 | 2 | 6 | 12.5 | 1 | 2 |
| 30 | Mixed breed | 8 | Tubular carcinoma | 11.2 | 3 | 6 | 28.3 | 2 | 4 | 10.2 | 2 | 4 |
| 31 | Poodle | 15 | Complex carcinoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 32 | Mixed breed | 9 | Tubular carcinoma | 3 | 1 | 1 | 25.9 | 1 | 2 | 10.3 | 2 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cacciola, N.A.; Sgadari, M.; Sepe, F.; Petillo, O.; Margarucci, S.; Martano, M.; Maiolino, P.; Restucci, B. Metabolic Flexibility in Canine Mammary Tumors: Implications of the Carnitine System. Animals 2021, 11, 2969. https://doi.org/10.3390/ani11102969
Cacciola NA, Sgadari M, Sepe F, Petillo O, Margarucci S, Martano M, Maiolino P, Restucci B. Metabolic Flexibility in Canine Mammary Tumors: Implications of the Carnitine System. Animals. 2021; 11(10):2969. https://doi.org/10.3390/ani11102969
Chicago/Turabian StyleCacciola, Nunzio Antonio, Mariafrancesca Sgadari, Fabrizia Sepe, Orsolina Petillo, Sabrina Margarucci, Manuela Martano, Paola Maiolino, and Brunella Restucci. 2021. "Metabolic Flexibility in Canine Mammary Tumors: Implications of the Carnitine System" Animals 11, no. 10: 2969. https://doi.org/10.3390/ani11102969






