Neonatal Proteinuria in Calves—A Quantitative Approach
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Analyses
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hjorth, L.; Helin, I.; Grubb, A. Age-related reference limits for urine levels of albumin, orosomucoid, immunoglobulin G and protein HC in children. Scand. J. Clin. Lab. Investig. 2000, 60, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Gudehithlu, K.P.; Pegoraro, A.A.; Dunea, G.; Arruda, J.A.L.; Singh, A.K. Degradation of albumin by the renal proximal tubule-cells and the subsequent fate of its fragments. Kidney Int. 2004, 65, 2113–2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falakaflaki, B.; Mousavinasab, S.N.; Mazloomzadeh, S. Dipstick urinalysis screening of healthy neonates. Pediatr. Neonatol. 2011, 52, 161–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, C.; Gattineni, J. Proteinuria and hematuria in the neonate. Curr. Opin. Pediatr. 2016, 28, 202–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dratwa-Chałupnik, A.; Wojdyła, K.; Ożgo, M.; Lepczyński, A.; Michałek, K.; Herosimczyk, A.; Rogowska-Wrzesińska, A. Urinary proteome of newborn calves—New potential in non-invasive neonatal diagnostic. Animals 2020, 10, 1257. [Google Scholar] [CrossRef]
- Viteri, B.; Reid-Adam, J. Hematuria and proteinuria in children. Pediatr. Rev. 2018, 39, 573–585. [Google Scholar] [CrossRef]
- Skrzypczak, W.F.; Ozgo, M.; Janus, K.; Skotnicka, E.; Jankowiak, D.; Muszczyński, Z.; Suszycka, J. The influence of increased protein content in the diet on renal functions in calves. Acta Vet. Brno 1996, 65, 115–121. [Google Scholar] [CrossRef] [Green Version]
- De Winter, D.; Salaets, T.; Gie, A.; Deprest, J.; Levtchenko, E.; Toelen, J. Glomerular developmental delay and proteinuria in the preterm neonatal rabbit. PLoS ONE 2020, 15, e0241384. [Google Scholar] [CrossRef]
- Tryggvason, K.; Pettersson, E. Causes and consequences of proteinuria: The kidney filtration barrier and progressive renal failure. J. Intern. Med. 2003, 254, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Drzezdzon, D.; Skrzypczak, W.F.; Ozgo, M. Introductory research on neonatal proteinuria in kids (in Polish, abstr. eng.). Folia Univ. Agric. Stetin. Zootech. 2003, 233, 93–102. [Google Scholar]
- Schäfer-Somi, S.; Bär-Schadler, S.; Aurich, J.E. Proteinuria and immunoglobulinuria in neonatal dogs. Vet. Rec. 2005, 157, 378–382. [Google Scholar] [CrossRef]
- Skrzypczak, W.F.; Drzezdzon, D.; Ozgo, M.; Michalek, K.; Dratwa-Chałupnik, A. Introductory research on renal filtration barrier tightness in kids in neonatal period. Folia Univ. Agric. Stetin. Zootech. 2005, 243, 153–160, (In Polish, Abstract in English). [Google Scholar]
- Ojala, R.; Ala-Houhala, M.; Harmoinen, A.P.T.; Luukkaala, T.; Uotila, J.; Tammela, O. Tubular proteinuria in pre-term and full-term infants. Pediatr. Nephrol. 2006, 21, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Ozgo, M.; Skrzypczak, W.F.; Drzezdzon, D.; Lepczynski, A.; Dratwa-Chalupnik, A.; Michalek, K.; Herosimczyk, A. Urinary excretion of low molecular weight proteins in goats during the neonatal period. J. Physiol. Pharmacol. 2009, 60, 119–125. [Google Scholar] [PubMed]
- Ciechanowicz, A.; Michalek, K.; Ozgo, M.; Dratwa-Chałupnik, A.; Kurpinska, A.; Klonowska, A.; Herosimczyk, A.; Lepczynski, A.; Niemcewicz, M.; Stanski, L.; et al. The concentration of selected protein fractions and theier percentage of the total blood plasma protein in neonatal calves. Acta Sci. Pol. Zootech. 2010, 9, 47–56, (In Polish, Abstract in English). [Google Scholar]
- Dratwa-Chałupnik, A.; Ozgo, M.; Herosimczyk, A.; Lepczyński, A.; Michałek, K.; Skrzypczak, W. Renal regulation of potassium homeostasis in calves in the first week of life including the role of atrial natriuretic peptide. Folia Biol. 2011, 59, 157–161. [Google Scholar] [CrossRef] [Green Version]
- D’Amico, G.; Bazzi, C. Pathophysiology of proteinuria. Kidney Int. 2003, 63, 809–825. [Google Scholar] [CrossRef] [Green Version]
- Zandi-Nejad, K.; Eddy, A.A.; Glassock, R.J.; Brenner, B.M. Why is proteinuria an ominous biomarker of progressive kidney disease? Kidney Int. Suppl. 2004, 66, S76–S89. [Google Scholar] [CrossRef] [Green Version]
- Hogg, R.J.; Portman, R.J.; Milliner, D.; Lemley, K.V.; Eddy, A.; Ingelfinger, J. Evaluation and management of proteinuria and nephrotic syndrome in children: Recommendations from a pediatric nephrology panel established at the National Kidney Foundation Conference on Proteinuria, Albuminuria, Risk, Assessment, Detection, and Eliminati. Pediatrics 2000, 105, 1242–1249. [Google Scholar] [CrossRef]
- Gubhaju, L.; Sutherland, M.R.; Horne, R.S.C.; Medhurst, A.; Kent, A.L.; Ramsden, A.; Moore, L.; Singh, G.; Hoy, W.E.; Black, M.J. Assessment of renal functional maturation and injury in preterm neonates during the first month of life. Am. J. Physiol. Ren. Physiol. 2014, 307, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Melandri, M.; Veronesi, M.C.; Alonge, S. Urinalysis in great dane puppies from birth to 28 days of age. Animals 2020, 10, 636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dariusz, D.; Skrzypczak, W.F.; Ziemak, J. Neonatal proteinuria in kids (in Polish, abstr. eng.). In Noworodek a Środowisko; Skrzypczak, W., Stefaniak, T., Zabielski, R., Eds.; Agricultural University of Wroclaw: Wroclaw, Poland, 2004; pp. 21–28. [Google Scholar]
- Stelloh, C.; Allen, K.P.; Mattson, D.L.; Lerch-Gaggl, A.; Reddy, S.; El-Meanawy, A. Prematurity in mice leads to reduction in nephron number, hypertension, and proteinuria. Transl. Res. 2012, 159, 80–89. [Google Scholar] [CrossRef] [Green Version]
- Saxena, I.; Shivankur, V.; Kumar, M. Urinary protein creatinine ratio in normal zero to three-day-old Indian neonates. J. Clin. Diagn. Res. 2016, 10, BC21–BC23. [Google Scholar] [CrossRef] [PubMed]
- Faulks, R.D.; Lane, I.F. Qualitative urinalyses in puppies 0 to 24 weeks of age. J. Am. Anim. Hosp. Assoc. 2003, 39, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Skrzypczak, W.F.; Drzezdzon, D. Kidney adaptation of kids and calves newborns to natremia regulation. Electron. J. Pol. Agric. Univ. 2001, 4, 2. [Google Scholar]
- Bauer, R.; Walter, B.; Bauer, K.; Klupsch, R.; Patt, S.; Zwiener, U. Intrauterine growth restriction reduces nephron number and renal excretory function in newborn piglets. Acta Physiol. Scand. 2002, 176, 83–90. [Google Scholar] [CrossRef]
- Slǔncheva, B.; Dimitrov, A.; Vakrilova, L. Development of renal function in low and extremely low birth weight infants: Correlation with gestational and postnatal age. Akusherstvo Ginekol. 2002, 41, 20–26. [Google Scholar]
- Dickson, L.E.; Wagner, M.C.; Sandoval, R.M.; Molitoris, B.A. The proximal tubule and albuminuria: Really! J. Am. Soc. Nephrol. 2014, 25, 443–453. [Google Scholar] [CrossRef] [Green Version]
- Birn, H.; Fyfe, J.C.; Jacobsen, C.; Mounier, F.; Verroust, P.J.; Ørskov, H.; Willnow, T.E.; Moestrup, S.K.; Christensen, E.I. Cubilin is an albumin binding protein important for renal tubular albumin reabsorption. J. Clin. Investig. 2000, 105, 1353–1361. [Google Scholar] [CrossRef] [Green Version]
- Zhai, X.Y.; Nielsen, R.; Birn, H.; Drumm, K.; Mildenberger, S.; Freudinger, R.; Moestrup, S.K.; Verroust, P.J.; Christensen, E.I.; Gekle, M. Cubilin- and megalin-mediated uptake of albumin in cultured proximal tubule cells of opossum kidney. Kidney Int. 2000, 58, 1523–1533. [Google Scholar] [CrossRef] [Green Version]
- Verroust, P.J.; Birn, H.; Nielsen, R.; Kozyraki, R.; Christensen, E.I. The tandem endocytic receptors megalin and cubilin are important proteins in renal pathology. Kidney Int. 2002, 62, 745–756. [Google Scholar] [CrossRef] [Green Version]
- Baran, D.; Tenstad, O.; Aukland, K. Aprotinin uptake in the proximal tubules in the rat kidney I. Length of proximal tubular uptake segment. J. Struct. Biol. 2003, 142, 402–408. [Google Scholar] [CrossRef]
- Baran, D.; Tenstad, O.; Aukland, K. Aprotinin uptake in the proximal tubules in the rat kidney. II. Uptake site relative to glomerulus. J. Struct. Biol. 2003, 142, 409–415. [Google Scholar] [CrossRef]
- Utsch, B.; Klaus, G. Urinanalyse im Kindesund Jugendalter. Dtsch. Arztebl. Int. 2014, 111, 617–626. [Google Scholar] [PubMed] [Green Version]


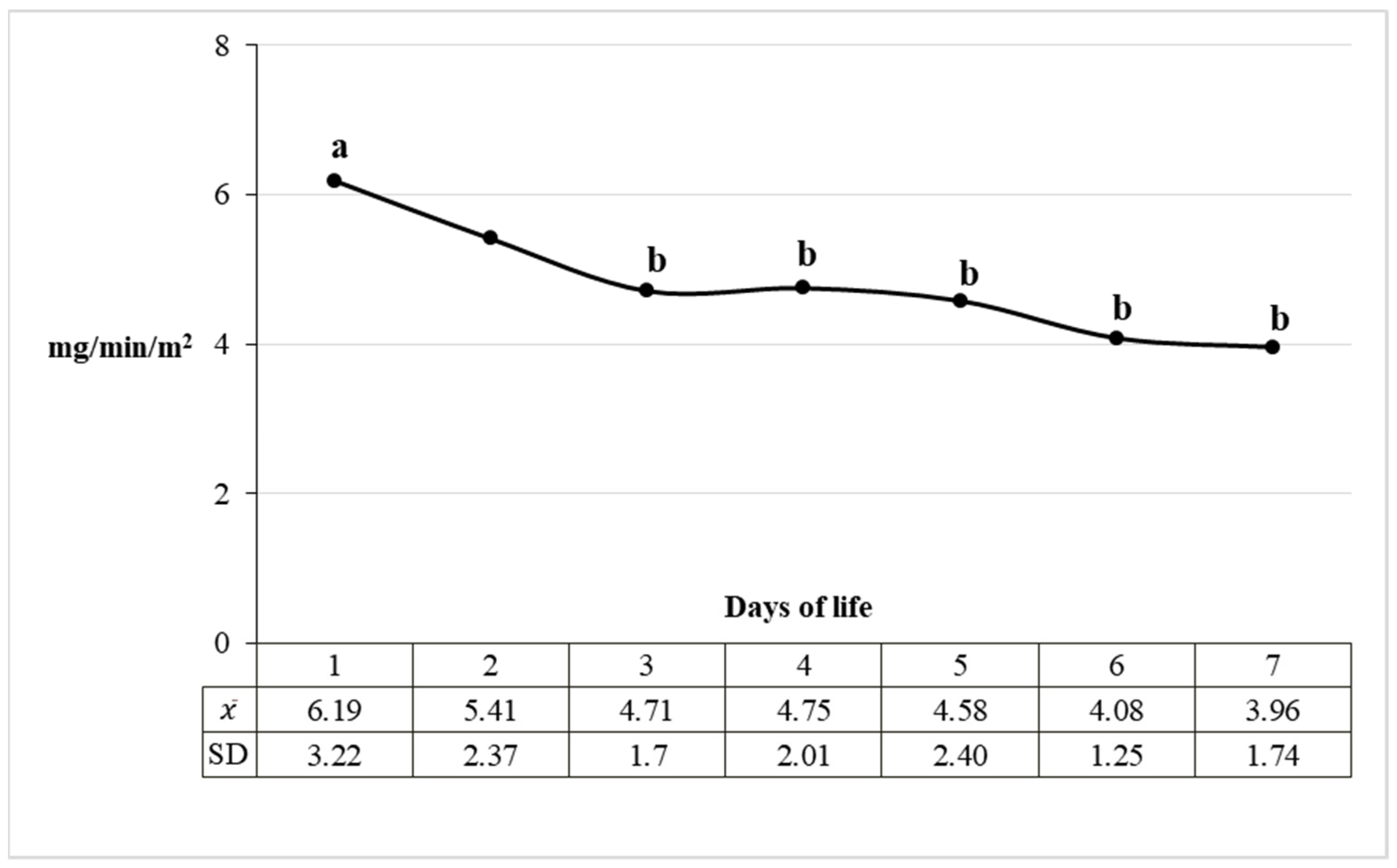



| Days of Life | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||||
| Percentage in urine (%) | LMW | 84.46 a,c | 75.07 b,c | 74.29 b,c | 72.22 b,c | 66.38 b,c | 69.65 b,c | 64.02 b | ||
| Albumin | 9.54 a,c | 13.27 b,c | 15.07 b,c | 15.27 b,c | 19.03 b | 15.37 b,c | 19.51 b | |||
| HMW | 6.68 a,c | 13.05 b,c | 11.88 b,c | 14.45 b,c | 18.04 b | 15.18 b,c | 18.13 b | |||
| Urinary excretion (mg/min/m2) | LMW | 4.30 a | 4.15 a | 3.87 a | 3.80 a | 3.14 a | 3.32 a | 2.52 b | ||
| SD | 2.49 | 1.93 | 1.92 | 2.02 | 1.98 | 1.81 | 1.64 | |||
| Albumin | 0.50 a,c | 0.70 c | 0.73 b,c | 0.63 a,c | 0.88 b | 0.70 a,c | 0.69 a,c | |||
| SD | 0.11 | 0.34 | 0.35 | 0.27 | 0.45 | 0.32 | 0.33 | |||
| HMW | 0.31 a,c | 0.71 b,c | 0.53 c | 0.79 bc | 0.92 b | 0.71 b,c | 0.69 b,c | |||
| SD | 0.23 | 0.73 | 0.43 | 0.5 | 0.63 | 0.46 | 0.26 | |||
| Percentage of protein fractions in daily urine excretion [%] | LMW | 84.1 a | 74.66 a | 75.47 a | 72.74 a | 63.48 b | 70.19 b | 67.40 b | ||
| Albumin | 9.78 a,c | 12.61 c | 14.23 b,c | 12.10 a,c | 17.84 b | 14.83 a,c | 17.65 a,c | |||
| HMW | 6.12 a,c | 12.73 b,c | 10.30 c | 15.16 b,c | 18.68 b | 14.98 b,c | 17.65 b,c | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skrzypczak, W.; Dratwa-Chałupnik, A.; Ożgo, M.; Boniecka, K. Neonatal Proteinuria in Calves—A Quantitative Approach. Animals 2021, 11, 3602. https://doi.org/10.3390/ani11123602
Skrzypczak W, Dratwa-Chałupnik A, Ożgo M, Boniecka K. Neonatal Proteinuria in Calves—A Quantitative Approach. Animals. 2021; 11(12):3602. https://doi.org/10.3390/ani11123602
Chicago/Turabian StyleSkrzypczak, Wiesław, Alicja Dratwa-Chałupnik, Małgorzata Ożgo, and Karolina Boniecka. 2021. "Neonatal Proteinuria in Calves—A Quantitative Approach" Animals 11, no. 12: 3602. https://doi.org/10.3390/ani11123602






