Effects of Dietary Supplementation of Conjugated Linoleic Acids and Their Inclusion in Semen Extenders on Bovine Sperm Quality
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Location and Ethical Clearance
2.2. Animals and Treatments
2.3. Sample Collection
2.4. Sample Processing
- Control: No supplementation into the Triladyl® base extender
- CLA c-9, t-11-50: Triladyl® base extender supplemented with 50 µM of CLA c-9, t-11
- CLA c-9, t-11-100: Triladyl® base extender supplemented with 100 µM of CLA c-9, t-11
- CLA t-10, c-12-50: Triladyl® base extender supplemented with 50 µM of CLA t-10, c-12
- CLA t-10, c-12-100: Triladyl® base extender supplemented with 100 µM of CLA t-10, c-12
- CLA mix: Triladyl® base extender supplemented with a mixture of the two isomers at 50 µM each
2.5. Analyses
2.6. IGF-I Levels in Blood and Seminal Plasma
2.7. Sperm Evaluation
2.8. Sperm Motility
2.9. Sperm Morphology
2.10. Sperm Viability, Mitochondrial Membrane Potential and Oxidative Stress
2.11. Experimental Design and Statistical Analyses
3. Results and Discussion
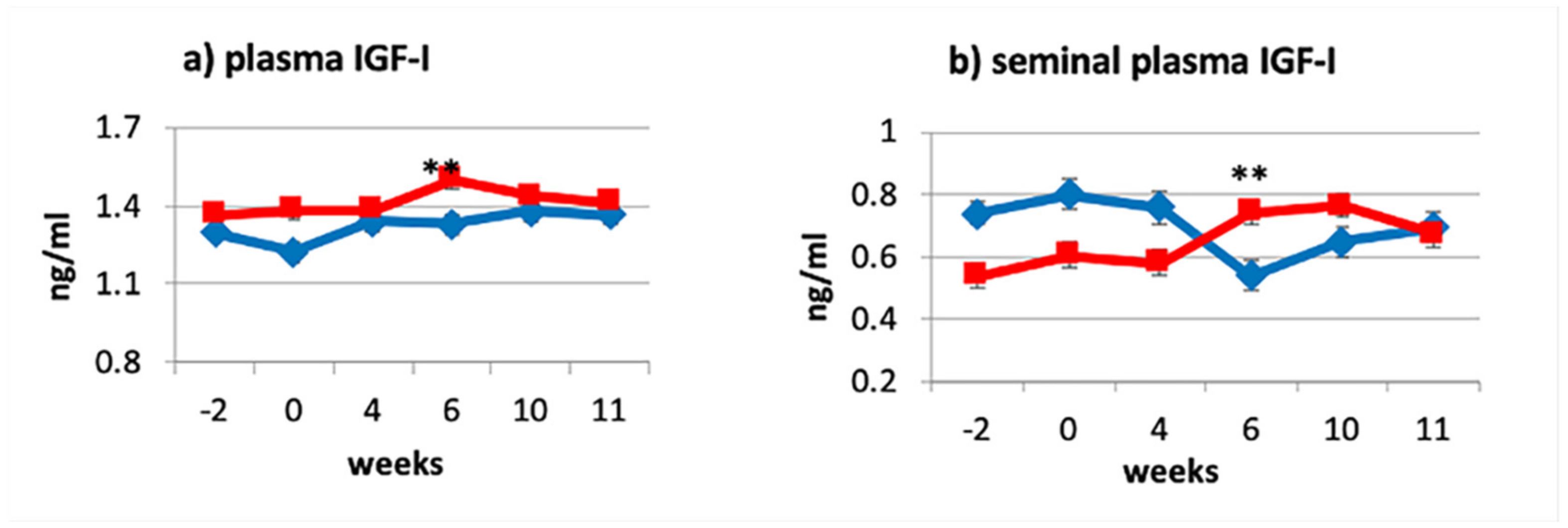
3.1. Effects of Dietary Treatments on Plasma and Seminal Plasma IGF-I Levels
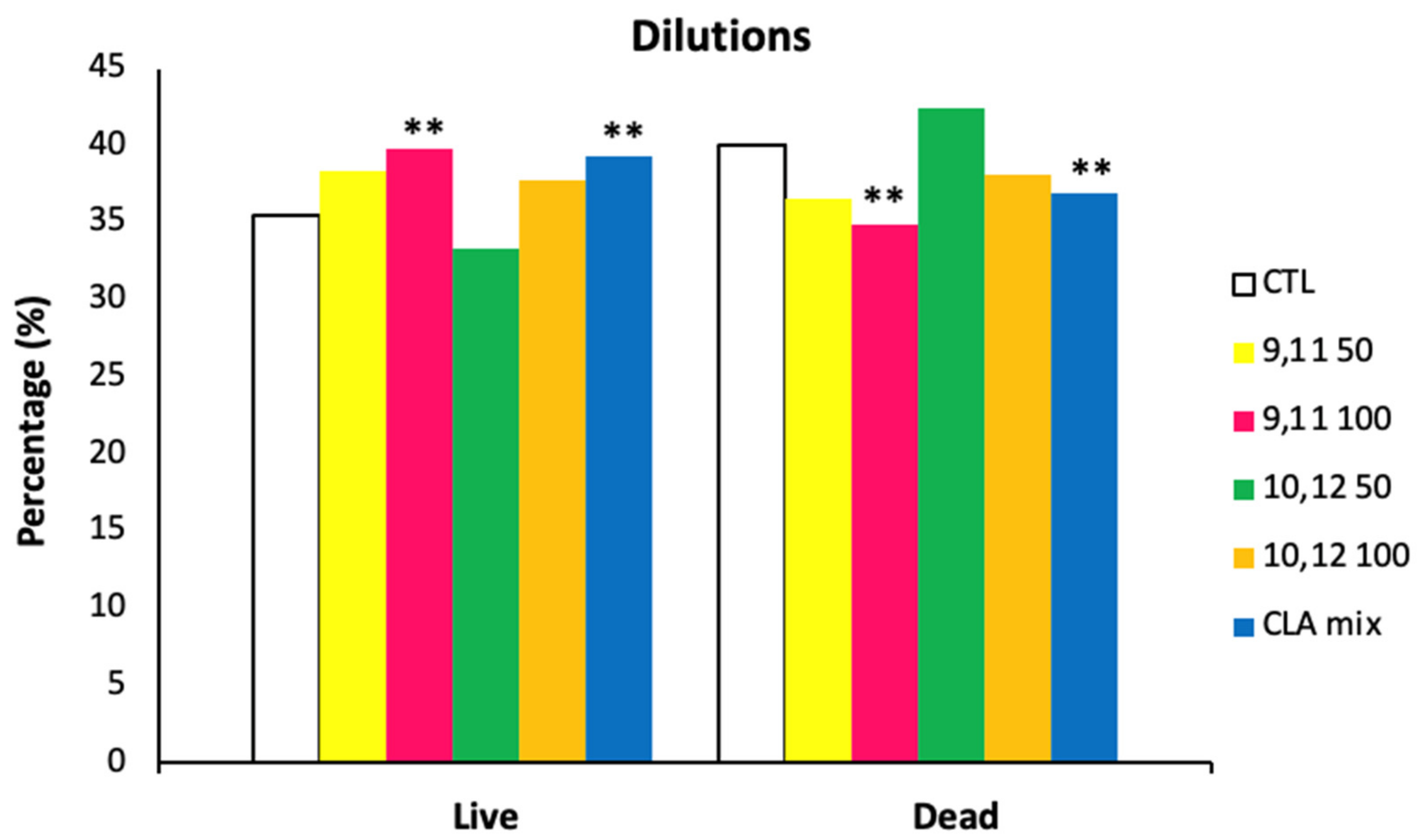
3.2. Effects of Dietary Treatments on Fresh Semen Characteristics
3.3. Sperm Morphological Defects
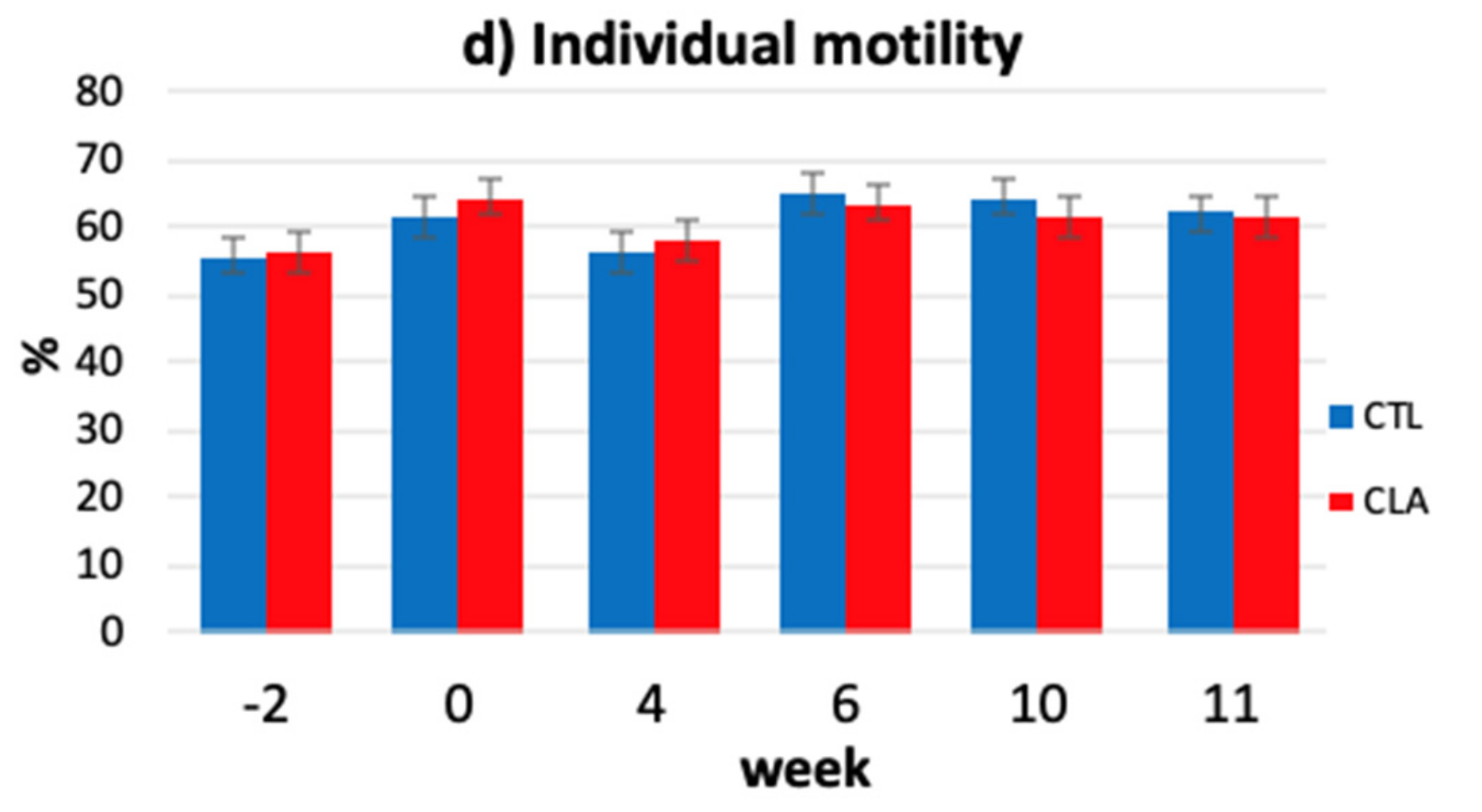
3.4. Kinematic Parameters
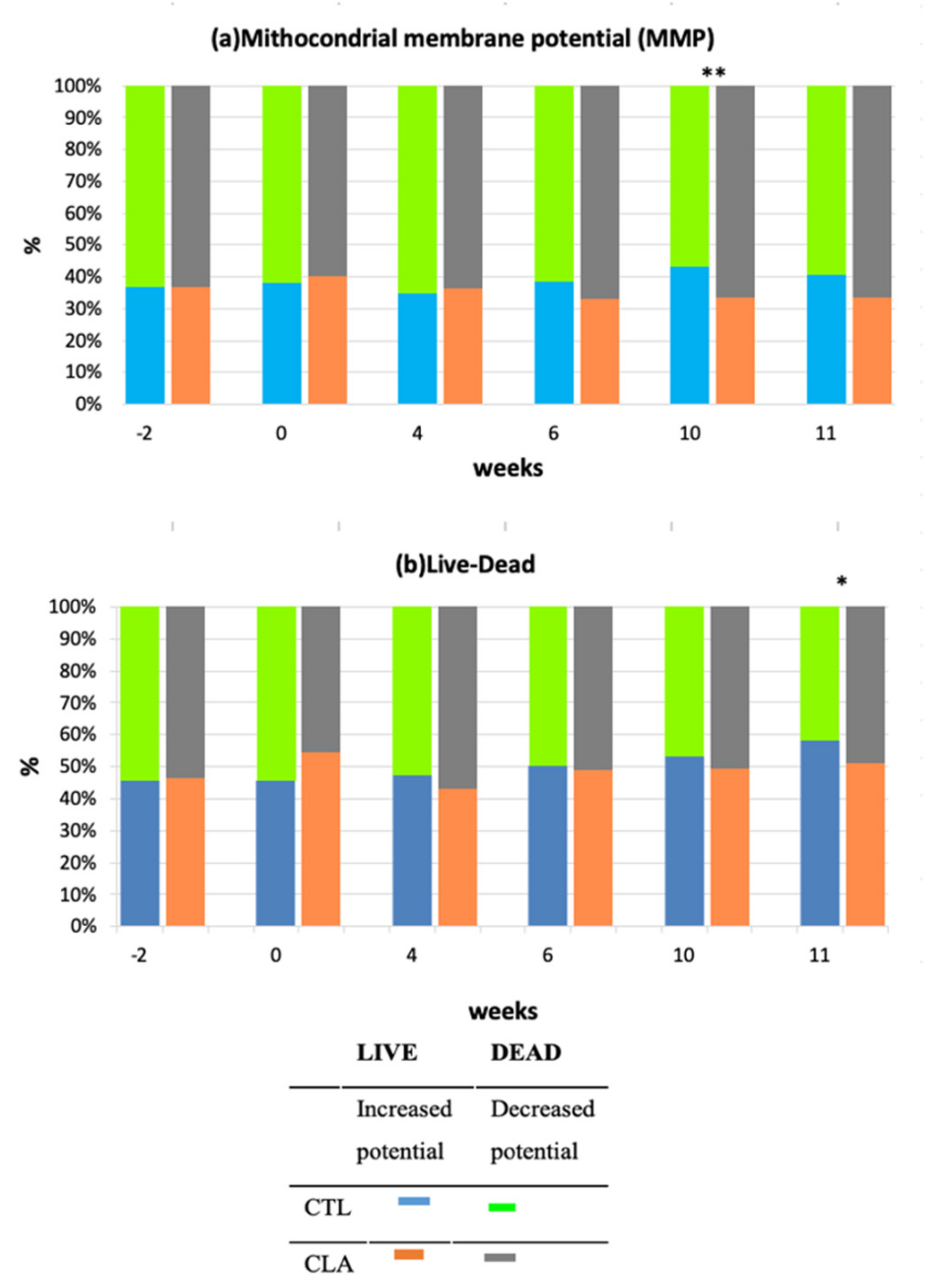
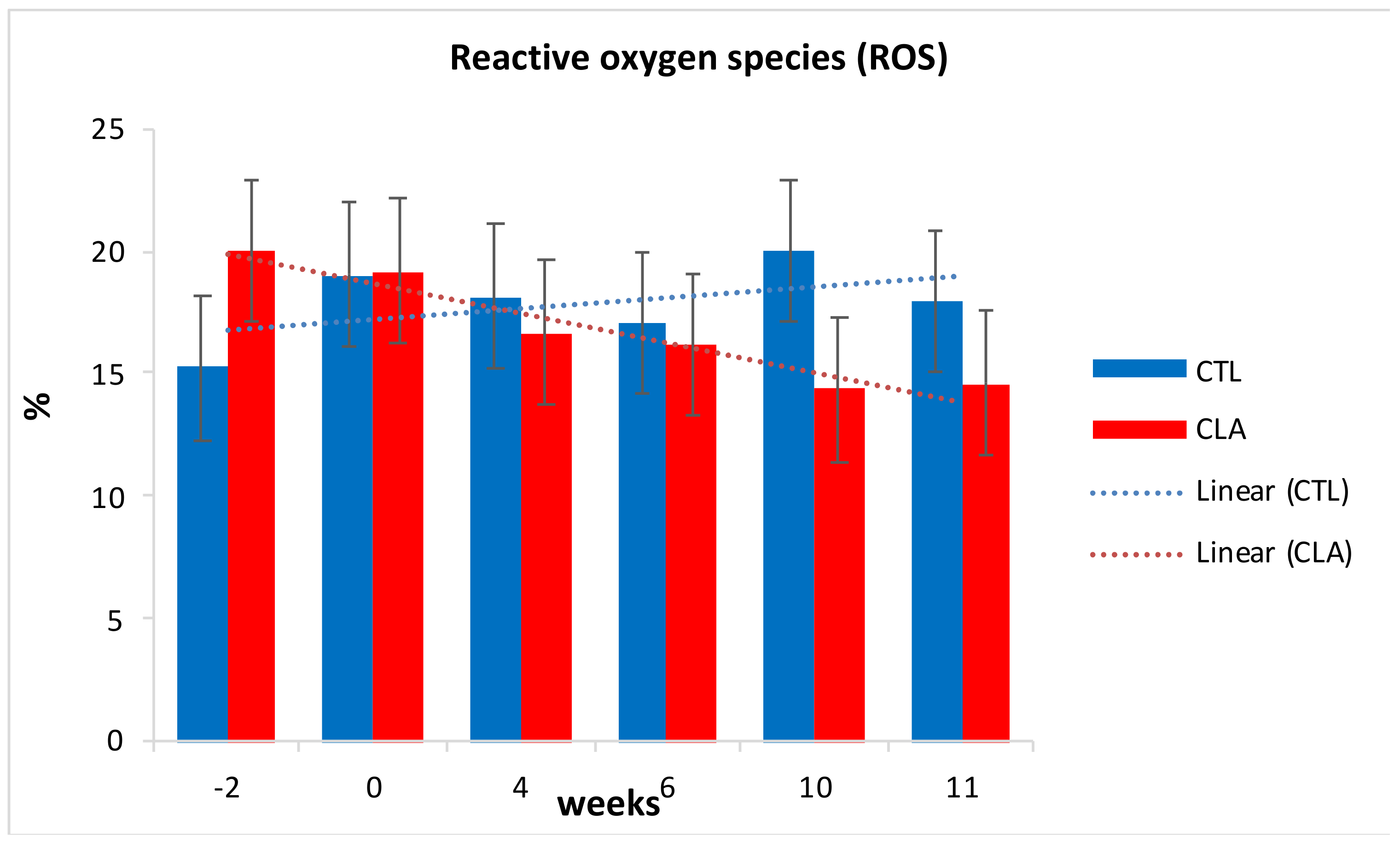
3.5. Flow Cytometry Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Kastelic, J.P. Male involvement in fertility and factors affecting semen quality in bulls. Anim. Front. 2013, 3, 20–25. [Google Scholar] [CrossRef]
- Mukhopadhyay, C.S.; Gupta, A.K.; Yadav, B.R.; Khate, K.; Raina, V.S.; Mohanty, T.K.; Dubey, P.P. Subfertility in males: An important cause of bull disposal in bovines. Australas. J. Anim. Sci. 2010, 23, 450–455. [Google Scholar] [CrossRef]
- Gouletsou, P.G.; Fthenakis, G.C. Clinical evaluation of reproductive ability of rams. Small Rumin. Res. 2010, 92, 45–51. [Google Scholar] [CrossRef]
- D’Amours, O.; Frenette, G.; Fortier, M.; Leclerc, P.; Sullivan, R. Proteomic comparison of detergent-extracted sperm proteins from bulls with different fertility indexes. Reproduction 2010, 139, 545–556. [Google Scholar] [CrossRef] [Green Version]
- López-Pérez, A.; Pérez-Clariget, R. Ram seminal plasma improves pregnancy rates in ewes cervically inseminated with ram semen stored at 5 °C for 24 hours. Theriogenology 2012, 77, 395–399. [Google Scholar] [CrossRef]
- Bernardini, A.; Hozbor, F.; Sanchez, E.; Fornés, M.W.; Alberio, R.H.; Cesari, A. Conserved ram seminal plasma proteins bind to the sperm membrane and repair cryopreservation damage. Theriogenology 2011, 76, 436–447. [Google Scholar] [CrossRef]
- Sauerweina, H.; Breier, B.H.; Gallaher, B.W.; Götz, C.; Küfner, G.; Montag, T.; Vickers, M.; Schallenberger, E. Growth hormone treatment of breeding bulls used for artificial insemination improves fertilization rates. Domest. Anim. Endocrinol. 2000, 18, 145–158. [Google Scholar] [CrossRef]
- Martin, G.B.; Blache, D.; Miller, D.W.; Vercoe, P.E. Interactions between nutrition and reproduction in the management of the mature male ruminant. Animal 2010, 4, 1214–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diskin, M.G.; Mackey, D.R.; Roche, J.F.; Sreenan, J.M. Effects of nutrition and metabolic status on circulating hormones and ovarian follicle development in cattle. Anim. Reprod. Sci. 2003, 78, 345–370. [Google Scholar] [CrossRef]
- Macpherson, M.L.; Simmen, R.C.M.; Simmen, F.A.; Hernandez, J.; Sheerin, B.R.; Varner, D.D.; Loomis, P.; Cadario, M.E.; Miller, C.D.; Brinsko, S.P.; et al. Insulin-Like Growth Factor-I and Insulin-Like Growth Factor Binding Protein-2 and -5 in Equine Seminal Plasma: Association with Sperm Characteristics and Fertility. Biol. Reprod. 2002, 67, 648–654. [Google Scholar] [CrossRef]
- Gupta, G.S. Proteomics of Spermatogenesis; Springer: Berlin, Germany, 2005. [Google Scholar]
- Griffin, J.E.; Ojeda, S.R. Textbook of Endocrine Physiology; Oxford University Press: Oxford, UK, 1992; ISBN 0195075609. [Google Scholar]
- Zhou, J.; Bondy, C. Anatomy of the insulin-like growth factor system in the human testis. Fertil. Steril. 1993, 60, 897–904. [Google Scholar] [CrossRef]
- Henricks, D.M.; Kouba, A.J.; Lackey, B.R.; Boone, W.R.; Gray, S.L. Identification of insulin-like growth factor I in bovine seminal plasma and its receptor on spermatozoa: Influence on sperm motility. Biol. Reprod. 1998, 59, 330–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, J.; Hardy, M.P.; Zhou, J.; Bondy, C.; Lupu, F.; Bellvé, A.R.; Efstratiadis, A. Effects of an Igf1 gene null mutation on mouse reproduction. Mol. Endocrinol. 1996, 10, 903–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvaraju, S.; Reddy, I.J.; Nandi, S.; Rao, S.B.N.; Ravindra, J.P. Influence of IGF-I on buffalo (Bubalus bubalis) spermatozoa motility, membrane integrity, lipid peroxidation and fructose uptake in vitro. Anim. Reprod. Sci. 2009, 113, 60–70. [Google Scholar] [CrossRef]
- Selvaraju, S.; Nandi, S.; Subramani, T.S.; Raghavendra, B.S.; Rao, S.B.N.; Ravindra, J.P. Improvement in buffalo (Bubalus bubalis) spermatozoa functional parameters and fertility in vitro: Effect of insulin-like growth factor-I. Theriogenology 2010, 73, 1–10. [Google Scholar] [CrossRef]
- Naz, R.K.; Padman, P. Identification of insulin-like growth factor (IGF)-1 receptor in human sperm cell. Arch. Androl. 1999, 43, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.B.; Walkden-Brown, S.W. Nutritional influences on reproduction in mature male sheep and goats. J. Reprod. Fertil. Suppl. 1995, 49, 437–449. [Google Scholar] [CrossRef]
- Webb, R.; Campbell, B.K.; Garverick, H.A.; Gong, J.G.; Gutierrez, C.G.; Armstrong, D.G. Molecular mechanisms regulating follicular re-cruitment and selection. J. Reprod. Fertil. Suppl. 1999, 54, 33–48. [Google Scholar]
- Fourie, P.J.; Schwalbach, L.M.; Neser, F.W.C.; der Wersthuizen, C.V. Scrotal, testicular and semen characteristics of young Dorper rams managed under intensive and extensive conditions. Small Rumin. Res. 2004, 54, 53–59. [Google Scholar] [CrossRef]
- Selvaraju, S.; Sivasubramani, T.; Raghavendra, B.S.; Raju, P.; Rao, S.B.N.; Dineshkumar, D.; Ravindra, J.P. Effect of dietary energy on seminal plasma insulin-like growth factor-I (IGF-I), serum IGF-I and testosterone levels, semen quality and fertility in adult rams. Theriogenology 2012, 78, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Gutiérrez, E.; Benefield, B.C.; de Veth, M.J.; Santos, N.R.; Gilbert, R.O.; Butler, W.R.; Bauman, D.E. Evaluation of the mechanism of action of conjugated linoleic acid isomers on reproduction in dairy cows. J. Dairy Sci. 2007, 90, 4253–4264. [Google Scholar] [CrossRef]
- Esposito, G.; Absalón Medina, V.A.; Schneider, A.; Gilbert, R.O.; Butler, W.R. Effect of dietary conjugated linoleic acid (CLA) on the metabolism and reproduction of dairy cows. S. Afr. J. Anim. Sci. 2013, 43. [Google Scholar] [CrossRef]
- Mehrdad, M.; Akram, M.; Khodaei, H. The Effect of Conjugated Linoleic Acid (CLA) on Male Reproductive Hormones in Mice. Adv. J. Food Sci. Technol. 2013, 5, 1618–1620. [Google Scholar]
- Karimi, R.; Towhidi, A.; Zeinoaldini, S.; Rezayazdi, K.; Mousavi, M.; Safari, H.; Martinez-Pastor, F. Effects of supplemental conjugated linoleic acids (CLA) on fresh and post-thaw sperm quality of Holstein bulls. Reprod. Domest. Anim. 2017, 52, 459–467. [Google Scholar] [CrossRef]
- Soares, M.P.; Brandelli, A.; Celeghini, E.C.C.; de Arruda, R.P.; Rodriguez, S.A.F. Effect of cis-9,trans-11 and trans-10,cis-12 isomers of conjugated linoleic acid on the integrity and functionality of cryopreserved bovine spermatozoa. Cryobiology 2013, 67, 102–105. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, S.M.P.; Chaveiro, A.E.N.; da Silva, J.F.M. Effect of trans-10, cis-12 isomer of conjugated linoleic acid on boar semen quality after cryopreservation. Anim. Reprod. 2017, 14, 400–405. [Google Scholar] [CrossRef]
- Staub, C.; Johnson, L. Review: Spermatogenesis in the bull. Animal 2018, 12, s27–s35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chenoweth, P. Bull Health and Breeding Soundness. In Bovine Medicine; John Wiley & Sons, Ltd.: Chichester, UK, 2015; pp. 246–261. [Google Scholar]
- Keating, A.F.; Kennelly, J.J.; Zhao, F.Q. Characterization and regulation of the bovine stearoyl-CoA desaturase gene promoter. Biochem. Biophys. Res. Commun. 2006, 344, 233–240. [Google Scholar] [CrossRef]
- Pérez-Pé, R.; Cebrián-Pérez, J.A.; Muiño-Blanco, T. Semen plasma proteins prevent cold-shock membrane damage to ram spermatozoa. Theriogenology 2001, 56, 425–434. [Google Scholar] [CrossRef]
- Companholi, S.P.; Monteiro, F.M.; Ribeiro Dias, E.A.; Zerlotti Mercadante, M.E.; Paro de Paz, C.C.; Dell’Aqua Junior, J.A.; Papa, F.O.; de Paula Freitas Dell’Aqua, C.; Vantini, R.; Garcia, J.M. Effect of seminal plasma removal before cryopreservation of bovine semen obtained by electroejaculation on semen quality and in vitro fertility. Theriogenology 2017, 89, 114–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barth, A.; Oko, R.J. Abnormal Morphology of Bovine Spermatozoa; Iowa State University Press: Ames, IA, USA, 1992; Volume 69, ISBN 0813801125. [Google Scholar]
- Nöthling, J.O.; Irons, P.C. A simple multidimensional system for the recording and interpretation of sperm morphology in bulls. Theriogenology 2008, 69, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Pastor, F.; Johannisson, A.; Gil, J.; Kaabi, M.; Anel, L.; Paz, P.; Rodriguez-Martinez, H. Use of chromatin stability assay, mitochondrial stain JC-1, and fluorometric assessment of plasma membrane to evaluate frozen-thawed ram semen. Anim. Reprod. Sci. 2004, 84, 121–133. [Google Scholar] [CrossRef]
- Gillan, L.; Evans, G.; Maxwell, W.M.C. Flow cytometric evaluation of sperm parameters in relation to fertility potential. Theriogenology 2005, 63, 445–457. [Google Scholar] [CrossRef]
- Hossain, M.S.; Johannisson, A.; Wallgren, M.; Nagy, S.; Siqueira, A.P.; Rodriguez-Martinez, H. Flow cytometry for the assessment of animal sperm integrity and functionality: State of the art. Asian J. Androl. 2011, 13, 406–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csillik, Z.; Faigl, V.; Keresztes, M.; Galamb, E.; Hammon, H.M.; Tröscher, A.; Fébel, H.; Kulcsár, M.; Husvéth, F.; Huszenicza, G.; et al. Effect of pre- and postpartum supplementation with lipid-encapsulated conjugated linoleic acid on reproductive performance and the growth hormone–insulin-like growth factor-I axis in multiparous high-producing dairy cows. J. Dairy Sci. 2017, 100, 5888–5898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breier, B.H.; Vickers, M.H.; Gravance, C.G.; Casey, P.J. Growth hormone (GH) therapy markedly increases the motility of spermatozoa and the concentration of insulin-like growth factor-I in seminal vesicle fluid in the male GH-deficient dwarf rat. Endocrinology 1996, 137, 4061–4064. [Google Scholar] [CrossRef] [Green Version]
- Rhoads, M.L.; Kim, J.W.; Collier, R.J.; Crooker, B.A.; Boisclair, Y.R.; Baumgard, L.H.; Rhoads, R.P. Effects of heat stress and nutrition on lactating Holstein cows: II. Aspects of hepatic growth hormone responsiveness. J. Dairy Sci. 2010, 93, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Wheelock, J.B.; Rhoads, R.P.; VanBaale, M.J.; Sanders, S.R.; Baumgard, L.H. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 2010, 93, 644–655. [Google Scholar] [CrossRef]
- Chaiyabutr, N.; Chanpongsang, S.; Suadsong, S. Effects of evaporative cooling on the regulation of body water and milk production in crossbred Holstein cattle in a tropical environment. Int. J. Biometeorol. 2008, 52, 575–585. [Google Scholar] [CrossRef]
- Titto, C.G.; Negrão, J.A.; Canaes, T.d.S.; Titto, R.M.; da Cunha Leme-dos Santos, T.M.; Henrique, F.L.; Calviello, R.F.; Pereira, A.M.F.; Titto, E.A.L. Heat stress and ACTH administration on cortisol and insulin-like growth factor I (IGF-I) levels in lactating Holstein cows. J. Appl. Anim. Res. 2017, 45, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Senger, P. Pathways to Pregnancy and Parturation, 3rd ed.; Current Conceptions: Pullman, WA, USA, 2012. [Google Scholar]
- Dimitriadis, F.; Tsiampali, C.; Chaliasos, N.; Tsounapi, P.; Takenaka, A.; Sofikitis, N. The sertoli cell as the orchestra conductor of spermatogenesis: Spermatogenic cells dance to the tune of testosterone. Hormones 2015, 14, 479–503. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.V.; Malla, B.A.; Sharma, A.N.; Kumar, S.; Tyagi, N.; Tyagi, A.K. Effect of omega-3 and omega-6 polyunsaturated fatty acid enriched diet on plasma IGF-1 and testosterone concentration, puberty and semen quality in male buffalo. Anim. Reprod. Sci. 2016, 173, 63–72. [Google Scholar] [CrossRef]
- Garner, D.L.; Johnson, L.A. Viability assessment of mammalian sperm using SYBR-14 and propidium iodide. Biol. Reprod. 1995, 53, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Vickers, M.H.; Casey, P.J.; Champion, Z.J.; Gravance, C.G.; Breier, B.H. IGF-I treatment increases motility and improves morphology of immature spermatozoa in the GH-deficient dwarf (dw/dw) rat. Growth Horm. Igf Res. 1999, 9, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Cancel, A.; Lobdel, D.; Mendola, P.; Perreault, S. Objective evaluation of hyperactivated motility in rat spermatozoa using computer-assisted sperm analysis. Hum. Reprod. 2000, 15, 1322–1328. [Google Scholar] [CrossRef] [Green Version]
- Antończyk, A.; Niżański, W.; Twardoń, J.; Kozdrowski, R.; Ochota, M.; Błasiak, K.; Mikołajewska, N.; Stañczyk, E. Current views on computer assisted sperm analysis. Med. Weter. 2010, 66, 663–667. [Google Scholar]
- Kathiravan, P.; Kalatharan, J.; Karthikeya, G.; Rengarajan, K.; Kadirvel, G. Objective Sperm Motion Analysis to Assess Dairy Bull Fertility Using Computer-Aided System—A Review. Reprod. Domest. Anim. 2011, 46, 165–172. [Google Scholar] [CrossRef]
- Dolatpanah, M.B.; Towhidi, A.; Farshad, A.; Rashidi, A.; Rezayazdi, A. Effects of dietary fish oil on semen quality of goats. Asian-Australas. J. Anim. Sci. 2008, 21, 29–34. [Google Scholar] [CrossRef]
- Esmaeili, V.; Shahverdi, A.H.; Alizadeh, A.R.; Alipour, H.; Chehrazi, M. Saturated, omega-6 and omega-3 dietary fatty acid effects on the characteristics of fresh, frozen-thawed semen and blood parameters in rams. Andrologia 2014, 46, 42–49. [Google Scholar] [CrossRef]
- Estienne, M.J.; Harper, A.F.; Crawford, R.J. Dietary supplementation with a source of omega-3 fatty acids increases sperm number and the duration of ejaculation in boars. Theriogenology 2008, 70, 70–76. [Google Scholar] [CrossRef]
- Adeel, M.; Ijaz, A.; Aleem, M.; Rehman, H.; Yousaf, M.S.; Jabbar, M.A. Improvement of liquid and frozen-thawed semen quality of Nili-Ravi buffalo bulls (Bubalus bubalis) through supplementation of fat. Theriogenology 2009, 71, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, G.V.; Lee, W.; Seth, A.; McCulloch, C.A. Role of mitochondrial membrane potential in concanavalin A-induced apoptosis in human fibroblasts. Exp. Cells Res. 1998, 245, 170–178. [Google Scholar] [CrossRef]
- Gholami, H.; Chamani, M.; Towhidi, A.; Fazeli, M.H. Effect of feeding a docosahexaenoic acid-enriched nutriceutical on the quality of fresh and frozen-thawed semen in Holstein bulls. Theriogenology 2010, 74, 1548–1558. [Google Scholar] [CrossRef] [PubMed]
- Meister, A. On the discovery of glutathione. Trends Biochem. Sci. 1988, 13, 185–188. [Google Scholar] [CrossRef]
- Kaps, M.; Lamberson, W.R. Hipothesis testing. In Biostatistics for Animal Science; Kaps, M., Lamberson, W.R., Eds.; CABI Publishing: Cambridge, MA, USA, 2004; pp. 92–107. [Google Scholar]



| Main Analysis | Unit | Alfalfa | Reprotech | Molasses |
|---|---|---|---|---|
| Hay | Mature 1 | Meal 2 | ||
| Dry matter | % as fed | 30.8 | 88.3 | - |
| Crude protein | % DM | 14.5 | 13 | 40 |
| Crude fibre | % DM | 34 | 6.03 | 100 |
| Crude fat | g/kg | - | 5.91 | - |
| NDF * | % DM | 48.2 | - | - |
| ADF ** | % DM | 46.1 | - | - |
| Lignin | % DM | 9.1 | - | - |
| Ether extract | % DM | 1.9 | - | - |
| Ash | % DM | 8.7 | 9.28 | - |
| Urea | g/kg | - | 10 | - |
| Calcium (Ca) | g/kg | - | 9 | 13 |
| Phosphorus (P) | g/kg | - | 3.5 | - |
| Ca: P Ratio | g/kg | - | 2-3:1 | - |
| Moisture | g/kg | - | 11.7 | 150 |
| Gross energy | MJ/kg DM | 18.5 | 10.77 | 10.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liman, M.S.; Franco, V.; Cardoso, C.L.; Longobardi, V.; Gasparrini, B.; Wheeler, M.B.; Rubessa, M.; Esposito, G. Effects of Dietary Supplementation of Conjugated Linoleic Acids and Their Inclusion in Semen Extenders on Bovine Sperm Quality. Animals 2021, 11, 483. https://doi.org/10.3390/ani11020483
Liman MS, Franco V, Cardoso CL, Longobardi V, Gasparrini B, Wheeler MB, Rubessa M, Esposito G. Effects of Dietary Supplementation of Conjugated Linoleic Acids and Their Inclusion in Semen Extenders on Bovine Sperm Quality. Animals. 2021; 11(2):483. https://doi.org/10.3390/ani11020483
Chicago/Turabian StyleLiman, Mohammed S., Vittoria Franco, Claudia L. Cardoso, Valentina Longobardi, Bianca Gasparrini, Matthew B. Wheeler, Marcello Rubessa, and Giulia Esposito. 2021. "Effects of Dietary Supplementation of Conjugated Linoleic Acids and Their Inclusion in Semen Extenders on Bovine Sperm Quality" Animals 11, no. 2: 483. https://doi.org/10.3390/ani11020483







