Relationship between Allelic Heterozygosity in BoLA-DRB3 and Proviral Loads in Bovine Leukemia Virus-Infected Cattle
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Samples
2.2. Real-Time PCR Quantification of BLV-Associated PVLs
2.3. Diagnosing BLV Infections Using BLV-Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Detecting Resistant and Susceptible BoLA-DRB3 Alleles
2.5. BoLA-DRB3 exon2 Cloning and Sequence Analysis
2.6. Statistical Analyses
3. Results
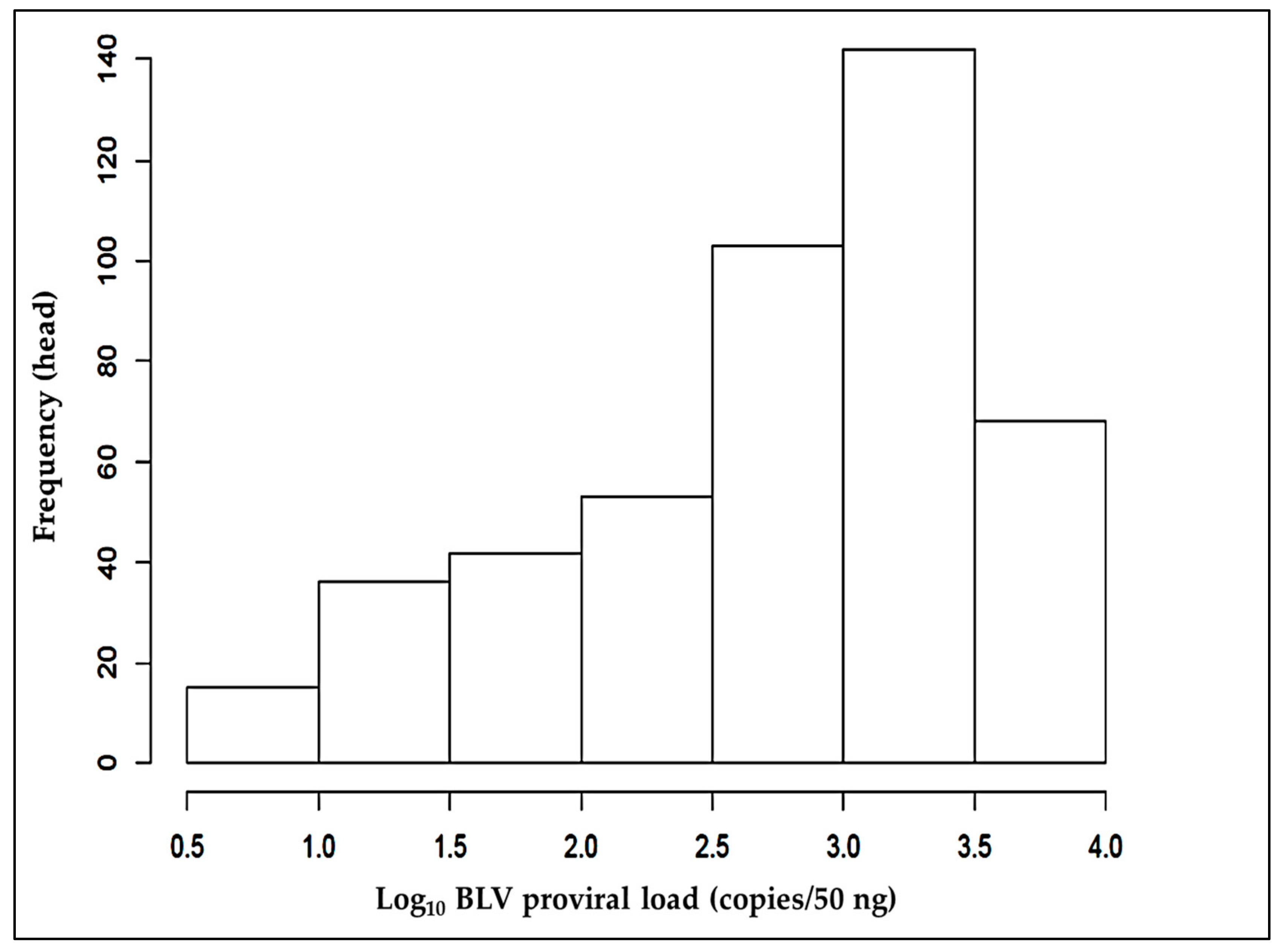
3.1. Quantifying BLV-Associated PVLs and Grouping Cattle According to These Loads
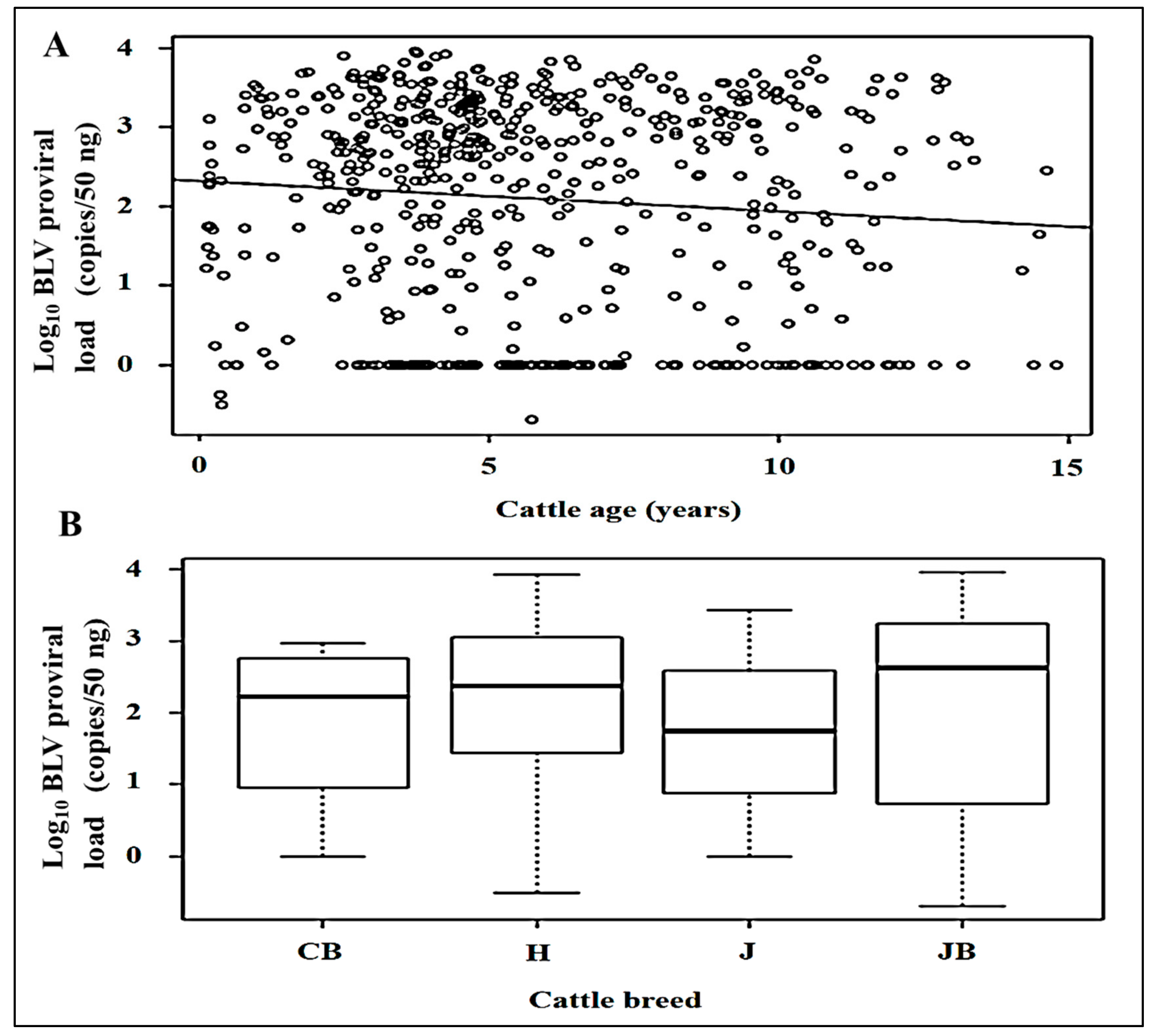
3.2. BLV PVLs and Their Relationships with Cattle Age and Breed
3.3. Relationships among BLV and BoLA-DRB3 Alleles, Age, and Breed
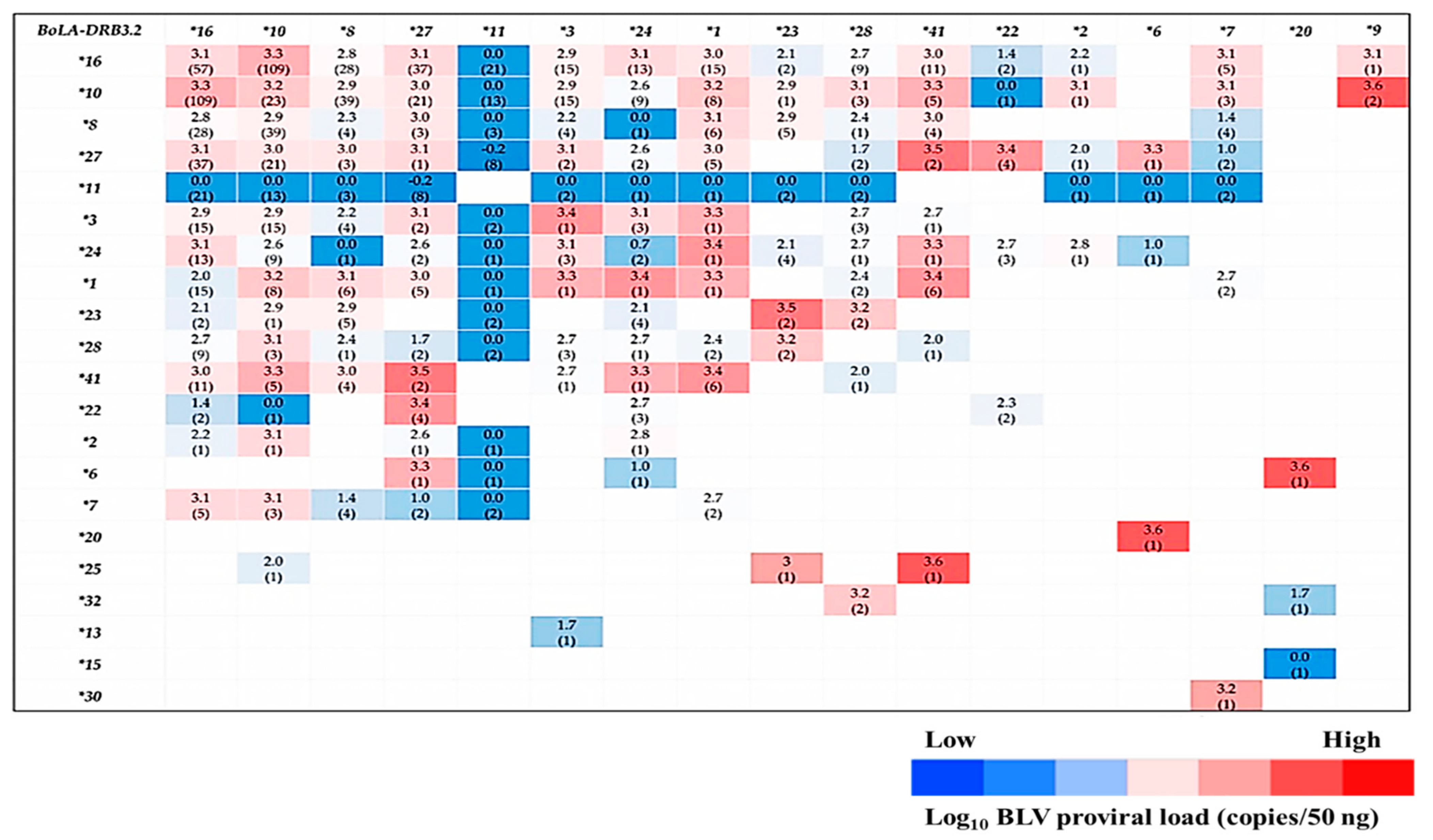
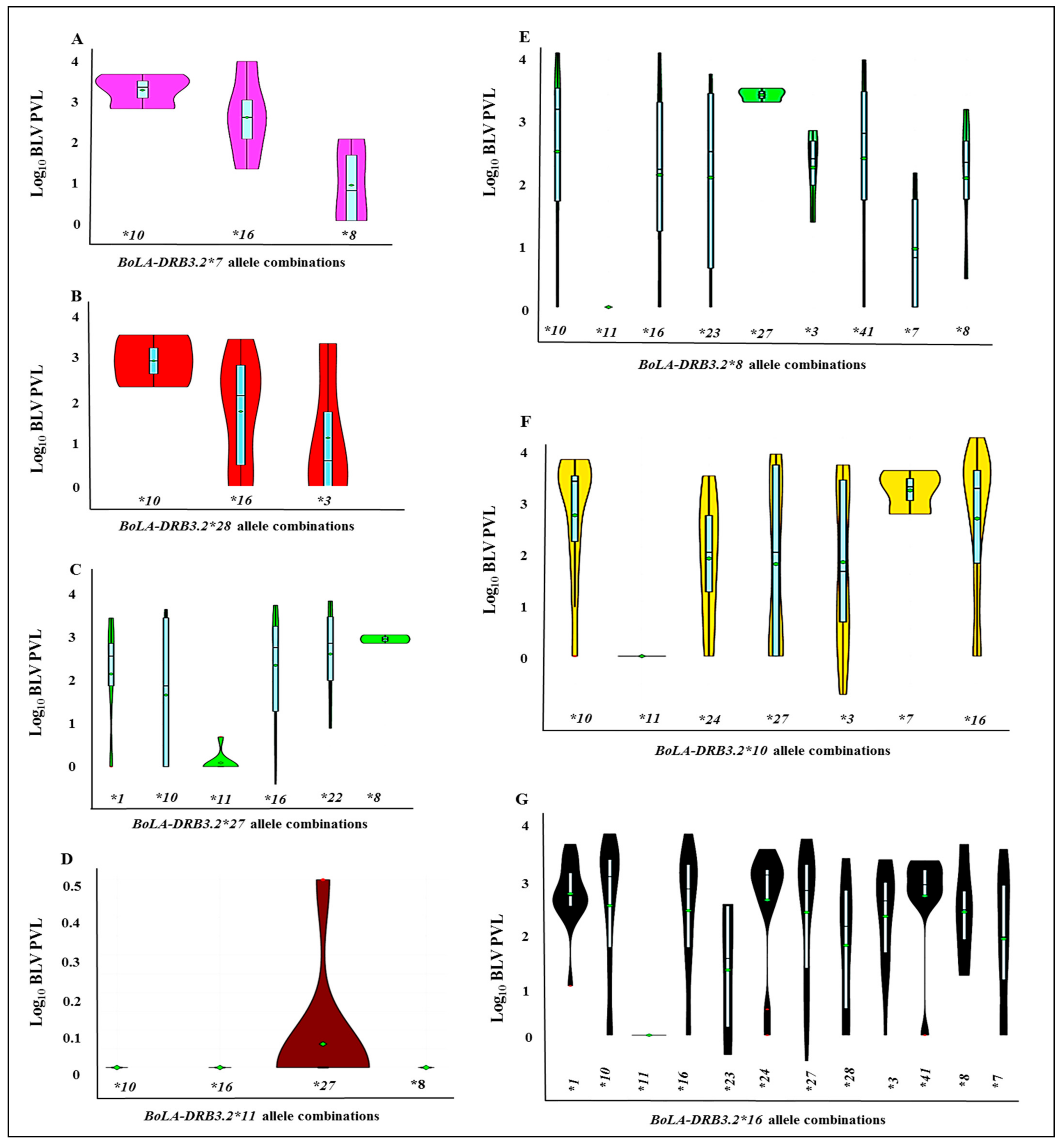
3.4. Analysis of the Relationship between BoLA-DRB3 Allele Combinations (Heterozygosity) and BLV-Associated PVLs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kale, M.; Bulut, O.; Yapkic, O.; Gulay, M.; Pehlivanoglu, F.; Ata, A.; Yavru, S. Effects of subclinical bovine leukemia virus infection on some production parameters in a dairy farm in southern Turkey. J. S. Afr. Vet. Assoc. 2007, 78, 130–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dequiedt, F.; Cantor, G.H.; Hamilton, V.T.; Pritchard, S.M.; Davis, W.C.; Kerkhofs, P.; Burny, A.; Kettmann, R.; Willems, L. Bovine leukemia virus-induced persistent lymphocytosis in cattle does not correlate with increased Ex Vivo survival of B lymphocytes. J. Virol. 1999, 73, 1127–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, S.M.; Florins, A.; Gillet, N.; de Brogniez, A.; Sánchez-Alcaraz, M.T.; Boxus, M.; Boulanger, F.; Gutiérrez, G.; Trono, K.; Alvarez, I.; et al. Preventive and therapeutic strategies for bovine leukemia virus: Lessons for HTLV. Viruses 2011, 3, 1210–1248. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, P.C.; Sordillo, L.M.; Byrem, T.M.; Norby, B.; Grooms, D.L.; Swenson, C.L.; Zalucha, J.; Erskine, R.J. Options for the control of bovine leukemia virus in dairy cattle. J. Am. Vet. Med. Assoc. 2014, 244, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Juliarena, M.A.; Gutierrez, S.E.; Ceriani, C. Determination of proviral load in bovine leukemia virus–infected cattle with and without lymphocytosis. Am. J. Vet. Res. 2007, 68, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Lewin, H.A.; Bernoco, D. Evidence for BoLA linked resistance and susceptibility to subclinical progression of bovine leukemia virus infection. Anim. Genet. 1986, 17, 297. [Google Scholar] [CrossRef]
- Miyasaka, T.; Takeshima, S.N.; Jimba, M.; Matsumoto, Y.; Kobayashi, N.; Matsuhashi, T.; Sentsui, H.; Aida, Y. Identification of bovine leukocyte antigen class II haplotypes associated with variations in bovine leukemia virus proviral load in Japanese Black cattle. Tissue Antigens 2013, 81, 72–82. [Google Scholar] [CrossRef]
- Juliarena, M.; Poli, M.; Sala, L.; Ceriani, C.; Gutierrez, S.; Dolcini, G.; Rodríguez, E.; Mariño, B.; Rodríguez-Dubra, C.; Esteban, E. Association of BLV infection profiles with alleles of the BoLADRB3.2 gene. Anim. Genet. 2008, 39, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Mekata, H.; Sekiguchi, S.; Kirino, Y.; Mitoma, S.; Honkawa, K.; Horii, Y.; Norimine, J. Cattle with the BoLA class II DRB3*0902 allele have significantly lower bovine leukemia proviral loads. J. Vet. Med. Sci. 2017, 79, 1552–1555. [Google Scholar] [CrossRef] [Green Version]
- Aida, Y.; Niimi, M.; Asahina, M.; Okada, K.; Nakai, Y.; Ogimoto, K. Identification of a new bovine MHC class II DRB allele by nucleotide sequencing and an analysis of phylogenetic relationships. Biochem. Biophys. Res. Commun. 1995, 209, 981–988. [Google Scholar] [CrossRef]
- Takeshima, S.-N.; Aida, Y. Structure, function and disease susceptibility of the bovine major histocompatibility complex. Anim. Sci. J. 2006, 77, 138–150. [Google Scholar] [CrossRef]
- Mekata, H.; Sekiguchi, S.; Konnai, S.; Kirino, Y.; Honkawa, K.; Nonaka, N.; Horii, Y.; Norimine, J. Evaluation of the natural perinatal transmission of bovine leukaemia virus. Vet. Rec. 2015, 176, 254. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; van Eijk, M.J.; Park, C.; Lewin, H.A. Polymorphism in BoLA-DRB3 exon 2 correlates with resistance to persistent lymphocytosis caused by bovine leukemia virus. J. Immunol. 1993, 151, 6977–6985. [Google Scholar]
- Eijk, M.J.T.; Stewart-Haynes, J.A.; Lewin, H.A. Extensive polymorphism of the BOLA-DRB3 gene distinguished by PCR-RFLP. Anim. Genet. 2009, 23, 483–496. [Google Scholar] [CrossRef]
- Gwynn, R.C.; Burnett, R.T.; Thurston, G.D. A time-series analysis of acidic particulate matter and daily mortality and mor-bidity in the buffalo, New York, region. Environ. Health. Perspect. 2000, 108, 125–133. [Google Scholar] [CrossRef]
- Mai, T.N.; Bui, T.P.; Le Huynh, T.M.; Sasaki, Y.; Mitoma, S.; El Daous, H.; Fahkrajang, W.; Norimine, J.; Sekiguchi, S. Evaluating the risk factors for porcine epidemic diarrhea virus infection in an endemic area of vietnam. Front. Vet. Sci. 2020, 7, 433. [Google Scholar] [CrossRef]
- Takeshima, S.-N.; Ohno, A.; Aida, Y. Bovine leukemia virus proviral load is more strongly associated with bovine major histocompatibility complex class II DRB3 polymorphism than with DQA1 polymorphism in Holstein cow in Japan. Retrovirology 2019, 16, 1–6. [Google Scholar] [CrossRef]
- Miyasaka, T.; Takeshima, S.-N.; Sentsui, H.; Aida, Y. Identification and diversity of bovine major histocompatibility complex class II haplotypes in Japanese Black and Holstein cattle in Japan. J. Dairy Sci. 2012, 95, 420–431. [Google Scholar] [CrossRef] [Green Version]
- Forletti, A.; Lützelschwab, C.M.; Cepeda, R.; Esteban, E.N.; Gutiérrez, S.E. Early events following bovine leukaemia virus infection in calves with different alleles of the major histocompatibility complex DRB3 gene. Vet. Res. 2020, 51, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Traul, D.L.; Li, H.; Dasgupta, N.; O’Toole, D.; Eldridge, J.A.; Besser, T.E.; Davies, C.J. Resistance to malignant catarrhal fever in American bison (Bison bison) is associated with MHC class IIa polymorphisms. Anim. Genet. 2007, 38, 141–146. [Google Scholar] [CrossRef]
- Úsuga-Monroy, C.; Echeverri Zuluaga, J.J.; López-Herrera, A. Association between genes BoLA-DRB3.2*8 and Bo-LA-DRB3.2*12 with resistance and Bola-DRB3.2*16 with susceptibility to infection by bovine leukemia virus. Pak. Vet. J. 2016, 36, 400–404. [Google Scholar]
- Brujeni, G.N.; Ghorbanpour, R.; Esmailnejad, A. Association of BoLA-DRB3.2 Alleles with BLV Infection Profiles (Persistent Lymphocytosis/Lymphosarcoma) and Lymphocyte Subsets in Iranian Holstein Cattle. Biochem. Genet. 2016, 54, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Esteban, E.N.; Poli, M.; Poiesz, B.; Ceriani, C.; Dube, S.; Gutierrez, S.; Dolcini, G.; Gagliardi, R.; Perez, S.; Lutzelschwab, C.; et al. Bovine leukemia virus (BLV), proposed control and eradication programs by marker assisted breeding of genetically resistant cattle. In Animal Genetics Hauppauge; Rechi, L.J., Ed.; Nova Science Publishers: New York, NY, USA, 2009; pp. 107–130. [Google Scholar]
- Lo, C.-W.; Borjigin, L.; Saito, S.; Fukunaga, K.; Saitou, E.; Okazaki, K.; Mizutani, T.; Wada, S.; Takeshima, S.-N.; Aida, Y. BoLA-DRB3 polymorphism is associated with differential susceptibility to bovine leukemia virus-induced lymphoma and proviral load. Viruses 2020, 12, 352. [Google Scholar] [CrossRef] [Green Version]
- Kabeya, H.; Ohashi, K.; Oyunbileg, N.; Nagaoka, Y.; Aida, Y.; Sugimoto, C.; Yokomizo, Y.; Onuma, M. Up-regulation of tumor necrosis factor alpha mRNA is associated with bovine-leukemia virus (BLV) elimination in the early phase of infection. Vet. Immunol. Immunop. 1999, 68, 255–265. [Google Scholar] [CrossRef]
- Pyeon, D.; O’Reilly, K.L.; Splitter, G.A. Increased interleukin-10 mRNA expression in tumor-bearing or persistently lymphocytotic animals infected with bovine leukemia virus. J. Virol. 1996, 70, 5706–5710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norimine, J.; Brown, W.C. Intrahaplotype and interhaplotype pairing of bovine leukocyte antigen DQA and DQB molecules generate functional DQ molecules important for priming CD4+ T-lymphocyte responses. Immunogenetics 2005, 57, 750–762. [Google Scholar] [CrossRef]

| Cattle Breeds | Number of Collected Samples | ||||
|---|---|---|---|---|---|
| Japanese Black | 523 | ||||
| Farm A | 7 | ||||
| Farm B | 10 | ||||
| Farm C | 437 | ||||
| Farm D | 69 | ||||
| Holstein Farm A | 68 | ||||
| Jersey Farm A | 3 | ||||
| Crossbreed Farm C | 4 | ||||
| Total | 598 | ||||
| Age (years) | |||||
| Min. | 1st Qu. | Median | Mean | 3rd Qu. | Max. |
| 0.1 | 3.5 | 5.7 | 8.0 | 14.8 | 4.9 |
| Clustering Groups of the log10 Proviral Load (Copies/50 ng) | Number of BLV-Positive Cattle |
|---|---|
| LPL grou; <=2.30 | 258 |
| MPL group; 2.31~2.69 | 62 |
| HPL group; 2.70~ | 278 |
| Total | 598 |
| BoLA-DRB3 Allele | Allele Frequency | Log10 PVL Median (Copies/50 ng) | CI 95% | Regression Coefficients | p-Value |
|---|---|---|---|---|---|
| DRB3.2* 10 | 277 (23 Homozygous) | 2.8 | (0.8–5.4) | Ref. | Ref. |
| DRB3.2* 16 | 383 (57 Homozygous) | 2.7 | (0.8–5.4) | 0.091 | NS |
| DRB3.2*8 | 106 (4 Homozygous) | 2.1 | (0.5–5.1) | −0.617 | <0.01 |
| DRB3.2*27 | 92 (1 Homozygous) | 2.6 | (0.5–5.6) | −0.134 | NS |
| DRB3.2*11 | 57 | 0 | (−1–0.3) | −9.343 | <0.0001 |
| DRB3.2*3 | 49 (1 Homozygous) | 2.4 | (0.4–5.4) | −1.0167 | <0.01 |
| DRB3.2*24 | 45 (2 Homozygous) | 2.6 | (0.4–5.4) | −0.764 | <0.05 |
| DRB3.2*1 | 49 (1 Homozygous) | 2.8 | (0.5–5.8) | 0.143 | NS |
| DRB3.2*23 | 21 (2 Homozygous) | 2.7 | (0.1–6.3) | 0.092 | NS |
| DRB3.2*28 | 28 | 2.2 | (0.2–5.4) | −1.149 | <0.01 |
| DRB3.2*41 | 32 | 2.9 | (0.4–6.1) | −0.143 | NS |
| DRB3.2*22 | 14 (2 Homozygous) | 2.3 | (−0.1–6.2) | −1.951 | <0.01 |
| DRB3.2*9 | 3 | 3.5 | (−0.1–7.1) | 0.572 | NS |
| DRB3.2*2 | 5 | 2.5 | (−0.3–5.7) | −1.215 | NS |
| DRB3.2*6 | 4 | 3 | (−0.3–6.6) | −0.283 | NS |
| DRB3.2*7 | 19 | 1.9 | (0.1–5.6) | −1.532 | <0.01 |
| DRB3.2*20 | 3 | 1.7 | (−0.6–6.7) | −3.904 | <0.05 |
| DRB3.2*25 | 3 | 3.3 | (−1.1–7.6) | 1.030 | NS |
| DRB3.2*32 | 3 | 3.1 | (−1.1–7.3) | 0.033 | NS |
| DRB3.2*13 | 1 | 1.7 | – | −3.278 | NS |
| DRB3.2*15 | 1 | 0 | – | −23.493 | NS |
| DRB3.2*30 | 1 | 3.2 | – | 0.223 | NS |
| Cattle age | 598 | −0.015 | NS | ||
| Crossbreed | 4 | Ref. | Ref. | ||
| Holstein | 68 | 1.034 | NS | ||
| Japanese Black | 523 | 1.209 | NS | ||
| Jersey | 3 | 0.989 | NS |
| BoLA-DRB3 Allele | Regression Coefficients | p-Value | OR | CI95% | Allele Frequencies | ||
|---|---|---|---|---|---|---|---|
| HPL Group | MPL Group | LPL Group | |||||
| DRB3.2*10 | Ref. | Ref. | 0.69 | (0.5–0.8) | 142 | 35 | 100 |
| DRB3.2*11 | −9.445 × 100 | <0.0001 | 61.42 | (8.4–446.7) | 0 | 0 | 57 |
| DRB3.2*8 | −1.319 × 100 | <0.001 | 1.40 | (0.9–2.2) | 43 | 7 | 56 |
| DRB3.2*28 | −1.094 × 100 | <0.01 | 1.89 | (0.8–4.6) | 8 | 5 | 15 |
| DRB3.2*7 | −1.681 × 100 | <0.01 | 1.98 | (0.7–5.0) | 6 | 2 | 11 |
| DRB3.2*3 | −1.055 × 100 | <0.01 | 1.33 | (0.7–2.4) | 20 | 4 | 25 |
| DRB3.2*24 | −8.777 × 10−1 | <0.01 | 1.32 | (0.7–2.5) | 16 | 7 | 22 |
| DRB3.2*22 | −1.974 × 100 | <0.01 | 2.42 | (0.7–7.9) | 4 | 1 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daous, H.E.; Mitoma, S.; Elhanafy, E.; Thi Nguyen, H.; Thi Mai, N.; Notsu, K.; Kaneko, C.; Norimine, J.; Sekiguchi, S. Relationship between Allelic Heterozygosity in BoLA-DRB3 and Proviral Loads in Bovine Leukemia Virus-Infected Cattle. Animals 2021, 11, 647. https://doi.org/10.3390/ani11030647
Daous HE, Mitoma S, Elhanafy E, Thi Nguyen H, Thi Mai N, Notsu K, Kaneko C, Norimine J, Sekiguchi S. Relationship between Allelic Heterozygosity in BoLA-DRB3 and Proviral Loads in Bovine Leukemia Virus-Infected Cattle. Animals. 2021; 11(3):647. https://doi.org/10.3390/ani11030647
Chicago/Turabian StyleDaous, Hala El, Shuya Mitoma, Eslam Elhanafy, Huyen Thi Nguyen, Ngan Thi Mai, Kosuke Notsu, Chiho Kaneko, Junzo Norimine, and Satoshi Sekiguchi. 2021. "Relationship between Allelic Heterozygosity in BoLA-DRB3 and Proviral Loads in Bovine Leukemia Virus-Infected Cattle" Animals 11, no. 3: 647. https://doi.org/10.3390/ani11030647
APA StyleDaous, H. E., Mitoma, S., Elhanafy, E., Thi Nguyen, H., Thi Mai, N., Notsu, K., Kaneko, C., Norimine, J., & Sekiguchi, S. (2021). Relationship between Allelic Heterozygosity in BoLA-DRB3 and Proviral Loads in Bovine Leukemia Virus-Infected Cattle. Animals, 11(3), 647. https://doi.org/10.3390/ani11030647






