Antibiotic-Resistant Escherichia coli and Salmonella from the Feces of Food Animals in the East Province of Rwanda
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fecal Sample Collection
2.2. Bacteria Isolation
2.3. Phenotypic Antibiotic Susceptibility Testing
2.4. Statistical Analysis
3. Results
3.1. Antibiotic Susceptibility
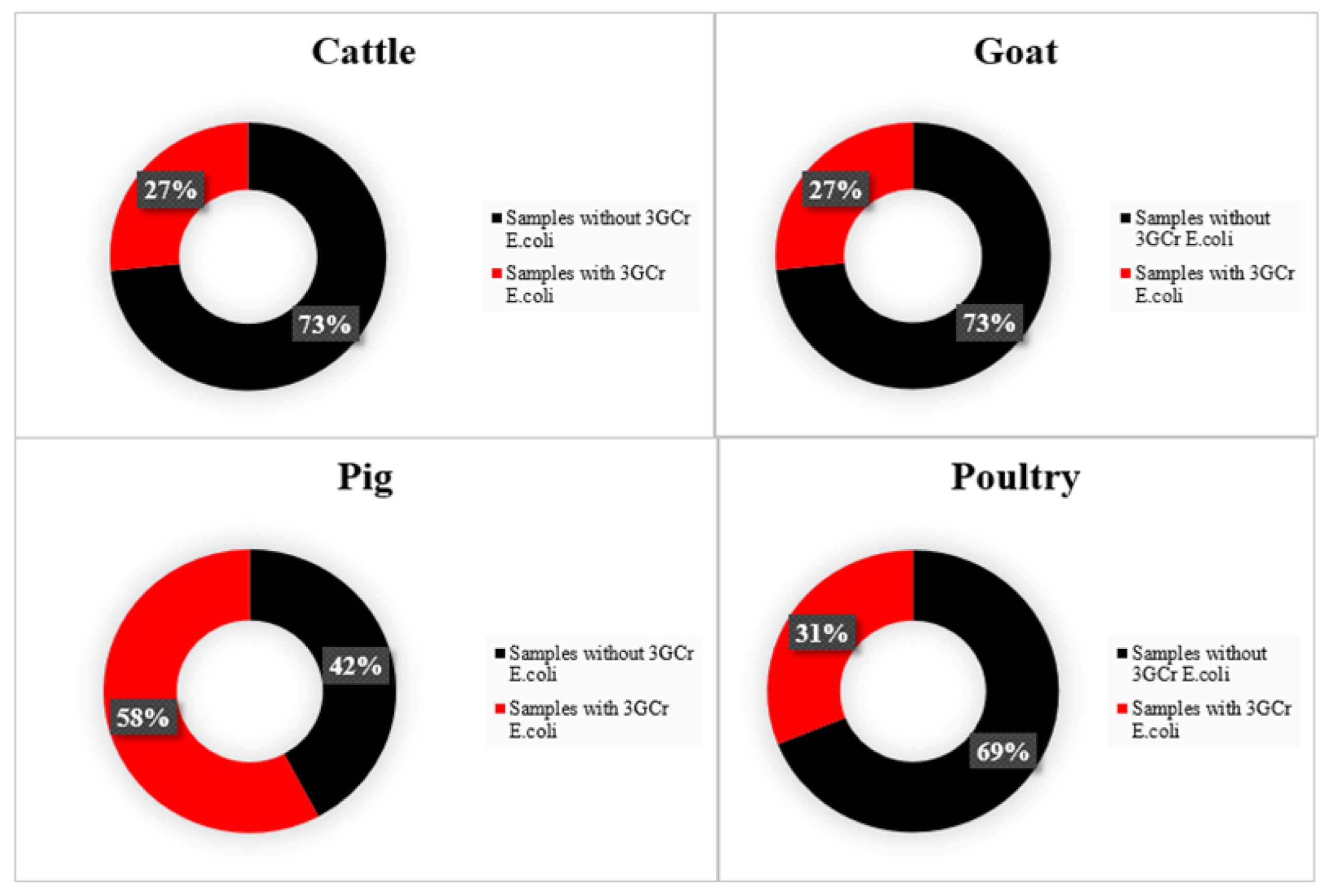
3.2. E. coli Producing ESBLs and Patterns of Resistance to Quinolones
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aarestrup, F.M. Antimicrobial Resistance in Bacteria of Animal Origin; ASM Press: Washington, DC, USA, 2006. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- World Health Organization. Integrated Surveillance of Antimicrobial Resistance in Foodborne Bacteria: Application of a One Health Approach; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Founou, L.L.; Amoako, D.G.; Founou, R.C.; Essack, S.Y. Antibiotic Resistance in Food Animals in Africa: A Systematic Review and Meta-Analysis. Microb. Drug Resist. 2018, 24, 648–665. [Google Scholar] [CrossRef] [PubMed]
- Kimera, Z.I.; Mshana, S.E.; Rweyemamu, M.M.; Mboera, L.E.G.; Matee, M.I.N. Antimicrobial Use and Resistance in Food- Producing Animals and the Environment: An African Perspective. Antimicrob. Resist. Infect. Control 2020, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omulo, S.; Thumbi, S.M.; Njenga, M.K.; Call, D.R. A Review of 40 Years of Enteric Antimicrobial Resistance Research in Eastern Africa: What Can Be Done Better? Antimicrob. Resist. Infect. Control 2015, 4, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katakweba, A.A.S.; Muhairwa, A.P.; Lupindu, A.M.; Damborg, P.; Rosenkrantz, J.T.; Minga, U.M.; Mtambo, M.M.A.; Olsen, J.E. First Report on a Randomized Investigation of Antimicrobial Resistance in Fecal Indicator Bacteria from Livestock, Poultry, and Humans in Tanzania. Microb. Drug Resist. 2018, 24, 260–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okubo, T.; Yossapol, M.; Maruyama, F.; Wampande, E.M.; Kakooza, S.; Ohya, K.; Tsuchida, S.; Asai, T.; Kabasa, J.D.; Ushida, K. Phenotypic and Genotypic Analyses of Antimicrobial Resistant Bacteria in Livestock in Uganda. Transbound. Emerg. Dis. 2018, 66, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, M.; Caudell, M.A.; Mair, C.; Davis, M.A.; Matthews, L.; Quinlan, R.J.; Quinlan, M.B.; Lyimo, B.; Buza, J.; Keyyu, J.; et al. Antimicrobial Resistant Enteric Bacteria Are Widely Distributed amongst People, Animals and the Environment in Tanzania. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef] [Green Version]
- Van den Honert, M.S.; Gouws, P.A.; Hoffman, L.C. Importance and Implications of Antibiotic Resistance Development in Livestock and Wildlife Farming in South Africa: A Review. S. Afr. J. Anim. Sci. 2018, 48, 401–412. [Google Scholar] [CrossRef] [Green Version]
- Hassell, J.M.; Ward, M.J.; Muloi, D.; Bettridge, J.M.; Robinson, T.P.; Kariuki, S.; Ogendo, A.; Kiiru, J.; Imboma, T.; Kang’ethe, E.K.; et al. Clinically Relevant Antimicrobial Resistance at the Wildlife–Livestock–Human Interface in Nairobi: An Epidemiological Study. Lancet Planet. Health 2019, 3, e259–e269. [Google Scholar] [CrossRef] [Green Version]
- Benavides, J.A.; Godreuil, S.; Bodenham, R.; Ratiarison, S.; Devos, C.; Petretto, M.O.; Raymond, M.; Escobar-Páramo, P. No Evidence for Transmission of Antibiotic-Resistant Escherichia Coli Strains from Humans to Wild Western Lowland Gorillas in Lopé National Park, Gabon. Appl. Environ. Microbiol. 2012, 78, 4281–4287. [Google Scholar] [CrossRef] [Green Version]
- Muvunyi, C.M.; Mutesa, L.; Masaisa, F.; Bayingana, C. Etiology and Antimicrobial Susceptibility Patterns of Acute Uncomplicated Cystitis from Primary Care Settings in Rwanda. Int. J. Infect. Dis. 2014, 21, 85–86. [Google Scholar] [CrossRef] [Green Version]
- Kurz, M.S.E.; Bayingana, C.; Ndoli, J.M.; Sendegeya, A.; Durst, A.; Pfuller, R.; Gahutu, J.B.; Mockenhaupt, F.P. Intense Pre-Admission Carriage and Further Acquisition of ESBL-Producing Enterobacteriaceae among Patients and Their Caregivers in a Tertiary Hospital in Rwanda. Trop. Med. Int. Health 2017, 22, 210–220. [Google Scholar] [CrossRef] [Green Version]
- Bogaerts, J.; Lepage, P.; Taelman, H.; Rouvroy, D.; Batungwanayo, J.; Kestelyn, P.; Hitimana, D.G.; Perre, P.V.; Vandepitte, J.; Verbist, L.; et al. Antimicrobial Susceptibility and Serotype Distribution of Streptococcus Pneumoniae from Rwanda, 1984-1990. J. Infect. 1993, 27, 157–168. [Google Scholar] [CrossRef]
- Bayingana, C.; Kayitare, E.; Nteziyaremye, J.; Sendegeya, A.; Ndoli, J.; Busumbigabo, A. Update on Antibiotic Activity on Bacterial Strains Isolated from Urine Samples at Butare University Teaching Hospital (Buth) Laboratory in Rwanda. Online Int. Interdiscip. Res. J. 2016, 6, 6–16. [Google Scholar]
- Rickard, J.; Ngarambe, C.; Ndayizeye, L.; Smart, B.; Riviello, R.; Majyambere, J.P.; Ghebre, R.G. Antibiotic Use and Antimicrobial Resistance of Surgical Patients with Peritonitis at a Tertiary Referral Hospital in Rwanda. Surg. Infect. 2018, 19, 382–387. [Google Scholar] [CrossRef] [Green Version]
- Muvunyi, C.M.; Masasaisa, F.; Bayingana, C.; Mutesa, L.; Musemakweri, A. Antimicrobial Resistance and Extended-Spectrum β- Lactamase (ESBL) Producing Clinical Isolates from Urinary Tract Infection at Two Teaching Hospitals in Rwanda. Rwanda J. Ser. F Health Sci. 2013, 1, 3–16. [Google Scholar] [CrossRef]
- Ntirenganya, C.; Manzi, O.; Muvunyi, C.M.; Ogbuagu, O. High Prevalence of Antimicrobial Resistance among Common Bacterial Isolates in a Tertiary Healthcare Facility in Rwanda. Am. J. Trop. Med. Hyg. 2015, 92, 865–870. [Google Scholar] [CrossRef]
- Sutherland, T.; Mpirimbanyi, C.; Nziyomaze, E.; Niyomugabo, J.P.; Niyonsenga, Z.; Muvunyi, C.M.; Mueller, A.; Bebell, L.M.; Nkubana, T.; Musoni, E.; et al. Widespread Antimicrobial Resistance among Bacterial Infections in a Rwandan Referral Hospital. PLoS ONE 2019, 14, e0221121. [Google Scholar] [CrossRef]
- Carroll, M.A. Five-Year Resistance Trends of Bacterial Isolates in Kigali, Rwanda. Master’s Thesis, School of Public Health, Yale University, New Haven, CT, USA, 2015. [Google Scholar]
- Niyonzima, E.; Ongol, M.P.; Brostaux, Y.; Korsak, N.; Daube, G.; Kimonyo, A.; Sindic, M.; Koulagenko, N.K.; Daube, G.; Kimonyo, A.; et al. Daily Intake and Bacteriological Quality of Meat Consumed in the Households of Kigali, Rwanda. Food Control 2016, 69, 108–114. [Google Scholar] [CrossRef]
- Kamana, O.; Ceuppens, S.; Jackesen, L.; Kimonyo, A.; Uuyttendaele, M. Microbiological Quality and Safety Assessment of the Rwandan Milk and Dairy Chain. J. Food Prot. 2014, 77, 299–307. [Google Scholar] [CrossRef]
- Manishimwe, R.; Buhire, M.; Uyisunze, A.; Turikumwenayo, J.B.; Tukei, M. Characterization of Antibiotic Resistant Escherichia Coli in Different Poultry Farming Systems in the Eastern Province and Kigali City of Rwanda. Rev. Elev. Med. Vet. Pays Trop. 2017, 70, 13–19. [Google Scholar] [CrossRef] [Green Version]
- National Institute of Statistics of Rwanda. Agricultural Household Survey 2017 Report; National Institute of Statistics Rwanda: Kigali, Rwanda, 2017.
- Rwandan Ministry of Agriculture and Animal Resources. Annual Report 2018–2019; Rwandan Ministry of Agriculture and Animal Resources: Kigali, Rwanda, 2019.
- National Institute of Statistics of Rwanda. Rwanda National Agriculture Survey 2008; National Institute of Statistics of Rwanda: Kigali, Rwanda, 2008; Volume 2008.
- Shapiro, B.I.; Gebru, G.; Desta, S.; Nigussie, K. Rwanda Livestock Master Plan. 2017. Available online: https://cgspace.cgiar.org/handle/10568/104049 (accessed on 10 January 2021).
- World Organisation for Animal Health. WAHIS Rwanda 2018. Available online: https://www.oie.int/wahis_2/public/wahid.php/Countryinformation/Animalsituation (accessed on 15 September 2020).
- Iraguha, B.; Hamudikuwanda, H.; Mushonga, B. Bovine Mastitis Prevalence and Associated Risk Factors in Dairy Cows in Nyagatare District, Rwanda. J. S. Afr. Vet. Assoc. 2015, 86, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ndahetuye, J.B.; Persson, Y.; Nyman, A.K.; Tukei, M.; Ongol, M.P.; Båge, R. Aetiology and Prevalence of Subclinical Mastitis in Dairy Herds in Peri-Urban Areas of Kigali in Rwanda. Trop. Anim. Health Prod. 2019, 51, 2037–2044. [Google Scholar] [CrossRef] [Green Version]
- Ndazigaruye, G.; Mushonga, B.; Kandiwa, E.; Samkange, A.; Segwagwe, B.E. Prevalence and Risk Factors for Brucellosis Seropositivity in Cattle in Nyagatare District, Eastern Province, Rwanda. J. S. Afr. Vet. Assoc. 2018, 89, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Manishimwe, R.; Ntaganda, J.; Habimana, R.; Nishimwe, K.; Byukusenge, M.; Dutuze, F.; Ayabagabo, J.D.; Lydia, U.; Rukundo, J.C. Comparison between Rose Bengal Plat Test and Competitive Enzyme Linked Immunosorbent Assay to Detect Bovine Brucellosis in Kigali City, Rwanda. J. Vet. Sci. Technol. 2015, 06, 1–4. [Google Scholar] [CrossRef]
- Rujeni, N.; Gasogo, A.; Mbanzamihigo, L. Contribution to the Study of the Prevalence of Brucellosis in Rwanda. Case Study of Huye District. In Proceedings of the 13th International Congress on Infectious Diseases, Kuala Lumpur, Malaysia, 19–22 June 2008; Volume 12, p. e128. [Google Scholar] [CrossRef] [Green Version]
- Bazarusanga, T.; Vercruysse, J.; Marcotty, T.; Geysen, D. Epidemiological Studies on Theileriosis and the Dynamics of Theileria Parva Infections in Rwanda. Vet. Parasitol. 2007, 143, 214–221. [Google Scholar] [CrossRef]
- Shyaka, A.; Ugirabe, M.A.; Wensman, J.J. Serological Evidence of Exposure to Peste Des Petits Ruminants in Small Ruminants in Rwanda. Front. Vet. Sci. 2021, 8, 186. [Google Scholar] [CrossRef]
- Rwanda Agriculture Board. Rwanda Agriculture Board Annual Report 2016–2017; Rwanda Agriculture Board: Kigali, Rwanda, 2017.
- Manishimwe, R.; Nishimwe, K.; Ojok, L. Assessment of Antibiotic Use in Farm Animals in Rwanda. Trop. Anim. Health Prod. 2017, 49. [Google Scholar] [CrossRef]
- Timothy, P.J. Snowball Sampling: Introduction. Wiley StatsRef Stat. Ref. Online 2014. [Google Scholar] [CrossRef]
- Manishimwe, R.; Paola, M.M.; Bugarel, M.; Scott, H.M.; Loneragan, G.H. Antibiotic Resistance among Escherichia Coli and Salmonella Isolated from Dairy Cattle Feces in Texas. bioRxiv 2020. [Google Scholar] [CrossRef]
- Hendriksen, R.S.; Bortolaia, V.; Pedersen, S.K.; Hasman, H.; Agersø, Y.; Cavaco, L.M.; Guerra-Roman, B. Isolation of ESBL-, AmpC- and Carbapenemase-Producing E. Coli from Caecal Samples. 2019. Available online: https://www.eurl-ar.eu/CustomerData/Files/Folders/21-protocols/529_esbl-ampc-cpeprotocol-version-meat-v7-09-12-19.pdf (accessed on 10 January 2021).
- Hara-kudo, Y. Loop-Mediated Isothermal Amplification for the Rapid Detection of Salmonella. FEMS Microbiol. Lett. 2005, 253, 155–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinical and Laboratory Standards Institute. M100 Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Galani, I.; Kontopidou, F.; Souli, M.; Rekatsina, P.; Koratzanis, E.; Deliolanis, J.; Giamarellou, H. Colistin Susceptibility Testing by Etest and Disk Diffusion Methods. Int. J. Antimicrob. Agents 2008, 31, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langata, L.M.; Maingi, J.M.; Musonye, H.A.; Kiiru, J.; Nyamache, A.K. Antimicrobial Resistance Genes in Salmonella and Escherichia Coli Isolates from Chicken Droppings in Nairobi, Kenya. BMC Res. Notes 2019, 12, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usui, M.; Ozawa, S.; Onozato, H.; Kuge, R.; Obata, Y.; Uemae, T. Antimicrobial Susceptibility of Indicator Bacteria Isolated from Chickens in Southeast Asian Countries (Vietnam, Indonesia and Thailand). J. Vet. Med. Sci. 2014, 76, 685–692. [Google Scholar] [CrossRef] [Green Version]
- Van Boeckel, T.P.; Pires, J.; Silvester, R.; Zhao, C.; Song, J.; Criscuolo, N.G.; Gilbert, M.; Bonhoeffer, S.; Laxminarayan, R. Global Trends in Antimicrobial Resistance in Animals in Low- And Middle-Income Countries. Science 2019, 365, 1252–1255. [Google Scholar] [CrossRef] [Green Version]
- Hounmanou, Y.M.G.; Mdegela, R.H. Current Situation for Antimicrobial Use, Antimicrobial Resistance and Antimicrobial Residues in the Food and Agriculture Sectors in Tanzania: A Review. Tanzania Vet. Assoc. 2017, 35, 58–62. [Google Scholar]
- Mitema, E.S.; Kikuvi, G.M.; Wegener, H.C.; Sthor, K. An Assessment of Antimicrobial Consumption in Food Producing Animals in Kenya. J. Vet. Pharmacol. Ther. 2001, 24, 385–390. [Google Scholar] [CrossRef]
- International Organization for Standarization. Microbiology of Food Chain-Horizontal Method for Detection, Enumeration and Serotyping of Salmonella; ISO-6579-1-2017; International Organization for Standarization: Geneva, Switzerland, 2017. [Google Scholar]
- Lúcia, V.; Rall, M.; Rall, R.; Aragon, L.C.; Guimarães, M. Evaluation of Three Enrichment Broths and Five Plating Media for Salmonella Detection in Poultry. Braz. J. Microbiol. 2005, 35, 147–150. [Google Scholar]
- Voogt, N.; Raes, M.; Wannet, W.J.B.; Henken, A.M.; Van De Giessen, A.W. Comparison of Selective Enrichment Media for the Detection of Salmonella in Poultry Faeces. Lett. Appl. Microbiol. 2001, 32, 89–92. [Google Scholar] [CrossRef] [Green Version]
- Kunze, D.J.; Loneragan, G.H.; Platt, T.M.; Miller, M.F.; Besser, T.E.; Koohmaraie, M.; Stephens, T.; Brashears, M.M. Salmonella Enterica Burden in Harvest-Ready Cattle Populations from the Southern High Plains of the United States. Appl. Environ. Microbiol. 2008, 74, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Likavec, T.; Pires, A.F.A.; Funk, J.A. Association between Thermal Environment and Salmonella in Fecal Samples from Dairy Cattle in Midwestern United States. Can. J. Vet. Res. 2016, 80, 183–188. [Google Scholar]
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the Mechanisms and Drivers of Antimicrobial Resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Doyle, M.P.; Loneragan, G.H.; Scott, H.M.; Singer, R.S. Antimicrobial Resistance: Challenges and Perspectives. Compr. Rev. Food Sci. Food Saf. 2013, 12, 234–248. [Google Scholar] [CrossRef]
- Boonyasiri, A.; Tangkoskul, T.; Seenama, C.; Saiyarin, J.; Tiengrim, S.; Thamlikitkul, V. Prevalence of Antibiotic Resistant Bacteria in Healthy Adults, Foods, Food Animals, and the Environment in Selected Areas in Thailand. Pathog. Glob. Health 2014, 108, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Bonnet, R. Growing Group of Extended-Spectrum -Lactamases: The CTX-M Enzymes. Antimicrob. Agents Chemother. 2004, 48, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Dellgren, L.; Claesson, C.; Högdahl, M.; Forsberg, J.; Hanberger, H.; Nilsson, L.E.; Hällgren, A. Phenotypic Screening for Quinolone Resistance in Escherichia Coli. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1765–1771. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Martínez, J.M.; López-Cerero, L.; Díaz-de-Alba, P.; Chamizo-López, F.J.; Polo-Padillo, J.; Pascual, Á. Assessment of a Phenotypic Algorithm to Detect Plasmid-Mediated Quinolone Resistance in Enterobacteriaceae. J. Antimicrob. Chemother. 2016, 71, 845–847. [Google Scholar] [CrossRef] [Green Version]
- Hang, B.P.T.; Wredle, E.; Börjesson, S.; Sjaunja, K.S.; Dicksved, J.; Duse, A. High Level of Multidrug-Resistant Escherichia Coli in Young Dairy Calves in Southern Vietnam. Trop. Anim. Health Prod. 2019, 51, 1405–1411. [Google Scholar] [CrossRef]
- World Health Organization. Critically Important Antimicrobials for Human Medicine; 6th Revision; 2018; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- World Organization for Animal Health. OIE List of Antimicrobial Agents of Veterinary Importance; World Organization for Animal Health: France, Paris, 2019. [Google Scholar]
- Xiong, W.; Sun, Y.; Zeng, Z. Antimicrobial Use and Antimicrobial Resistance in Food Animals. Environ. Sci. Pollut. Res. 2018, 25, 18377–18384. [Google Scholar] [CrossRef]

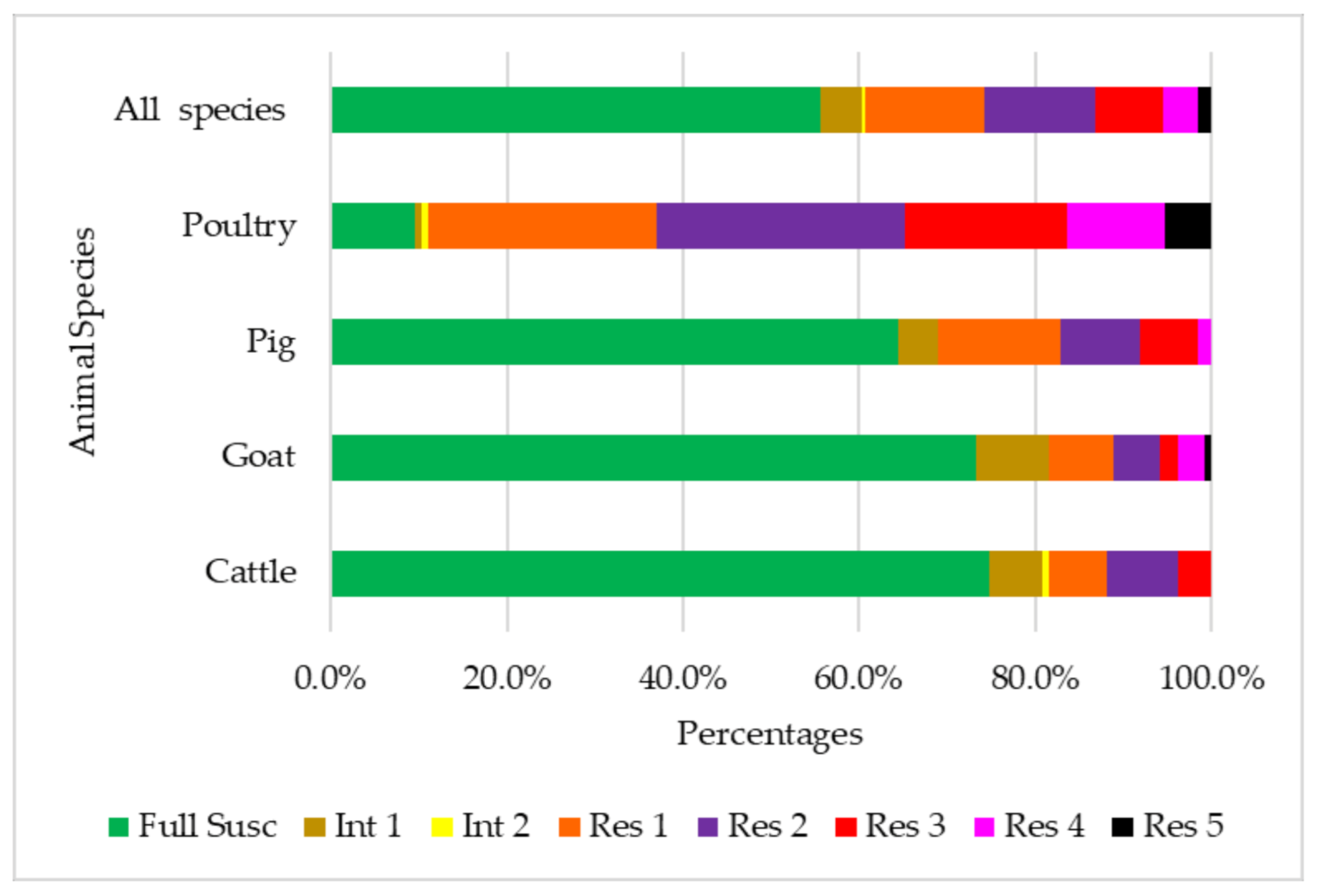

| ATB | R | I | S | Distribution (Number) in Each Inhibition Zone: Diameter (in mm) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | % | 0 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | >25 | |
| AMC | 0.7 | 0.3–1.9 | 1.1 | 98.1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 9 | 12 | 33 | 63 | 87 | 90 | 80 | 69 | 87 | ||||
| AMP | 19.6 | 16.5–23.2 | 2.0 | 78.3 | 99 | 1 | 1 | 2 | 1 | 2 | 1 | 5 | 5 | 9 | 38 | 57 | 81 | 73 | 64 | 53 | 24 | 12 | 12 | ||
| AZI | 1.1 | 0.5–2.4 | 98.9 | 1 | 3 | 2 | 2 | 5 | 7 | 6 | 11 | 35 | 40 | 39 | 44 | 64 | 70 | 66 | 1 | 144 | |||||
| FOX | 0.7 | 0.3–1.9 | 0.6 | 98.7 | 3 | 1 | 1 | 2 | 1 | 2 | 3 | 17 | 41 | 72 | 101 | 105 | 191 | ||||||||
| CRO | 0.0 | 0.0–0.7 | 0.4 | 99.6 | 1 | 1 | 3 | 8 | 527 | ||||||||||||||||
| CHL | 3.3 | 2.1–5.2 | 0.2 | 96.5 | 12 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 9 | 23 | 40 | 65 | 380 | |||||||
| CIP | 3.1 | 2.0–5.0 | 1.7 | 95.2 | 3 | 1 | 2 | 3 | 3 | 4 | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 3 | 4 | 6 | 13 | 486 | |||
| COL | 2.0 | 1.1–3.6 | 40.4 | 57.6 | 1 | 10 | 80 | 138 | 156 | 106 | 39 | 9 | 1 | ||||||||||||
| MER | 0.0 | 0.0–0.7 | 0.2 | 99.8 | 1 | 1 | 1 | 7 | 530 | ||||||||||||||||
| NAL | 6.5 | 4.7–8.9 | 4.3 | 89.3 | 25 | 1 | 2 | 4 | 2 | 1 | 3 | 7 | 7 | 6 | 8 | 8 | 23 | 24 | 53 | 66 | 72 | 228 | |||
| STR | 16.5 | 13.6–19.8 | 6.1 | 77.4 | 57 | 6 | 4 | 11 | 4 | 7 | 5 | 7 | 21 | 55 | 101 | 88 | 89 | 52 | 21 | 5 | 4 | 2 | 1 | ||
| TET | 35.6 | 31.6–39.7 | 1.3 | 63.1 | 101 | 22 | 32 | 23 | 11 | 3 | 5 | 1 | 1 | 1 | 4 | 13 | 35 | 44 | 47 | 58 | 50 | 89 | |||
| Cattle (n = 135) | Goats (n = 135) | Pigs (n = 135) | Poultry (n = 135) | Total (n = 540) | |
|---|---|---|---|---|---|
| Antibiotic | Number (%) | Number (%) | Number (%) | Number (%) | Number (%) |
| Amoxicillin–clavulanic acid | 1 (0.7) | 2 (1.5) | 0 (0.0) | 1 (0.7) | 4 (0.7) |
| Ampicillin * | 6 (4.4) | 10 (7.4) | 17 (12.6) | 73 (54.1) | 106 (19.6) |
| Azithromycin | 0 (0.0) | 1 (0.7) | 1 (0.7) | 4 (3.0) | 6 (1.1) |
| Cefoxitin | 1 (0.7) | 2 (1.5) | 1 (0.7) | 0 (0.0) | 4 (0.7) |
| Ceftriaxone | 0 (0.0) | 2 (1.5) | 0 (0.0) | 0 (0.0) | 2 (0.4) |
| Chloramphenicol * | 0 (0.0) | 3 (2.2) | 3 (2.2) | 12 (8.9) | 18 (3.3) |
| Ciprofloxacin * | 1 (0.7) | 2 (1.5) | 0 (0.0) | 14 (10.4) | 17 (3.1) |
| Colistin | 2 (0.4) | 5 (0.9) | 2 (0.4) | 2 (0.4) | 11 (2.0) |
| Meropenem | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nalidixic acid * | 1 (0.7) | 3 (2.2) | 0 (0.0) | 31 (23.0) | 35 (6.5) |
| Streptomycin * | 15 (11.1) | 12 (8.9) | 18 (13.3) | 44 (32.6) | 89 (16.5) |
| Tetracycline * | 22 (16.3) | 19 (14.1) | 36 (26.7) | 115 (85.2) | 192 (35.6) |
| Pan-susceptible * | 101 (74.8) | 99 (73.3) | 87 (64.4) | 13 (13.6) | 300 (55.6) |
| ATB | R | I | S | Distribution (Number) in Each Inhibition Zones: Diameters (in mm) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | % | 0 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | >25 | |
| AMC | 1.6 | 0.3–8.3 | 7.8 | 90.6 | 1 | 1 | 1 | 1 | 2 | 8 | 10 | 10 | 11 | 11 | 5 | 2 | 1 | ||||||||
| AMP | 100.0 | 94.3–100.0 | 0 | 0 | 64 | ||||||||||||||||||||
| AZI | 14.1 | 7.6–24.6 | 85.9 | 3 | 1 | 1 | 4 | 1 | 1 | 4 | 1 | 6 | 2 | 8 | 11 | 5 | 4 | 12 | |||||||
| FOX | 1.6 | 0.3–8.3 | 0 | 98.4 | 1 | 1 | 2 | 3 | 7 | 11 | 14 | 12 | 13 | ||||||||||||
| CRO | 100.0 | 94.3–100.0 | 0 | 0 | 18 | 1 | 2 | 12 | 8 | 16 | 5 | 2 | |||||||||||||
| CHL | 0.0 | 0.0–5.7 | 0 | 100 | 1 | 2 | 1 | 6 | 54 | ||||||||||||||||
| CIP | 15.6 | 8.7–26.4 | 4.7 | 79.7 | 8 | 1 | 1 | 1 | 2 | 2 | 3 | 2 | 4 | 9 | 31 | ||||||||||
| COL | 0.0 | 0.0–5.7 | 32.8 | 67.2 | 8 | 13 | 22 | 17 | 4 | ||||||||||||||||
| MER | 0.0 | 0.0–5.7 | 0 | 100 | 1 | 2 | 61 | ||||||||||||||||||
| NAL | 29.7 | 19.9–41.8 | 6.3 | 64.1 | 15 | 1 | 2 | 1 | 1 | 2 | 1 | 8 | 2 | 9 | 5 | 10 | 3 | 4 | |||||||
| STR | 84.4 | 73.6–91.3 | 4.7 | 10.9 | 26 | 9 | 9 | 6 | 3 | 1 | 1 | 2 | 3 | 2 | 2 | ||||||||||
| TET | 87.5 | 77.2–93.5 | 0 | 12.5 | 22 | 4 | 14 | 11 | 2 | 3 | 1 | 1 | 2 | 2 | 2 | ||||||||||
| ATB | R | I | S | Distribution (Number) in Each Inhibition Zones: Diameters (in mm) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | % | 0 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | >25 | |
| AMC | 0 | 0.0–4.2 | 2.3 | 97.7 | 2 | 8 | 6 | 16 | 16 | 13 | 11 | 8 | 5 | 2 | |||||||||||
| AMP | 55.2 | 44.7–65.2 | 1.1 | 42.5 | 46 | 1 | 1 | 1 | 5 | 3 | 4 | 9 | 11 | 3 | 1 | 1 | 1 | ||||||||
| AZI | 13.8 | 8.1–22.6 | 86.2 | 2 | 5 | 5 | 1 | 5 | 6 | 1 | 3 | 8 | 5 | 12 | 7 | 7 | 7 | 5 | 4 | 4 | |||||
| FOX | 1.1 | 0.2–6.2 | 2.3 | 96.6 | 1 | 2 | 5 | 4 | 10 | 15 | 10 | 15 | 14 | 11 | |||||||||||
| CRO | 1.1 | 0.2–6.2 | 0 | 98.9 | 1 | 2 | 1 | 3 | 80 | ||||||||||||||||
| CHL | 17.2 | 10.7–26.5 | 0 | 82.8 | 10 | 2 | 2 | 1 | 1 | 3 | 4 | 5 | 11 | 10 | 38 | ||||||||||
| CIP | 58.6 | 48.1–68.4 | 20.7 | 19.5 | 24 | 2 | 3 | 8 | 8 | 4 | 2 | 1 | 4 | 5 | 8 | 2 | 3 | 5 | 4 | 3 | 1 | ||||
| COL | 3.4 | 1.2–9.7 | 52.9 | 43.7 | 3 | 16 | 30 | 30 | 5 | 2 | 1 | ||||||||||||||
| MER | 0 | 0.0–4.2 | 0 | 100.0 | 1 | 4 | 82 | ||||||||||||||||||
| NAL | 100.0 | 95.8–100.0 | 0 | 0 | 63 | 3 | 2 | 8 | 4 | 4 | 3 | ||||||||||||||
| STR | 48.3 | 38.1–58.6 | 13.8 | 37.9 | 32 | 4 | 3 | 1 | 1 | 1 | 1 | 2 | 9 | 9 | 12 | 4 | 4 | 3 | 1 | ||||||
| TET | 95.4 | 88.8–98.2 | 0 | 4.6 | 53 | 9 | 7 | 7 | 5 | 2 | 2 | 2 | |||||||||||||
| ATB | R | I | S | Distribution (Number) in Each Inhibition Zones: Diameters (in mm) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | % | 0 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | >28 | |
| AMK | 0 | 0.0–5.1 | 0 | 100.0 | 1 | 3 | 5 | 10 | 6 | 17 | 14 | 8 | 5 | 1 | 2 | |||||||||||||
| CFZ | 98.6 | 92.5–99.8 | 1.4 | 71 | 1 | |||||||||||||||||||||||
| FEP | 45.8 | 34.8–57.3 | 50 | 4.2 | 2 | 2 | 2 | 2 | 2 | 5 | 4 | 2 | 7 | 5 | 11 | 12 | 9 | 1 | 1 | 2 | 1 | 2 | ||||||
| CTX | 98.6 | 92.5–99.8 | 0 | 1.4 | 34 | 2 | 6 | 6 | 8 | 7 | 3 | 2 | 1 | 1 | 1 | 1 | ||||||||||||
| CTX-CLA | 1 | 2 | 3 | 1 | 3 | 1 | 5 | 5 | 5 | 10 | 8 | 13 | 15 | |||||||||||||||
| CAZ | 31.9 | 22.3–43.4 | 43.1 | 25 | 1 | 3 | 7 | 12 | 14 | 11 | 6 | 5 | 5 | 4 | 1 | 2 | 1 | |||||||||||
| CAZ-CLA | 1 | 1 | 1 | 3 | 12 | 13 | 14 | 27 | ||||||||||||||||||||
| FOS | 0 | 0.0–5.1 | 0 | 100.0 | 2 | 5 | 6 | 9 | 5 | 10 | 9 | 26 | ||||||||||||||||
| GEN | 6.9 | 3.0–15.2 | 0 | 93.1 | 1 | 3 | 1 | 1 | 1 | 7 | 16 | 10 | 16 | 10 | 4 | 1 | 1 | |||||||||||
| IMP | 0 | 0.0–5.1 | 0 | 100.0 | 2 | 3 | 5 | 7 | 9 | 7 | 39 | |||||||||||||||||
| SSS | 95.8 | 88.5–98.6 | 0 | 4.2 | 67 | 1 | 1 | 2 | 1 | |||||||||||||||||||
| SXT | 88.9 | 79.6–94.3 | 0 | 11.1 | 63 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | |||||||||||||||
| ESBL | 98.6 | 92.5–99.8 | ||||||||||||||||||||||||||
| AmpC | 0 | 0.0–5.1 | ||||||||||||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manishimwe, R.; Moncada, P.M.; Musanayire, V.; Shyaka, A.; Scott, H.M.; Loneragan, G.H. Antibiotic-Resistant Escherichia coli and Salmonella from the Feces of Food Animals in the East Province of Rwanda. Animals 2021, 11, 1013. https://doi.org/10.3390/ani11041013
Manishimwe R, Moncada PM, Musanayire V, Shyaka A, Scott HM, Loneragan GH. Antibiotic-Resistant Escherichia coli and Salmonella from the Feces of Food Animals in the East Province of Rwanda. Animals. 2021; 11(4):1013. https://doi.org/10.3390/ani11041013
Chicago/Turabian StyleManishimwe, Rosine, Paola M. Moncada, Vestine Musanayire, Anselme Shyaka, H. Morgan Scott, and Guy H. Loneragan. 2021. "Antibiotic-Resistant Escherichia coli and Salmonella from the Feces of Food Animals in the East Province of Rwanda" Animals 11, no. 4: 1013. https://doi.org/10.3390/ani11041013








