Integrated Use of Molecular Techniques to Detect and Genetically Characterise DNA Viruses in Italian Wolves (Canis lupus italicus)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sampling
2.2. Detection of Carnivore Protoparvovirus 1, Canine Adenovirus, and Canine Circovirus DNA
2.3. Genetic Characterisation of the Viruses Identified
3. Results
3.1. Study Population
3.2. Detection of Carnivore Protoparvovirus 1, Canine Adenovirus, and Canine Circovirus DNA
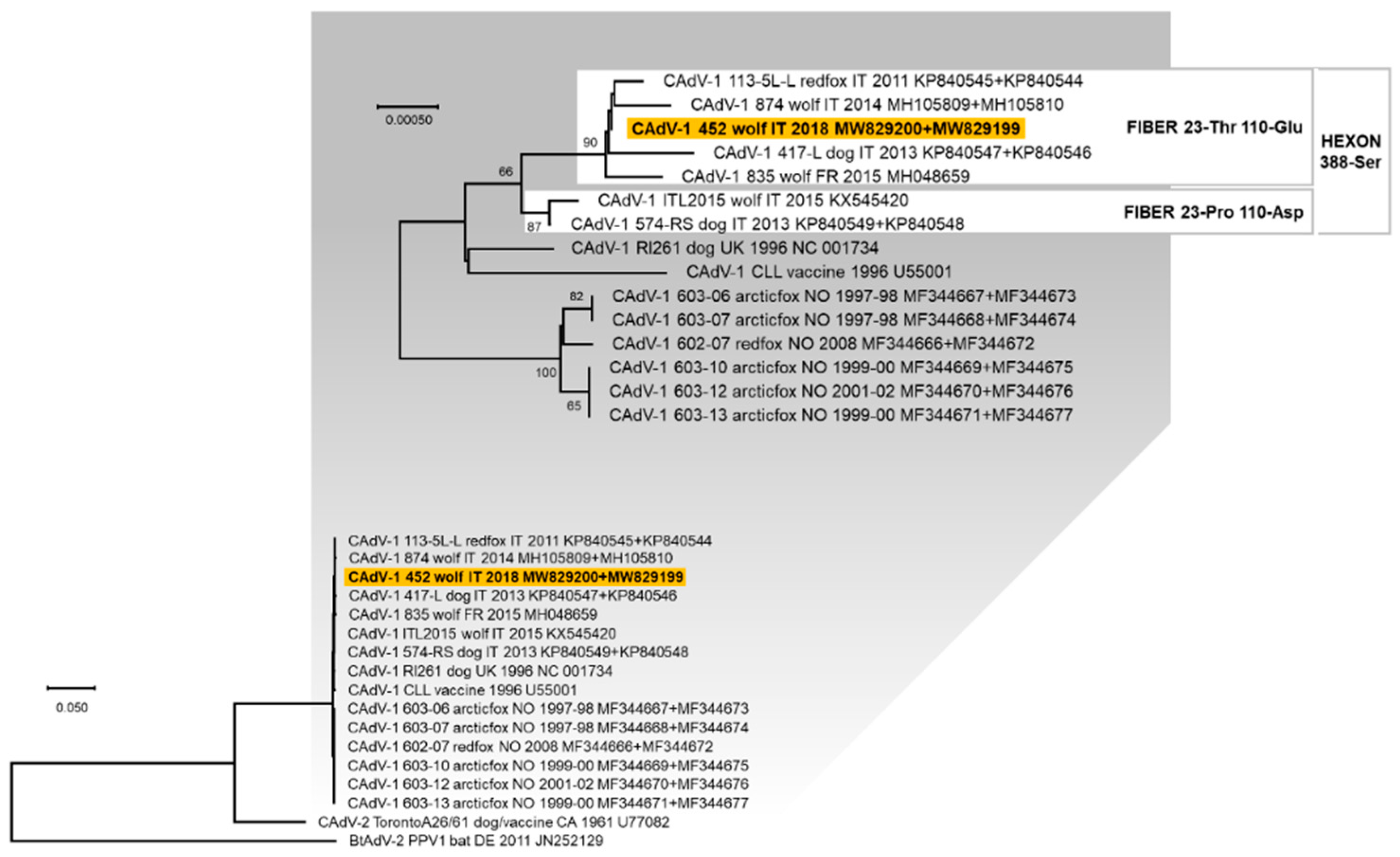
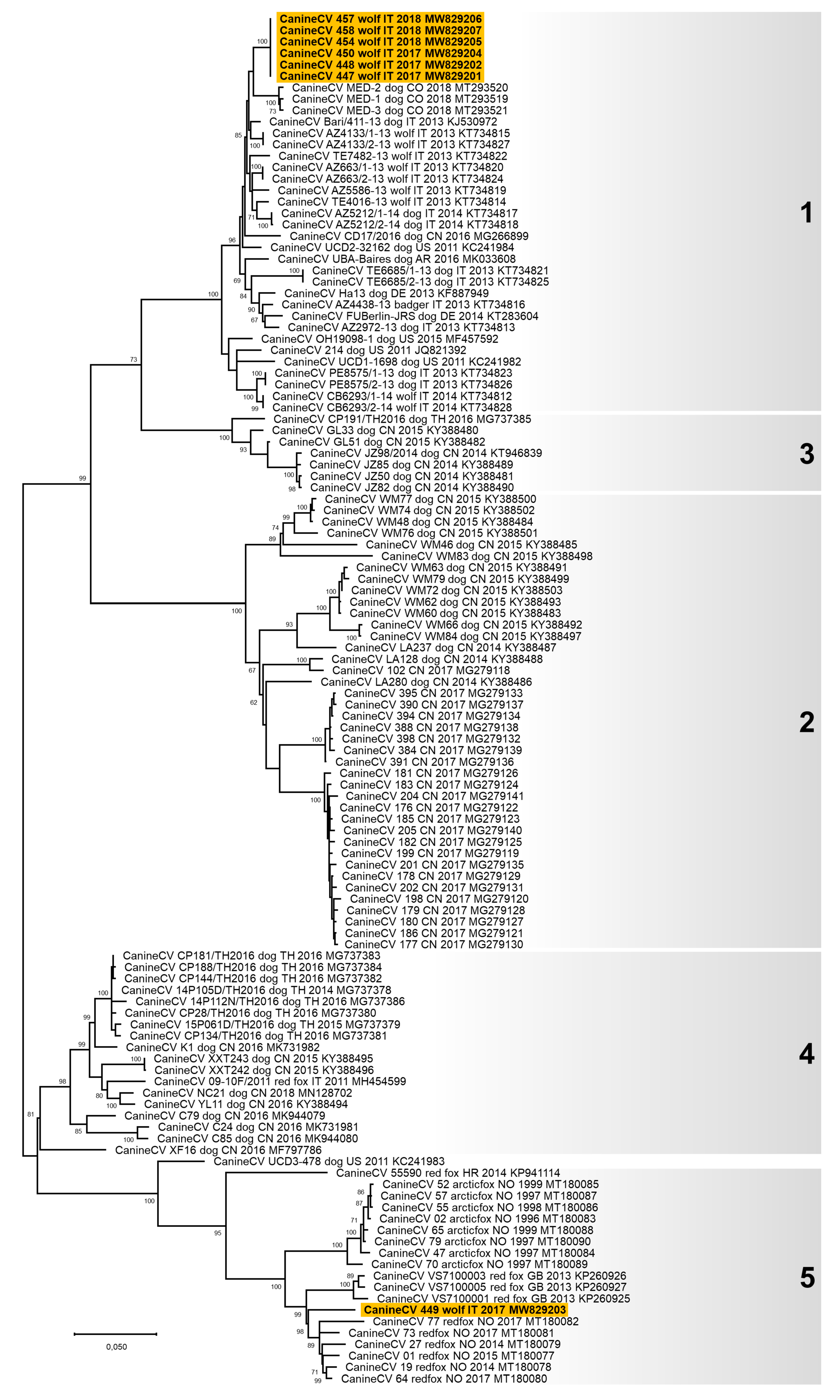
3.3. Genetic Characterisation of the Viruses Identified
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pedersen, A.B.; Jones, K.E.; Nunn, C.L.; Altizer, S. Infectious diseases and extinction risk in wild mammals. Conserv. Biol. 2007, 21, 1269–1279. [Google Scholar] [CrossRef]
- Smith, K.F.; Acevedo-Whitehouse, K.; Pedersen, A.B. The role of infectious diseases in biological conservation. Conserv. Biol. 2009, 12, 1–12. [Google Scholar] [CrossRef]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging infectious diseases of wildlife—Threats to biodiversity and human health. Science 2000, 287, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Murray, D.L.; Kapke, C.A.; Evermann, J.F.; Fuller, T.K. Infectious disease and the conservation of free-ranging large carnivores. Anim. Conserv. 1999, 2, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Balboni, A.; Savini, F.; Scagliarini, A.; Berti, E.; Naldi, M.; Urbani, L.; Fontana, M.C.; Carra, E.; Gibelli, L.R.M.; Gobbo, F.; et al. Persistent natural distemper infection in stone martens (Martes foina): From infection to neutralizing antibodies. Res. Vet. Sci. 2021, 138, 196–200. [Google Scholar] [CrossRef]
- Sobrino, R.; Arnal, M.C.; Luco, D.F.; Gortázar, C. Prevalence of antibodies against canine distemper virus and canine parvovirus among foxes and wolves from Spain. Vet. Microbiol. 2008, 126, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Miranda, C.; Thompson, G. Canine parvovirus: The worldwide occurrence of antigenic variants. J. Gen. Virol. 2016, 97, 2043–2057. [Google Scholar] [CrossRef]
- Almberg, E.S.; Mech, L.D.; Smith, D.W.; Sheldon, J.W.; Crabtree, R.L. A serological survey of infectious disease in Yellowstone National Park′s canid community. PLoS ONE 2009, 4, e7042. [Google Scholar] [CrossRef] [PubMed]
- Frölich, K.; Streich, W.J.; Fickel, J.; Jung, S.; Truyen, U.; Hentschke, J.; Dedek, J.; Prager, D.; Latz, N. Epizootiologic investigations of parvovirus infections in free-ranging carnivores from Germany. J. Wildl. Dis. 2005, 41, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Cotmore, S.F.; Agbandje-McKenna, M.; Canuti, M.; Chiorini, J.A.; Eis-Hubinger, A.M.; Hughes, J.; Mietzsch, M.; Modha, S.; Ogliastro, M.; Pénzes, J.J.; et al. Ictv Report Consortium. ICTV Virus Taxonomy Profile: Parvoviridae. J. Gen. Virol. 2019, 100, 367–368. [Google Scholar] [CrossRef]
- Eugster, A.K.; Nairn, C. Diarrhea in puppies: Parvovirus-like particles demonstrated in their feces. Southwest Vet. 1977, 30, 59. [Google Scholar]
- Truyen, U.; Gruenberg, A.; Chang, S.F.; Obermaier, B.; Veijalainen, P.; Parrish, C.R. Evolution of the feline-subgroup parvoviruses and the control of canine host range in vivo. J. Virol. 1995, 69, 4702–4710. [Google Scholar] [CrossRef] [Green Version]
- Ndiana, L.A.; Lanave, G.; Desario, C.; Berjaoui, S.; Alfano, F.; Puglia, I.; Fusco, G.; Colaianni, M.L.; Vincifori, G.; Camarda, A.; et al. Circulation of diverse protoparvoviruses in wild carnivores, Italy. Transbound. Emerg. Dis. 2020. Online ahead of print. [Google Scholar] [CrossRef]
- Decaro, N.; Buonavoglia, C. Canine parvovirus--a review of epidemiological and diagnostic aspects, with emphasis on type 2c. Vet. Microbiol. 2012, 155, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Steinel, A.; Parrish, C.R.; Bloom, M.E.; Truyen, U. Parvovirus infections in wild carnivores. J. Wildl. Dis. 2001, 37, 594–607. [Google Scholar] [CrossRef]
- Calatayud, O.; Esperón, F.; Velarde, R.; Oleaga, A.; Llaneza, L.; Ribas, A.; Negre, N.; de la Torre, A.; Rodríguez, A.; Millán, J. Genetic characterization of Carnivore Parvoviruses in Spanish wildlife reveals domestic dog and cat-related sequences. Transbound. Emerg. Dis. 2020, 67, 626–634. [Google Scholar] [CrossRef]
- Alfano, F.; Dowgier, G.; Valentino, M.P.; Galiero, G.; Tinelli, A.; Decaro, N.; Fusco, G. Identification of pantropic canine coronavirus in a wolf (Canis lupus italicus) in Italy. J. Wildl. Dis. 2019, 55, 504–508. [Google Scholar]
- Miranda, C.; Santos, N.; Parrish, C.; Thompson, G. Genetic characterization of canine parvovirus in sympatric free-ranging wild carnivores in Portugal. J. Wildl. Dis. 2017, 53, 824–831. [Google Scholar] [CrossRef]
- Zaccaria, G.; Malatesta, D.; Scipioni, G.; Di Felice, E.; Campolo, M.; Casaccia, C.; Savini, G.; Di Sabatino, D.; Lorusso, A. Circovirus in domestic and wild carnivores: An important opportunistic agent? Virology 2016, 490, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Molnar, B.; Duchamp, C.; Möstl, K.; Diehl, P.-A.; Betschart, B. Comparative survey of canine parvovirus, canine distemper virus and canine enteric coronavirus infection in free-ranging wolves of central Italy and south-eastern France. Eur. J. Wildl. Res. 2014, 60, 613–624. [Google Scholar] [CrossRef]
- Martinello, F.; Galuppo, F.; Ostanello, F.; Guberti, V.; Prosperi, S. Detection of canine parvovirus in wolves from Italy. J. Wildl. Dis. 1997, 33, 628–631. [Google Scholar] [CrossRef]
- Viscardi, M.; Santoro, M.; Clausi, M.T.; Cozzolino, L.; Decaro, N.; Colaianni, M.L.; Fusco, G. Molecular detection and characterization of carnivore parvoviruses in free-ranging Eurasian otters (Lutra lutra) in southern Italy. Transbound. Emerg. Dis. 2019, 66, 1864–1872. [Google Scholar] [CrossRef]
- Duarte, M.D.; Henriques, A.M.; Barros, S.C.; Fagulha, T.; Mendonça, P.; Carvalho, P.; Monteiro, M.; Fevereiro, M.; Basto, M.P.; Rosalino, L.M.; et al. Snapshot of viral infections in wild carnivores reveals ubiquity of parvovirus and susceptibility of Egyptian mongoose to feline panleukopenia virus. PLoS ONE 2013, 8, e59399. [Google Scholar] [CrossRef]
- Steinel, A.; Munson, L.; van Vuuren, M.; Truyen, U. Genetic characterization of feline parvovirus sequences from various carnivores. J. Gen. Virol. 2000, 81, 345–350. [Google Scholar] [CrossRef]
- Balboni, A.; Musto, C.; Kaehler, E.; Verin, R.; Caniglia, R.; Fabbri, E.; Carra, E.; Cotti, C.; Battilani, M.; Delogu, M. Genetic characterization of canine adenovirus type 1 detected by real-time polymerase chain reaction in an oral sample of an Italian wolf (Canis lupus). J. Wildl. Dis. 2019, 55, 737–741. [Google Scholar] [CrossRef]
- Balboni, A.; Tryland, M.; Mørk, T.; Killengreen, S.T.; Fuglei, E.; Battilani, M. Unique genetic features of canine adenovirus type 1 (CAdV-1) infecting red foxes (Vulpes vulpes) in northern Norway and arctic foxes (Vulpes lagopus) in Svalbard. Vet. Res. Commun. 2019, 43, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Dowgier, G.; Lahoreau, J.; Lanave, G.; Losurdo, M.; Varello, K.; Lucente, M.S.; Ventriglia, G.; Bozzetta, E.; Martella, V.; Buonavoglia, C.; et al. Sequential circulation of canine adenoviruses 1 and 2 in captive wild carnivores, France. Vet. Microbiol. 2018, 221, 67–73. [Google Scholar] [CrossRef]
- Walker, D.; Fee, S.A.; Hartley, G.; Learmount, J.; O′Hagan, M.J.H.; Meredith, A.L.; de C. Bronsvoort, B.M.; Porphyre, T.; Sharp, C.P.; Philbey, A.W. Serological and molecular epidemiology of canine adenovirus type 1 in red foxes (Vulpes vulpes) in the United Kingdom. Sci. Rep. 2016, 6, 36051. [Google Scholar] [CrossRef] [Green Version]
- Millán, J.; López-Bao, J.V.; García, E.J.; Oleaga, Á.; Llaneza, L.; Palacios, V.; de la Torre, A.; Rodríguez, A.; Dubovi, E.J.; Esperón, F. Patterns of exposure of Iberian wolves (Canis lupus) to canine viruses in human-dominated landscapes. Ecohealth 2016, 13, 123–134. [Google Scholar] [CrossRef]
- García Marín, J.F.; Royo, L.J.; Oleaga, A.; Gayo, E.; Alarcia, O.; Pinto, D.; Martínez, I.Z.; González, P.; Balsera, R.; Marcos, J.L.; et al. Canine adenovirus type 1 (CAdV-1) in free-ranging European brown bear (Ursus arctos arctos): A threat for Cantabrian population? Transbound. Emerg. Dis. 2018, 65, 2049–2056. [Google Scholar] [CrossRef]
- Woods, L.W. Adenoviral diseases. In Infectious Diseases of Wild Mammals, 3rd ed.; Williams, E.S., Barker, I.K., Eds.; Wiley-Blackwell: Ames, IA, USA, 2001; pp. 202–212. [Google Scholar]
- Pizzurro, F.; Marcacci, M.; Zaccaria, G.; Orsini, M.; Cito, F.; Rosamilia, A.; Di Renzo, L.; Malatesta, D.; Di Sabatino, D.; Lorusso, A. Genome sequence of canine adenovirus type 1 isolated from a wolf (Canis lupus) in southern Italy. Genome Announc. 2017, 5, e00225-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Francesco, C.E.; Smoglica, C.; Paoletti, B.; Angelucci, S.; Innocenti, M.; Antonucci, A.; Di Domenico, G.; Marsilio, F. Detection of selected pathogens in Apennine wolf (Canis lupus italicus) by a non-invasive GPS-based telemetry sampling of two packs from Majella National Park, Italy. Eur. J. Wildl. Res. 2019, 65, 84. [Google Scholar] [CrossRef] [PubMed]
- Melegari, I.; Sarchese, V.; Di Profio, F.; Robetto, S.; Carella, E.; Bermudez Sanchez, S.; Orusa, R.; Martella, V.; Marsilio, F.; Di Martino, B. First molecular identification of kobuviruses in wolves (Canis lupus) in Italy. Arch. Virol. 2018, 163, 509–513. [Google Scholar] [CrossRef]
- Balboni, A.; Verin, R.; Morandi, F.; Poli, A.; Prosperi, S.; Battilani, M. Molecular epidemiology of canine adenovirus type 1 and type 2 in free-ranging red foxes (Vulpes vulpes) in Italy. Vet. Microbiol. 2013, 162, 551–557. [Google Scholar] [CrossRef]
- Decaro, N.; Martella, V.; Buonavoglia, C. Canine adenoviruses and herpesvirus. Vet. Clin. North. Am. Small Anim. Pract. 2008, 38, 799–814. [Google Scholar] [CrossRef]
- Oleaga, A.; Balseiro, A.; Espí, A.; Royo, L.J. Wolf (Canis lupus) as canine adenovirus type 1 (CAdV-1) sentinel for the endangered cantabrian brown bear (Ursus arctos arctos). Transbound. Emerg. Dis. 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- Kotsias, F.; Bucafusco, D.; Nuñez, D.A.; Borisovsky, L.A.L.; Rodriguez, M.; Bratanich, A.C. Genomic characterization of canine circovirus associated with fatal disease in dogs in South America. PLoS ONE 2019, 14, e0218735. [Google Scholar] [CrossRef]
- Anderson, A.; Hartmann, K.; Leutenegger, C.M.; Proksch, A.L.; Mueller, R.S.; Unterer, S. Role of canine circovirus in dogs with acute haemorrhagic diarrhoea. Vet. Rec. 2017, 180, 542. [Google Scholar] [CrossRef]
- Dowgier, G.; Lorusso, E.; Decaro, N.; Desario, C.; Mari, V.; Lucente, M.S.; Lanave, G.; Buonavoglia, C.; Elia, G. A molecular survey for selected viral enteropathogens revealed a limited role of canine circovirus in the development of canine acute gastroenteritis. Vet. Microbiol. 2017, 204, 54–58. [Google Scholar] [CrossRef]
- Bexton, S.; Wiersma, L.C.; Getu, S.; van Run, P.R.; Verjans, G.M.; Schipper, D.; Schapendonk, C.M.; Bodewes, R.; Oldroyd, L.; Haagmans, B.L.; et al. Detection of Circovirus in Foxes with Meningoencephalitis, United Kingdom, 2009–2013. Emerg. Infect. Dis. 2015, 21, 1205–1208. [Google Scholar] [CrossRef]
- Li, L.; McGraw, S.; Zhu, K.; Leutenegger, C.M.; Marks, S.L.; Kubiski, S.; Gaffney, P.; Dela Cruz, F.N., Jr.; Wang, C.; Delwart, E.; et al. Circovirus in tissues of dogs with vasculitis and hemorrhage. Emerg. Infect. Dis. 2013, 19, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Dubovi, E.J.; Henriquez-Rivera, J.A.; Lipkin, W.I. Complete genome sequence of the first canine circovirus. J. Virol. 2012, 86, 7018. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.S.; Lin, T.H.; Wu, H.Y.; Lin, L.S.; Chung, C.S.; Chiou, M.T.; Lin, C.N. High detection rate of dog circovirus in diarrheal dogs. BMC Vet. Res. 2016, 12, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbani, L.; Tryland, M.; Ehrich, D.; Fuglei, E.; Battilani, M.; Balboni, A. Ancient origin and genetic segregation of canine circovirus infecting arctic foxes (Vulpes lagopus) in Svalbard and red foxes (Vulpes vulpes) in Northern Norway. Transbound. Emerg. Dis. 2021, 68, 1283–1293. [Google Scholar] [CrossRef]
- De Arcangeli, S.; Balboni, A.; Kaehler, E.; Urbani, L.; Verin, R.; Battilani, M. Genomic characterization of canine circovirus detected in red foxes (Vulpes vulpes) from Italy using a new Real-Time PCR assay. J. Wildl. Dis. 2020, 56, 239–242. [Google Scholar] [CrossRef]
- Zimen, E.; Boitani, L. Number and distribution of wolves in Italy. Z. Säugetierkunde 1975, 40, 102–112. [Google Scholar]
- Galaverni, M.; Caniglia, R.; Fabbri, E.; Milanesi, P.; Randi, E. One, no one, or one hundred thousand: How many wolves are there currently in Italy? Mamm. Res. 2016, 61, 13–24. [Google Scholar] [CrossRef]
- Mörner, T.; Eriksson, H.; Bröjer, C.; Nilsson, K.; Uhlhorn, H.; Agren, E.; af Segerstad, C.H.; Jansson, D.S.; Gavier-Widén, D. Diseases and mortality in free-ranging brown bear (Ursus arctos), gray wolf (Canis lupus), and wolverine (Gulo gulo) in Sweden. J. Wildl. Dis. 2005, 41, 298–303. [Google Scholar] [CrossRef] [Green Version]
- Balboni, A.; Bassi, F.; De Arcangeli, S.; Zobba, R.; Dedola, C.; Alberti, A.; Battilani, M. Molecular analysis of carnivore Protoparvovirus detected in white blood cells of naturally infected cats. BMC Vet. Res. 2018, 14, 41. [Google Scholar] [CrossRef] [Green Version]
- Balboni, A.; Dondi, F.; Prosperi, S.; Battilani, M. Development of a SYBR Green real-time PCR assay with melting curve analysis for simultaneous detection and differentiation of canine adenovirus type 1 and type 2. J. Virol. Methods 2015, 222, 34–40. [Google Scholar] [CrossRef]
- Battilani, M.; Modugno, F.; Mira, F.; Purpari, G.; Di Bella, S.; Guercio, A.; Balboni, A. Molecular epidemiology of canine parvovirus type 2 in Italy from 1994 to 2017: Recurrence of the CPV-2b variant. BMC Vet. Res. 2019, 15, 393. [Google Scholar] [CrossRef]
- Balboni, A.; Dondi, F.; Agnoli, C.; Verin, R.; Gruarin, M.; Morini, M.; Battilani, M. Novel sequence variants of viral hexon and fibre genes in two dogs with canine adenovirus type 1-associated disease. Vet. J. 2017, 223, 73–75. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Buonavoglia, C.; Martella, V.; Pratelli, A.; Tempesta, M.; Cavalli, A.; Buonavoglia, D.; Bozzo, G.; Elia, G.; Decaro, N.; Carmichael, L. Evidence for evolution of canine parvovirus type 2 in Italy. J. Gen. Virol. 2001, 82, 3021–3025. [Google Scholar] [CrossRef]
- Parrish, C.R.; Aquadro, C.F.; Strassheim, M.L.; Evermann, J.F.; Sgro, J.Y.; Mohammed, H.O. Rapid antigenic-type replacement and DNA sequence evolution of canine parvovirus. J. Virol. 1991, 65, 6544–6552. [Google Scholar] [CrossRef] [Green Version]
- Truyen, U.; Evermann, J.F.; Vieler, E.; Parrish, C.R. Evolution of canine parvovirus involved loss and gain of feline host range. Virology 1996, 215, 186–189. [Google Scholar] [CrossRef] [Green Version]
- Decaro, N.; Martella, V.; Desario, C.; Lanave, G.; Circella, E.; Cavalli, A.; Elia, G.; Camero, M.; Buonavoglia, C. Genomic characterization of a circovirus associated with fatal hemorrhagic enteritis in dog, Italy. PLoS ONE 2014, 9, e105909. [Google Scholar] [CrossRef]
- Piewbang, C.; Jo, W.K.; Puff, C.; van der Vries, E.; Kesdangsakonwut, S.; Rungsipipat, A.; Kruppa, J.; Jung, K.; Baumgärtner, W.; Techangamsuwan, S.; et al. Novel canine circovirus strains from Thailand: Evidence for genetic recombination. Sci. Rep. 2018, 8, 7524. [Google Scholar] [CrossRef]
- Niu, L.; Wang, Z.; Zhao, L.; Wang, Y.; Cui, X.; Shi, Y.; Chen, H.; Ge, J. Detection and molecular characterization of canine circovirus circulating in northeastern China during 2014–2016. Arch. Virol. 2020, 165, 137–143. [Google Scholar] [CrossRef]
- Thaiwong, T.; Wise, A.G.; Maes, R.K.; Mullaney, T.; Kiupel, M. Canine Circovirus 1 (CaCV-1) and Canine Parvovirus 2 (CPV-2): Recurrent Dual Infections in a Papillon Breeding Colony. Vet. Pathol. 2016, 53, 1204–1209. [Google Scholar] [CrossRef] [Green Version]
- Rosa, G.M.; Santos, N.; Grøndahl-Rosado, R.; Fonseca, F.P.; Tavares, L.; Neto, I.; Cartaxeiro, C.; Duarte, A. Unveiling patterns of viral pathogen infection in free-ranging carnivores of northern Portugal using a complementary methodological approach. Comp. Immunol. Microbiol. Infect. Dis. 2020, 69, 101432. [Google Scholar] [CrossRef]
- McKnight, C.A.; Maes, R.K.; Wise, A.G.; Kiupel, M. Evaluation of tongue as a complementary sample for the diagnosis of parvoviral infection in dogs and cats. J. Vet. Diagn. Investig. 2007, 19, 409–413. [Google Scholar] [CrossRef] [Green Version]
- Dei Giudici, S.; Cubeddu, T.; Giagu, A.; Sanna, G.; Rocca, S.; Oggiano, A. First molecular characterization of canine parvovirus strains in Sardinia, Italy. Arch. Virol. 2017, 162, 3481–3486. [Google Scholar] [CrossRef]
- Tucciarone, C.M.; Franzo, G.; Mazzetto, E.; Legnardi, M.; Caldin, M.; Furlanello, T.; Cecchinato, M.; Drigo, M. Molecular insight into Italian canine parvovirus heterogeneity and comparison with the worldwide scenario. Infect. Genet. Evol. 2018, 66, 171–179. [Google Scholar] [CrossRef]
- Allison, A.B.; Kohler, D.J.; Fox, K.A.; Brown, J.D.; Gerhold, R.W.; Shearn-Bochsler, V.I.; Dubovi, E.J.; Parrish, C.R.; Holmes, E.C. Frequent cross-species transmission of parvoviruses among diverse carnivore hosts. J. Virol. 2013, 87, 2342–2347. [Google Scholar] [CrossRef] [Green Version]
- Verin, R.; Forzan, M.; Schulze, C.; Rocchigiani, G.; Balboni, A.; Poli, A.; Mazzei, M. Multicentric molecular and pathologic study on Canine Adenovirus Type 1 in red foxes (Vulpes vulpes) in three European countries. J. Wildl. Dis. 2019, 55, 935–939. [Google Scholar] [CrossRef]
- Breitbart, M.; Delwart, E.; Rosario, K.; Segalés, J.; Varsani, A.; Ictv Report Consortium. ICTV Virus Taxonomy Profile: Circoviridae. J. Gen. Virol. 2017, 98, 1997–1998. [Google Scholar] [CrossRef]
- Rosario, K.; Breitbart, M.; Harrach, B.; Segalés, J.; Delwart, E.; Biagini, P.; Varsani, A. Revisiting the taxonomy of the family Circoviridae: Establishment of the genus Cyclovirus and removal of the genus Gyrovirus. Arch. Virol. 2017, 162, 1447–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variables | Total | Carnivore protoparvovirus 1 | CAdV | CanineCV | Coinfections |
|---|---|---|---|---|---|
| Number of wolves | 23 | 21 (91.3) | 4 (17.4) | 11 (47.8) | 11 (47.8) |
| Sex | |||||
| Male | 14 (60.9) | 13 | 4 | 7 | 8 |
| Female | 9 (39.1) | 8 | 0 | 4 | 3 |
| Age (months) | 24 (3–48) | 24 (5–48) | 30 (12–36) | 24 (3–48) | 24 (5–48) |
| Puppies (≤12) | 10 (43.5) | 8 | 1 | 5 | 4 |
| Sub-adults (13–24) | 7 (30.4) | 7 | 1 | 3 | 3 |
| Adults (>24) | 6 (26.1) | 6 | 2 | 3 | 4 |
| Geographical origin | |||||
| Emilia Romagna | 13 (56.5) | 12 | 1 | 8 | 7 |
| Tuscany | 8 (34.8) | 7 | 1 | 2 | 2 |
| Calabria | 2 (8.7) | 2 | 2 | 1 | 2 |
| Year of sampling | |||||
| 2017 | 6 (26.1) | 4 | 1 | 5 | 4 |
| 2018 | 13 (56.5) | 13 | 2 | 6 | 6 |
| 2019 | 4 (17.4) | 4 | 1 | 0 | 1 |
| Wolf | Carnivore protoparvovirus 1 | CAdV-1 | CAdV-2 | CanineCV | |||||
|---|---|---|---|---|---|---|---|---|---|
| Tongue | Intestine | Tongue | Spleen | Tongue | Spleen | Intestine | Spleen | ||
| 447/2017 | - | - | - | - | - | - | P | P S | |
| 448/2017 | P | - | - | - | - | - | - | P S | |
| 449/2017 | - | P | - | - | - | - | P | P S | |
| 450/2017 | P | P | - | - | - | - | P S | P | |
| 451/2017 | P S | P | - | - | - | - | P | P | |
| 452/2017 | - | - | - | P S | - | - | - | - | |
| 453/2018 | P S | - | - | - | - | - | - | - | |
| 454/2018 | P | P S | - | - | P | - | P S | P | |
| 455/2018 | P | P | - | - | - | - | - | P | |
| 456/2018 | - | P S | - | - | - | - | P | - | |
| 457/2018 | P | P S | - | - | - | - | P S | P | |
| 458/2018 | P | P S | - | - | - | - | P S | P | |
| 188/2018 | P S | P | - | - | - | - | - | - | |
| 189/2018 | P S | P | - | - | - | - | - | - | |
| 190/2018 | - | P S | - | P | - | - | - | P | |
| 191/2018 | P | - | - | - | - | - | - | - | |
| 193/2018 | - | P S | - | - | - | - | - | - | |
| 194/2019 | P | - | - | - | - | - | - | - | |
| 195/2018 | P | P S | - | - | - | - | - | - | |
| 196/2018 | - | P | - | - | - | - | - | - | |
| 197/2019 | P | P S | - | - | - | - | - | - | |
| 198/2019 | P S | - | - | - | P | - | - | - | |
| 199/2019 | P | P | - | - | - | - | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balboni, A.; Urbani, L.; Delogu, M.; Musto, C.; Fontana, M.C.; Merialdi, G.; Lucifora, G.; Terrusi, A.; Dondi, F.; Battilani, M. Integrated Use of Molecular Techniques to Detect and Genetically Characterise DNA Viruses in Italian Wolves (Canis lupus italicus). Animals 2021, 11, 2198. https://doi.org/10.3390/ani11082198
Balboni A, Urbani L, Delogu M, Musto C, Fontana MC, Merialdi G, Lucifora G, Terrusi A, Dondi F, Battilani M. Integrated Use of Molecular Techniques to Detect and Genetically Characterise DNA Viruses in Italian Wolves (Canis lupus italicus). Animals. 2021; 11(8):2198. https://doi.org/10.3390/ani11082198
Chicago/Turabian StyleBalboni, Andrea, Lorenza Urbani, Mauro Delogu, Carmela Musto, Maria Cristina Fontana, Giuseppe Merialdi, Giuseppe Lucifora, Alessia Terrusi, Francesco Dondi, and Mara Battilani. 2021. "Integrated Use of Molecular Techniques to Detect and Genetically Characterise DNA Viruses in Italian Wolves (Canis lupus italicus)" Animals 11, no. 8: 2198. https://doi.org/10.3390/ani11082198
APA StyleBalboni, A., Urbani, L., Delogu, M., Musto, C., Fontana, M. C., Merialdi, G., Lucifora, G., Terrusi, A., Dondi, F., & Battilani, M. (2021). Integrated Use of Molecular Techniques to Detect and Genetically Characterise DNA Viruses in Italian Wolves (Canis lupus italicus). Animals, 11(8), 2198. https://doi.org/10.3390/ani11082198






