Vitamins A and D and Zinc Affect the Leshmanicidal Activity of Canine Spleen Leukocytes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Dogs and Sample Collection
2.2. Real-Time PCR
2.3. Serum Micronutrient Assays
2.4. Cell Culture
2.5. Flow Cytometry
2.6. ELISA
2.7. Parasite Load by Count under a Light Microscope
2.8. Reagents and Assays
2.9. Statistical Analysis
3. Results
3.1. Reduction of Retinol and Zn and Increase in 25(OH)VD3 Serum
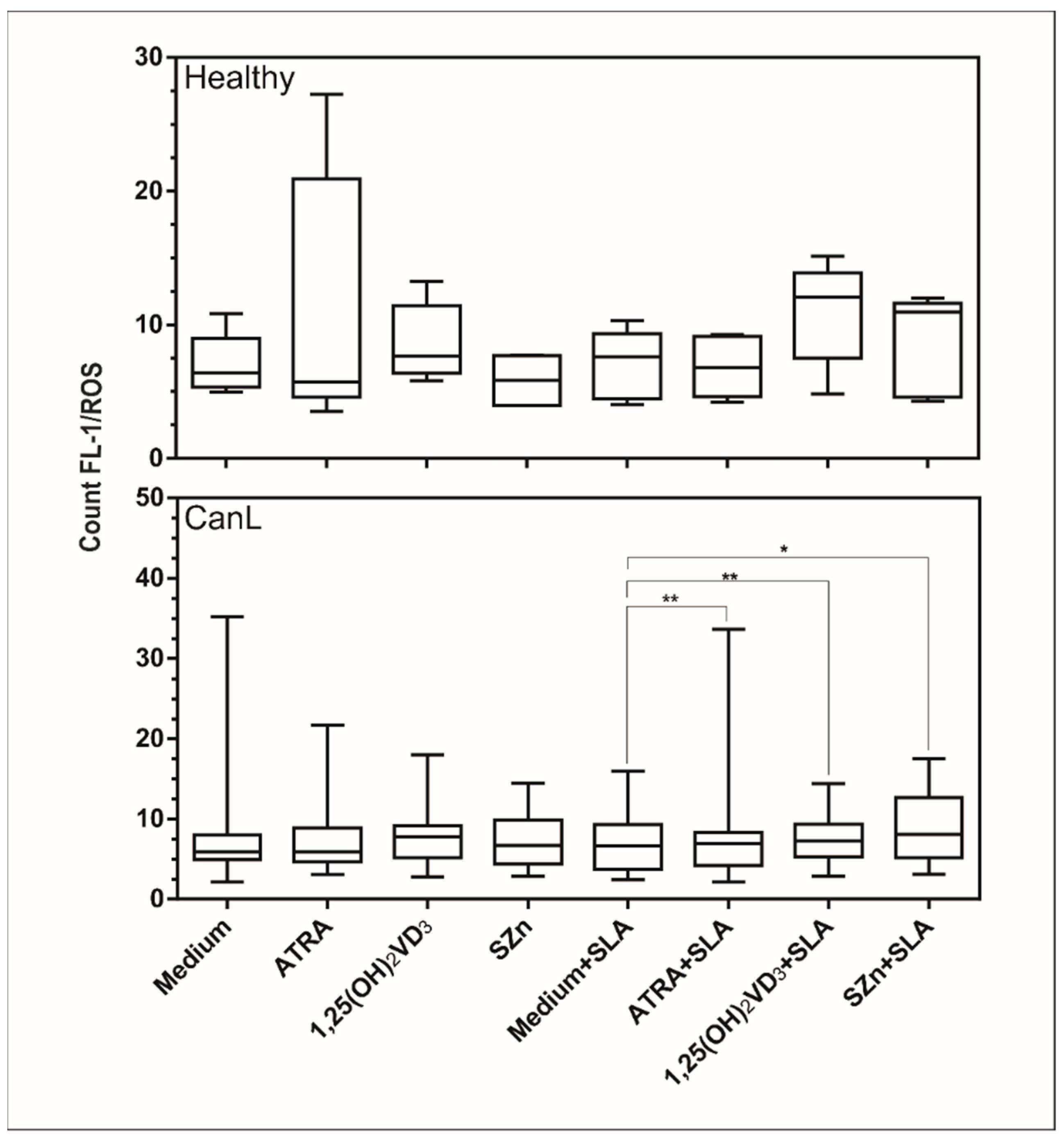
3.2. In Vitro Supplementation of Spleen Leukocytes with ATRA, 1,25(OH)2VD3, and SZn Increased Production of NO and ROS in the CanL Group Stimulated with SLA
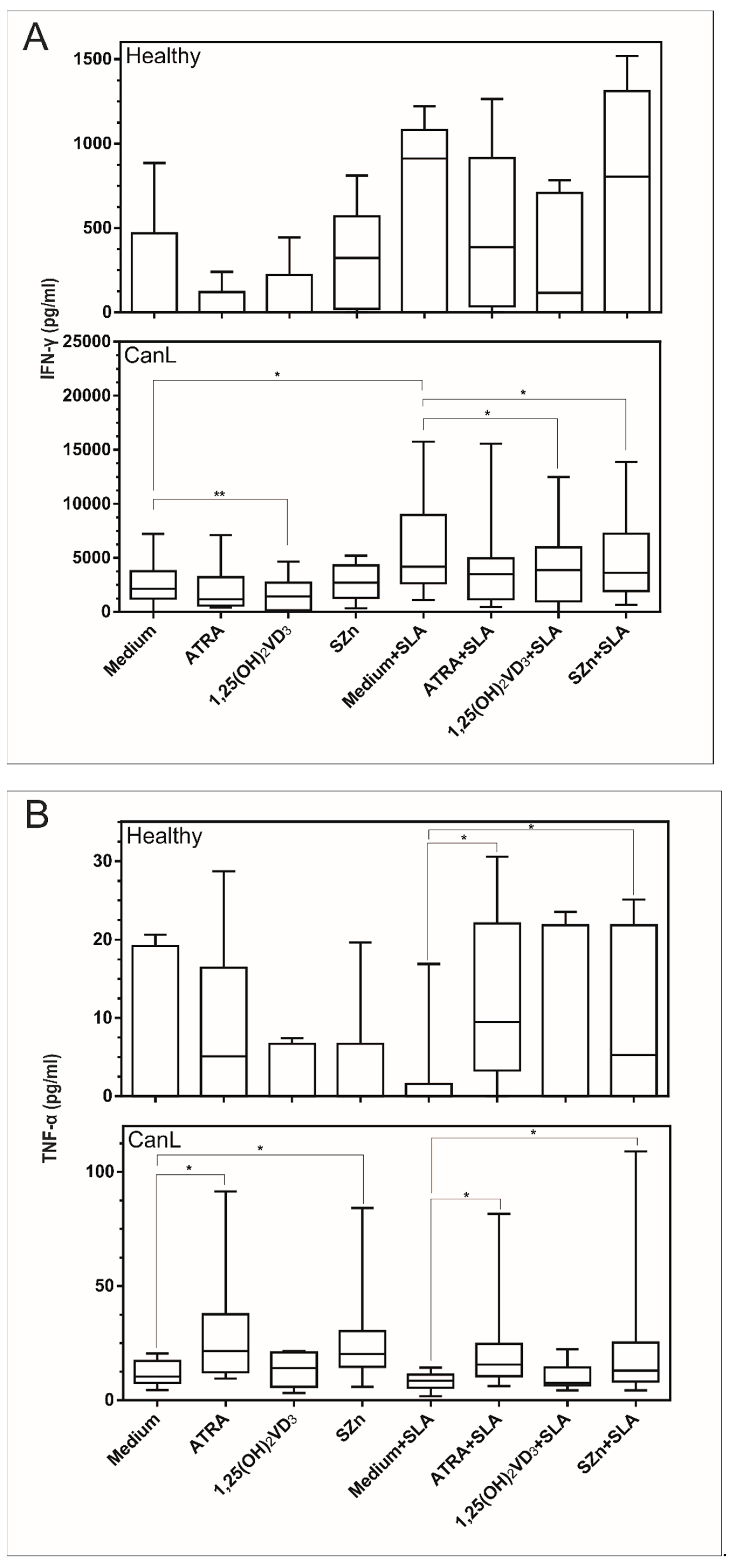
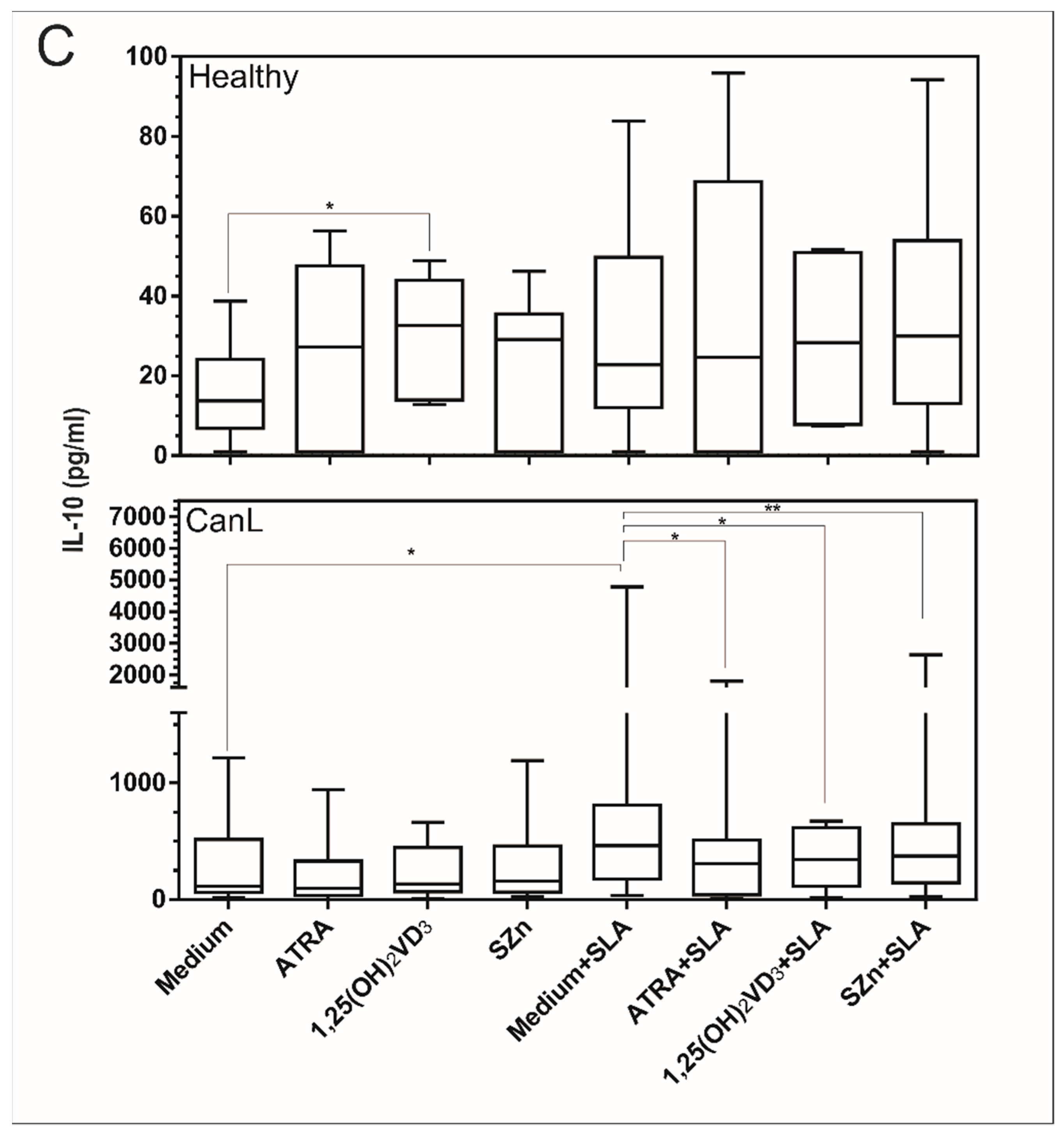
3.3. In Vitro Supplementation of Spleen Leukocytes with 1,25(OH)2VD3 and SZn Reduced IFN-γ; ATRA and SZn Increased TNF-α; and ATRA, 1,25(OH)2VD3, and SZn Reduced IL-10 in the CanL Group Stimulated with SLA
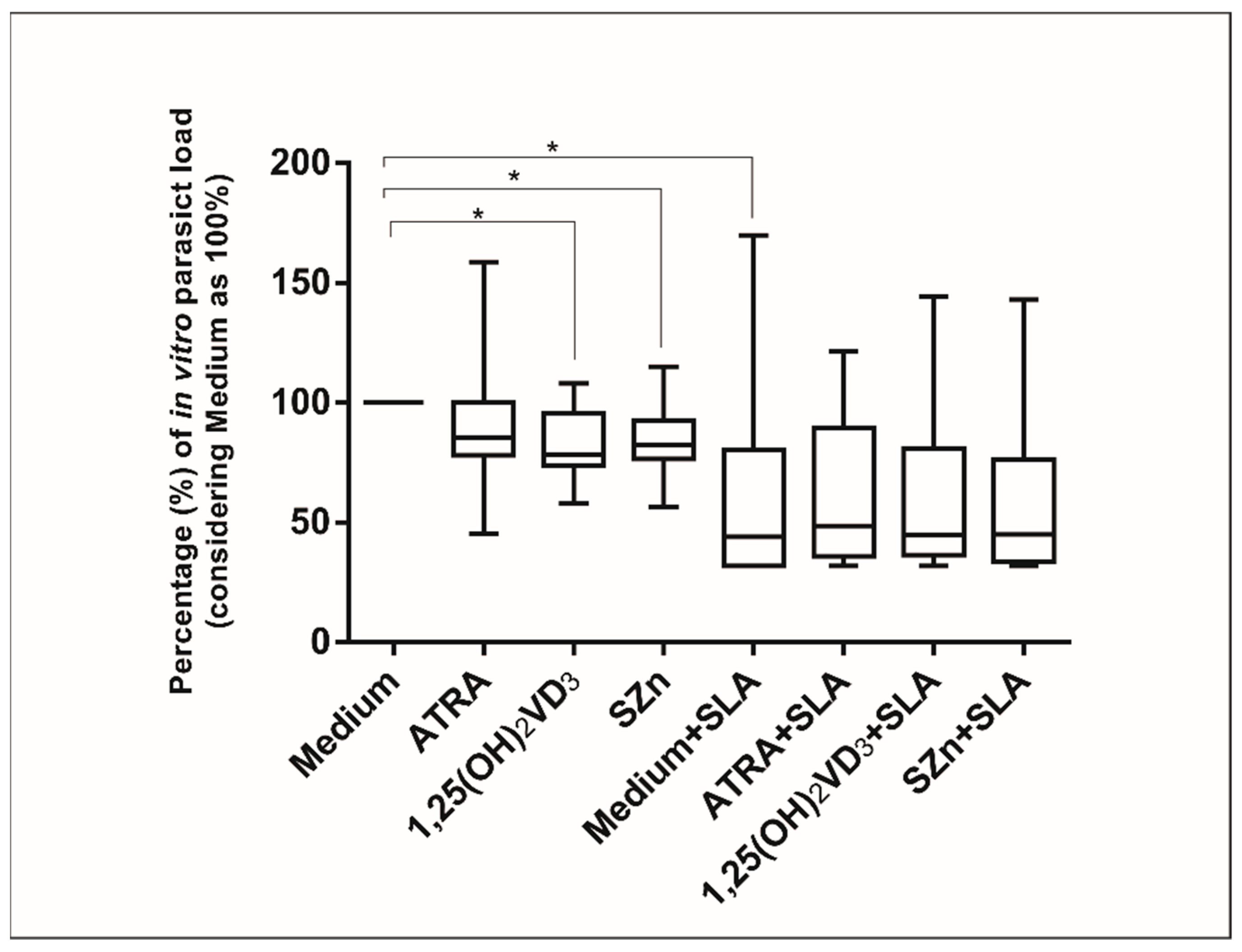
3.4. In Vitro Supplementation of Spleen Leukocytes in the CanL Group Stimulated with 1,25(OH)2VD3 and SZn Reduced the Parasite Load, but after the Stimulation with SLA, No Effect Was Observed
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Magnitude of the Problem. Leishmaniasis. 2019. Available online: https://www.who.int/leishmaniasis/burden/magnitude/burden_magnitude/en/ (accessed on 26 November 2019).
- Lima, V.M.F.; Fattori, K.R.; Souza, F.; Eugenio, F.R.; Patto, O.S.; Rozza, D.B.; Machado, G.F. Apoptosis in T lymphocytes from spleen tissue and peripheral blood of L.(L.) chagasi naturally infected dogs. Vet. Parasitol. 2012, 184, 147–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, K.L.O.; Andrade, M.M.C.; Melo, L.M.; Perosso, J.; Vasconcelos, R.O.; Lima, V.M.F. CD4 + FOXP3 + cells produce IL-10 in the spleens of dogs with visceral leishmaniasis. Vet. Parasitol. 2014, 202, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Santos, W.L.C.; Jesus, E.E.; Paranhos-Silva, M.; Pereira, A.M.; Santos, J.C.; Baleeiro, C.O.; Nascimento, E.G.; Moreira, E.D.; Oliveira, G.G.S.; Pontes de Carvalho, L.C. Associations among immunological, parasitological and clinical parameters in canine visceral leishmaniasis: Emaciation, spleen parasitism, specific antibodies and leishmanin skin test reaction. Vet. Immunol. Immunophatol. 2008, 123, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Hailu, A.; Baarle, D.V.; Knol, G.J.; Berhe, N. T cell subset and cytokine profiles in human visceral leishmaniasis during active and asymptomatic or sub-clinical infection with Leishmania donovani. Clin Immunol. 2005, 117, 182–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, A.; Carrillo, E.; San, J.V.; Botana, L.; Molina, L.; Matía, B.; Fernandez, L.; Horrillo, L.; Ibarra-Meneses, A.; Sanchez, C.; et al. Lymphoproliferative response after stimulation with soluble leishmania antigen (SLA) as a predictor of visceral leishmaniasis (VL) relapse in HIV + patients. Acta Trop. 2016, 164, 345–351. [Google Scholar] [CrossRef]
- Alvar, J.; Cañavate, C.; Molina, R.; Moreno, J.; Nieto, J. Canine leishmaniasis. Adv. Parasitol. 2004, 57, 88. [Google Scholar] [CrossRef]
- Baneth, G.; Koutinas, A.F.; Solano-Gallego, L.; Bourdeau, P.; Ferrer, L. Canine leishmaniosis—New concepts and insights on an expanding zoonosis: Part one. Trends 2008, 24, 324–330. [Google Scholar] [CrossRef]
- Carneiro, P.P.; Conceição, J.; Macedo, M.; Magalhães, V.; Carvalho, E.M.; Bacellar, O. The role of nitric oxide and reactive oxygen species in the killing of Leishmania braziliensis by monocytes from patients with cutaneous leishmaniasis. PLoS ONE 2016, 11, e0148084. [Google Scholar] [CrossRef] [Green Version]
- Miao, L.; St Clair, D.K. Regulation of superoxide dismutase genes: Implications in disease. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gantt, K.R.; Goldman, T.L.; McCormick, M.L.; Miller, M.A.; Jeronimo, S.M.B.; Nascimento, E.T.; Britigan, B.E.; Wilson, M.E. Oxidative Responses of Human and Murine Macrophages During Phagocytosis of Leishmania chagasi. J. Immunol. 2001, 167, 893–901. [Google Scholar] [CrossRef] [Green Version]
- Jewitt, D.E.; Maurer, B.J.; Hubner, P.J.B. Inhibitory effect of superoxide-generating quinones on superoxide dismutase. Br. Med. J. 1984, 1, 795–796. [Google Scholar] [CrossRef] [Green Version]
- Baneth, G.; Shaw, S.E. Chemotherapy of canine leishmaniosis. Vet. Parasitol. 2002, 106, 315–324. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Koutinas, A.; Miró, G.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet. Parasitol. 2009, 165, 1–18. [Google Scholar] [CrossRef]
- Mafra, D.; Cozzolino, S.M.F. Importância do zinco na nutrição humana. Rev. Nutr. 2004, 17, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Paradies, P.; Lubas, G.; Iarussi, F.; Pezzuto, E.; Sasanelli, M. Comparison of standard protocols for the treatment of canine leishmaniasis in an endemic area with and without zinc oral supplementation. Ann. Clin. Cytol. Pathol. 2017, 3, 1066–1072. [Google Scholar]
- Villamor, E.; Fawzi, W.W. Effects of vitamin A supplementation on immune responses and correlation with clinical outcomes. Clin. Microbiol. Rev. 2005, 18, 446–464. [Google Scholar] [CrossRef] [Green Version]
- Hojyo, S.; Fukada, T. Roles of Zinc Signaling in the Immune System. J. Immunol. Res. 2016, 2016, 6762343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Cortes, A.; Martori, C.; Martinez-Florez, A.; Clop, A.; Amills, M.; Kubejko, J.; Llull, J.; Nadal, J.M.; Alberola, J. Canine leishmaniasis progression is associated with Vitamin D deficiency. Sci. Rep. 2017, 7, 3346. [Google Scholar] [CrossRef] [Green Version]
- Maciel, B.L.L.; Valverde, J.G.; Rodrigues-Neto, J.F.; Freire-Neto, F.; Keesen, T.S.L.; Jeronimo, S.M.B. Dual Immune modulatory effect of vitamin a in human visceral leishmaniasis. PLoS ONE 2014, 9, e107564. [Google Scholar] [CrossRef] [Green Version]
- Gogulamudi, V.R.; Dubey, M.L.; Kaul, D.; Hubert, D.J.; Kandimalla, R.; Sehgal, R. Vitamins (A&D) and Isoprenoid (Chenodeoxycholic acid) molecules are accompanied by Th1 immunostimulatory response and therapeutic cure in vivo: Possible antileishmanial drugs. Sci. Rep. 2019, 9, 8531. [Google Scholar]
- Lima, V.M.F.; Gonçalves, M.E.; Ikeda, F.A.; Luvizotto, M.C.R.; Feitosa, M.M. Anti-leishmania antibodies in cerebrospinal fluid from dogs with visceral leishmaniasis. Braz. J. Med. Biol. Res. 2003, 36, 485–489. [Google Scholar] [CrossRef] [Green Version]
- Perosso, J.; Silva, K.L.O.; Ferreira, S.Í.D.S.; Avanço, S.V.; dos Santos, P.S.P.; Eugênio, F.D.R.; Almeida, B.F.M.; Lima, V.M.F. Alteration of sFAS and sFAS ligand expression during canine visceral leishmaniosis. Vet. Parasitol. 2014, 205, 417–423. [Google Scholar] [CrossRef]
- Arnaud, J.; Fortis, I.; Blachier, S.; Kia, D.F.A. Simultaneous determination of retinol, alpha-tocopherol and beta-carotene in serum by isocratic high-performance liquid chromatography. J. Chromatogr. 1991, 572, 103–116. [Google Scholar] [CrossRef]
- Batista, B.L.; Rodrigues, J.L.; Nunes, J.A.; de Oliveira, S.V.C.; Barbosa, F. Exploiting dynamic reaction cell inductively coupled plasma mass spectrometry (DRC-ICP-MS) for sequential determination of trace elements in blood using a dilute-and-shoot procedure. Anal. Chim. Acta 2009, 639, 13–18. [Google Scholar] [CrossRef]
- Ehrchen, J.; Helming, L.; Varga, G.; Pasche, B.; Loser, K.; Gunzer, M.; Sunderkötter, C.; Sorg, C.; Roth, J.; Lengeling, A. Vitamin D receptor signaling contributes to susceptibility to infection with Leishmania major. FASEB J. 2007, 21, 3208–3218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzman-Rivero, M.; Verduguez-Orellana, A.; Montaño, K.; Cloetens, L.; Rojas, E.; Åkesson, B.; Sejas, E. The immune response in patients with cutaneous leishmaniasis and the influence of zinc supplementation. Biomed. Pharmacother. 2015, 69, 56–62. [Google Scholar] [CrossRef]
- Murray, B.H.W.; Nathan, C.F. Macrophage Microbicidal Mechanisms In vivo: Reactive. Differences. J. Exp. Med. 1999, 189, 741–746. [Google Scholar] [CrossRef]
- Pinelli, E.; Gebhard, D.; Mommaas, A.M.; Van Hoeij, M.; Langermans, J.A.M.; Ruitenberg, E.J.; Rutten, V.P. Infection of a canine macrophage cell line with Leishmania infantum: Determination of nitric oxide production and anti-leishmanial activity. Vet. Parasitol. 2000, 92, 181–189. [Google Scholar] [CrossRef]
- Stephensen, C.B.; Jiang, X.; Freytag, T. Vitamin A deficiency increases the in vivo development of IL-10–positive Th2 cells and decreases development of T1 cells in mice. J. Nutr. 2018, 134, 2660–2666. [Google Scholar] [CrossRef]
- Kuwata, T.; Wang, I.M.; Tamura, T.; Ponnamperuma, R.M.; Levine, R.; Holmes, K.L.; Morse, H.C.; De Luca, L.M.; Ozato, K. Vitamin A deficiency in mice causes a systemic expansion of myeloid cells. Blood 2000, 95, 3349–3356. [Google Scholar] [CrossRef] [PubMed]
- Santana, C.C.; Vassallo, J.; De Freitas, L.A.R.; Oliveira, G.G.S.; Pontes-De-Carvalho, L.C.; Dos-Santos, W.L.C. Inflammation and structural changes of splenic lymphoid tissue in visceral leishmaniasis: A study on naturally infected dogs. Parasite Immunol. 2008, 30, 515–524. [Google Scholar] [CrossRef] [Green Version]
- Souza, C.C.; Barreto, T.D.O.; da Silva, S.M.; Pinto, A.W.J.; Figueiredo, M.M.; Rocha, O.G.F.; Cangussú, S.D.; Tafuri, W.L. A potential link among antioxidant enzymes, histopathology and trace elements in canine visceral leishmaniasis. Int. J. Exp. Pathol. 2014, 95, 260–270. [Google Scholar] [CrossRef]
- Heidarpour, M.; Soltani, S.; Mohri, M.; Khoshnegah, J. Canine visceral leishmaniasis: Relationships between oxidative stress, liver and kidney variables, trace elements, and clinical status. Parasitol. Res. 2012, 111, 1491–1496. [Google Scholar] [CrossRef]
- Pasa, S.; Kargin, F.; Bildik, A.; Seyrek, K.; Ozbel, Y.; Ozensoy, S. Serum and hair levels of zinc and other elements in dogs with visceral leishmaniasis. Biol. Trace Elem. Res. 2003, 94, 141–147. [Google Scholar] [CrossRef]
- Lal, C.S.; Kumar, S.; Ranjan, A.; Rabidas, V.N.; Verma, N.; Pandey, K.; Verma, R.B.; Das, S.; Singh, D.; Das, P. Comparative analysis of serum zinc, Copper, Magnesium, Calcium and iron level in acute and chronic patients of visceral leishmaniasis. J. Trace Elem. Med. Biol. 2013, 27, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Bern, C.; Haque, R.; Chowdhury, R.; Ali, M.; Kurkjian, K.M.; Vaz, L.; Amann, J.; Wahed, M.A.; Wagatsuma, Y.; Breimam, R.F.; et al. The epidemiology of visceral leishmaniasis and asymptomatic leishmanial infection in a highly endemic Bangladeshi village. Am. J. Trop. Med. Hyg. 2007, 76, 909–914. [Google Scholar] [CrossRef]
- Wirth, J.J.; Fraker, P.J.; Kierszenbaum, F. Zinc requirement for macrophage function: Effect of zinc deficiency on uptake and killing of a protozoan parasite. Immunology 1989, 68, 114–119. [Google Scholar]
- Shankar, A.H.; Prasad, A.S. Zinc and immune function: The biological basis of altered. Am. J. Clin. Nutr. 1998, 68, 447–463. [Google Scholar] [CrossRef] [Green Version]
- Christian, P.; West, K.P. Interactions between zinc and vitamin A: An update. Am. J. Clin. Nutr. 1998, 68, 435–441. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Liu, Y.; Qi, G.; Brand, D.; Zheng, S. Role of Vitamin A in the Immune System. J. Clin. Med. 2018, 7, 258. [Google Scholar] [CrossRef] [Green Version]
- Almeida, B.F.M.; Narciso, L.G.; Melo, L.M.; Preve, P.P.; Bosco, A.M.; Lima, V.M.F.; Ciarlini, P.C. Leishmaniasis causes oxidative stress and alteration of oxidative metabolism and viability of neutrophils in dogs. Vet. J. 2013, 198, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.R.; Iwata, M.; Von Andrian, U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craig, T.A.; Benson, L.M.; Naylor, S.; Kumar, R.; Gaskell, S. Modulation effects of zinc on the formation of vitamin D receptor and retinoid X receptor α-DNA transcription complexes: Analysis by microelectrospray mass spectrometry. Rapid Commun. Mass Spectrom. 2001, 15, 1011–1016. [Google Scholar] [CrossRef]
- Xiao-dan, Y.; Chong-huai, Y.; Xiao-gang, Y.; Yu, G.; Jian, X.X.S. Effect of Zinc Deficiency on the Protein Expression of Vitamin D Receptor and Calcium Binding Protein in Growth-Stage Rats Duodenal Mucosa. Chin. J. Pediatr. 2006, 44, 11–14. [Google Scholar]
- Zafra, R.; Jaber, J.R.; Pérez-Écija, R.A.; Barragán, A.; Martínez-Moreno, A.; Pérez, J. High iNOS expression in macrophages in canine leishmaniasis is associated with low intracellular parasite burden. Vet. Immunol. Immunopathol. 2008, 123, 353–359. [Google Scholar] [CrossRef]
- Zou, F.; Liu, Y.; Liu, L.; Wu, K.; Wei, W.; Zhu, Y.; Wu, J. Retinoic acid activates human inducible nitric oxide synthase gene through binding of RARα/RXRα heterodimer to a novel retinoic acid response element in the promoter. Biochem. Biophys. Res. Commun. 2007, 355, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Seguin-Devaux, C.; Devaux, Y.; Latger-Cannard, V.; Grosjean, S.; Rochette-Egly, C.; Zannad, F.; Meistelman, C.; Mertes, P.M.; Longrois, D. Enhancement of the inducible NO synthase activation by retinoic acid is mimicked by RARα agonist in vivo. Am. J. Physiol. Endocrinol. Metab. 2002, 283, 525–535. [Google Scholar] [CrossRef] [Green Version]
- Shahmuradov, I.A.; Solovyev, V.V. Nsite, NsiteH and NsiteM computer tools for studying transcription regulatory elements. Bioinformatics 2015, 31, 3544–3545. [Google Scholar] [CrossRef] [Green Version]
- Alignment and Genome Comparison. 2020. Available online: http://www.softberry.com/ (accessed on 22 April 2020).
- Strauss-Ayalia, D.; Baneth, G.J.C. Splenic immune responses during canine visceral leishmaniasis. Vet. Res. 2007, 38, 547–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrêa, A.P.F.L.; Dossi, A.C.S.; Vasconcelos, R.O.; Munari, D.P.; de Lima, V.M.F. Evaluation of transformation growth factor β1, interleukin-10, and interferon-γ in male symptomatic and asymptomatic dogs naturally infected by Leishmania (Leishmania) chagasi. Vet. Parasitol. 2007, 143, 267–274. [Google Scholar] [CrossRef]
- Lage, R.S.; Oliveira, G.C.; Busek, S.U.; Guerra, L.L.; Giunchetti, R.C.; Corrêa-Oliveira, R.; Reis, A.B. Analysis of the cytokine profile in spleen cells from dogs naturally infected by Leishmania chagasi. Vet. Immunol. Immunopathol. 2007, 115, 135–145. [Google Scholar] [CrossRef]
- Ramos-Martínez, E.; Villaseñor-Cardoso, M.I.; López-Vancell, M.R.; García-Vázquez, F.J.; Pérez-Torres, A.; Salaiza-Suazo, N.; Pérez-Tamayo, R. Effect of 1,25(OH)2D3 on BALB/c mice infected with Leishmania mexicana. Exp. Parasitol. 2013, 134, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Whitcomb, J.P.; Deagostino, M.; Ballentine, M.; Fu, J.; Tenniswood, M.; Welsh, J.; Cantotna, M.; McDowell, M.A. The role of vitamin D and vitamin D receptor in immunity to Leishmania major infection. J. Parasitol. Res. 2012, 2012, 134645. [Google Scholar] [CrossRef]
- Khoo, A.L.; Chai, L.; Koenen, H.; Joosten, I.; Netea, M.; Van Der Ven, A. Translating the role of vitamin D 3 in infectious diseases. Crit. Rev. Microbiol. 2012, 38, 122–135. [Google Scholar] [CrossRef]
- Helming, L.; Böse, J.; Ehrchen, J.; Schiebe, S.; Frahm, T.; Geffers, R.; Probst-Kepper, M.; Balling, R.; Lengeling, A. 1α,25-dihydroxyvitamin D3 is a potent suppressor of interferon γ-mediated macrophage activation. Blood 2005, 106, 4351–4358. [Google Scholar] [CrossRef] [Green Version]
- Zella, J.B.; McCary, L.C.; DeLuca, H.F. Oral administration of 1,25-dihydroxyvitamin D3 completely protects NOD mice from insulin-dependent diabetes mellitus. Arch. Biochem. Biophys. 2003, 417, 77–80. [Google Scholar] [CrossRef]
- Gradoni, L. Canine Leishmania vaccines: Still a long way to go. Vet. Parasitol. 2015, 208, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.L.; Hung, T.C.; Hsieh, B.S.; Chen, Y.H.; Chen, T.F.; Cheng, H.L. Zinc at pharmacologic concentrations affects cytokine expression and induces apoptosis of human peripheral blood mononuclear cells. Nutrition 2006, 22, 465–474. [Google Scholar] [CrossRef]
- Nascimento, P.R.P.; Martins, D.R.A.; Monteiro, G.R.G.; Queiroz, P.V.; Freire-Neto, F.P.; Queiroz, J.W.; Lima, A.L.M.; Jeronimo, S.M.B. Association of pro-inflammatory cytokines and iron regulatory protein 2 (IRP2) with leishmania burden in canine visceral leishmaniasis. PLoS ONE 2013, 8, e73873. [Google Scholar] [CrossRef] [Green Version]
- Prasad, A.S. Zinc: Role in immunity, oxidative stress and chronic inflammation. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 646–652. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez, F.M.d.O.; Santos, M.O.; Venturin, G.L.; Bragato, J.P.; Rebech, G.T.; Melo, L.M.; Costa, S.F.; de Freitas, J.H.; Siqueira, C.E.; Morais, D.A.; et al. Vitamins A and D and Zinc Affect the Leshmanicidal Activity of Canine Spleen Leukocytes. Animals 2021, 11, 2556. https://doi.org/10.3390/ani11092556
Hernandez FMdO, Santos MO, Venturin GL, Bragato JP, Rebech GT, Melo LM, Costa SF, de Freitas JH, Siqueira CE, Morais DA, et al. Vitamins A and D and Zinc Affect the Leshmanicidal Activity of Canine Spleen Leukocytes. Animals. 2021; 11(9):2556. https://doi.org/10.3390/ani11092556
Chicago/Turabian StyleHernandez, Fabiana M. de O., Marilene O. Santos, Gabriela L. Venturin, Jaqueline P. Bragato, Gabriela T. Rebech, Larissa M. Melo, Sidnei F. Costa, Jéssica H. de Freitas, Carlos Eduardo Siqueira, Déborah A. Morais, and et al. 2021. "Vitamins A and D and Zinc Affect the Leshmanicidal Activity of Canine Spleen Leukocytes" Animals 11, no. 9: 2556. https://doi.org/10.3390/ani11092556






