WGCNA Analysis of Important Modules and Hub Genes of Compound Probiotics Regulating Lipid Metabolism in Heat-Stressed Broilers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Feeding Management and Experimental Design
2.3. Sample Collection and Processing
2.4. Production Performance
2.5. Meat Quality Test
- (1)
- Dripping loss: We took the chest muscle, weighed it as W1, tied one end of the meat sample with a thin wire, sealed it and hung it in a 4 °C refrigerator. After 24 h, the meat sample was wiped with dry filter paper and weighed as W2, and dripping lose was calculated according to the formula Dripping loss = [(W1 − W2)/W1] × 100%.
- (2)
- Crude moisture: We took the chest muscle and determined crude moisture through direct drying according to GB 5009.3—2016 National food safety standard Determination of moisture in foods.
- (3)
- Cooking loss: We took out the chest muscle sample stored in a 4 °C refrigerator for 24 h, making the sample temperature consistent with the room temperature, peeled off the surrounding fat and broken meat and weighed it as M1. We placed the sample in a plastic bag. Finally, the sample was placed in a 75 °C water bath for 45 min, removed and cooled to room temperature, wiped with filter paper and weighed as M2. Cooking loss was calculated according to the formula Cooking loss = [(M1 − M2)/M1] × 100%.
- (4)
- Cooked meat rate: We weighed the chest muscle sample as S1, steamed it in a pot of boiling water for 30 min, took out the sample, cooled it for 15 min and dried it with filter paper. It was weighed as S2 and the cooked meat rate was calculated according to the formula Cooked meat rate = (S2/S1) × 100%.
- (5)
- Shear force: We took the chest muscle according to the national standard NY_T 1180—2006 Determination of meat tenderness Shear force method.
- (6)
- Intramuscular fat: We took the chest muscle and measured it according to GB 5009.6—2016 National food safety standard Determination of fat in food.
- (7)
- The abdominal fat rate was calculated using the following formula: AF rate = AF weight/(eviscerated weight + AF weight) × 100%. The eviscerated weight was determined according to the national standard NY/T 823-2004 Performance Terms and Measurements for Poultry.
2.6. Blood Lipid Test
2.7. WGCNA Analysis
2.7.1. Construction of Gene Co-Expression Network
2.7.2. Screening Key Modules and Hub Genes
2.8. Statistical Analysis
3. Results
3.1. Production Performance
3.2. Meat Quality
3.3. Blood Lipid Quality
3.4. WGCNA Analysis
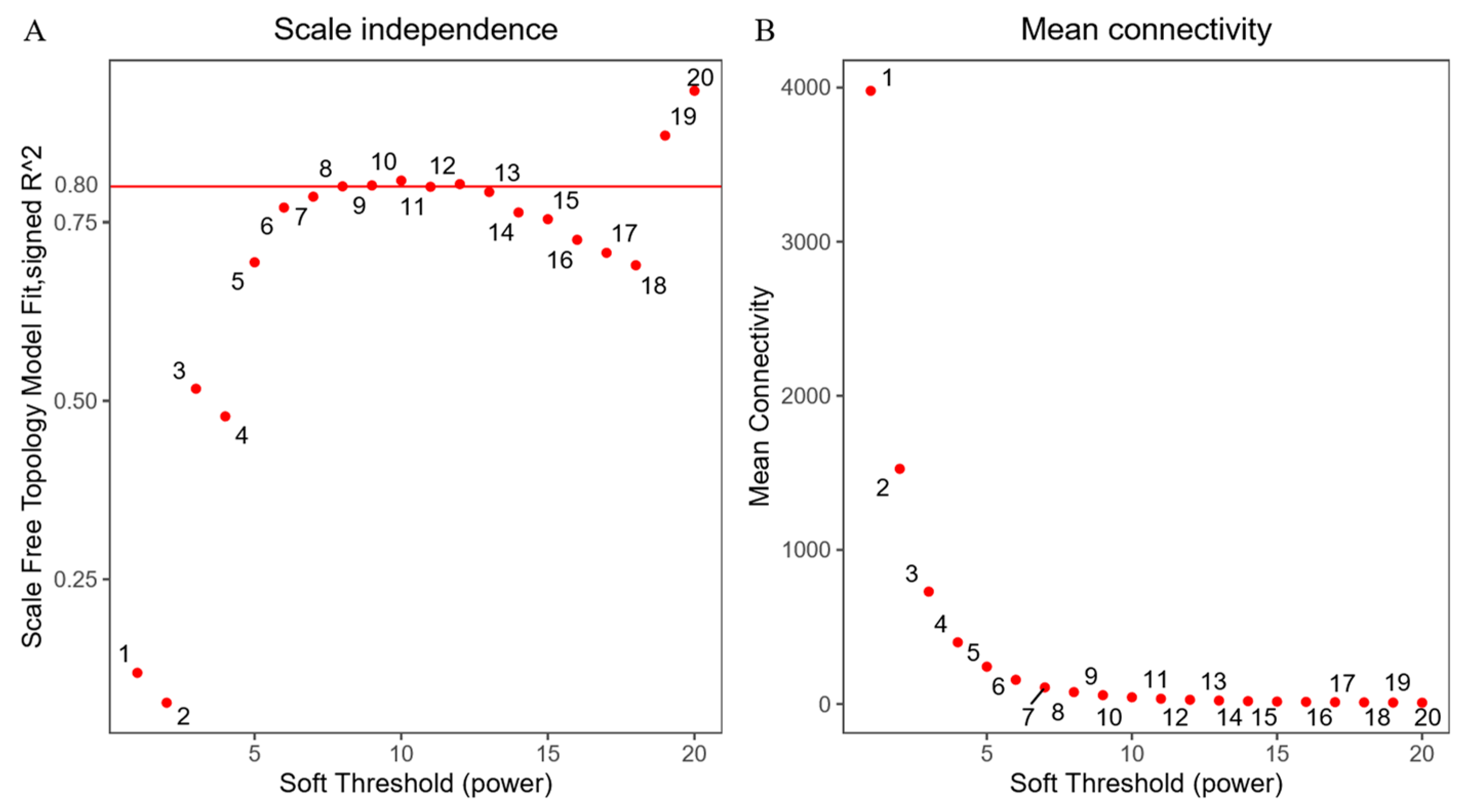
3.4.1. Construction and Module Division of Gene Co-Expression Network
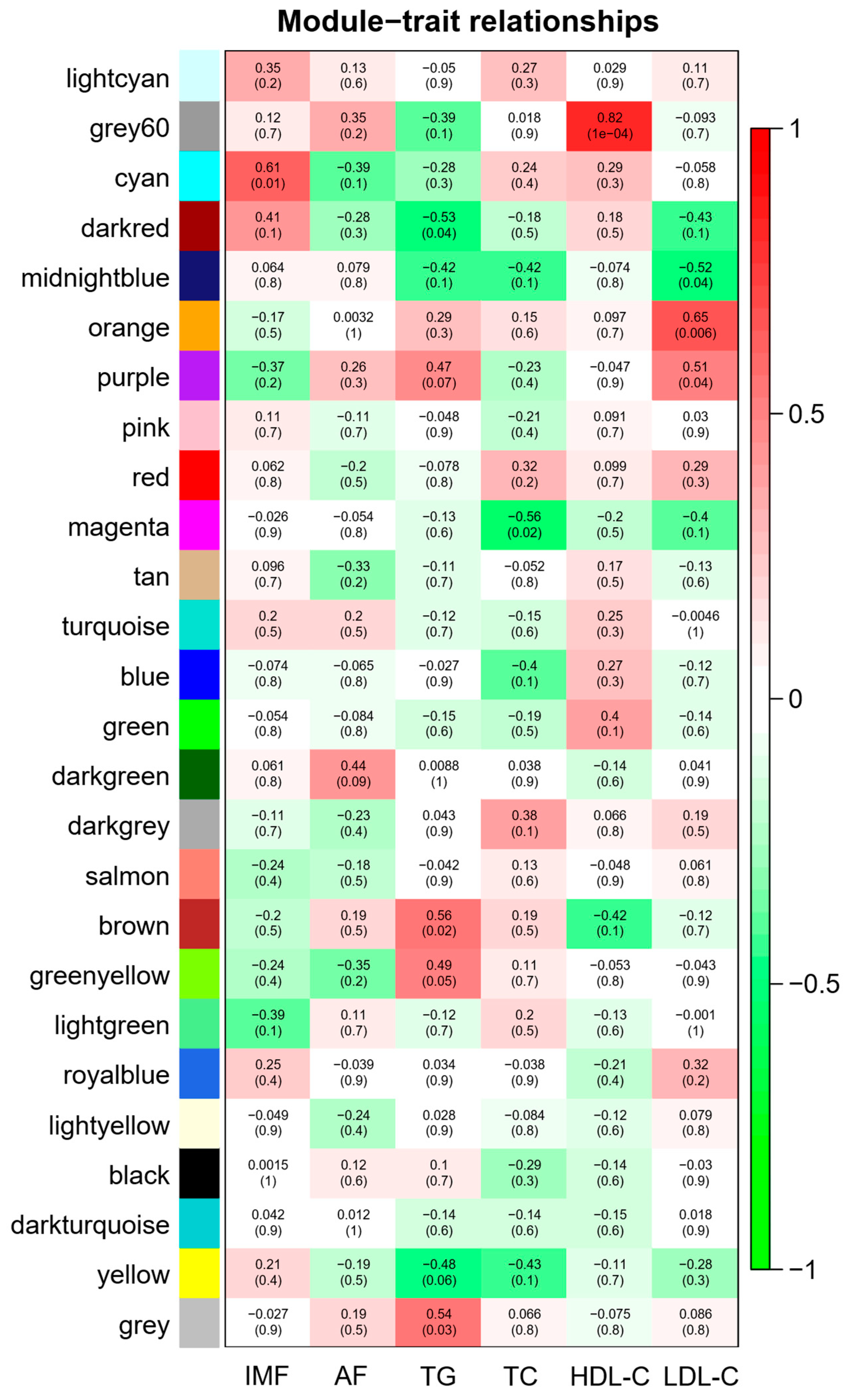
3.4.2. Correlation between Gene Module and Phenotype Data
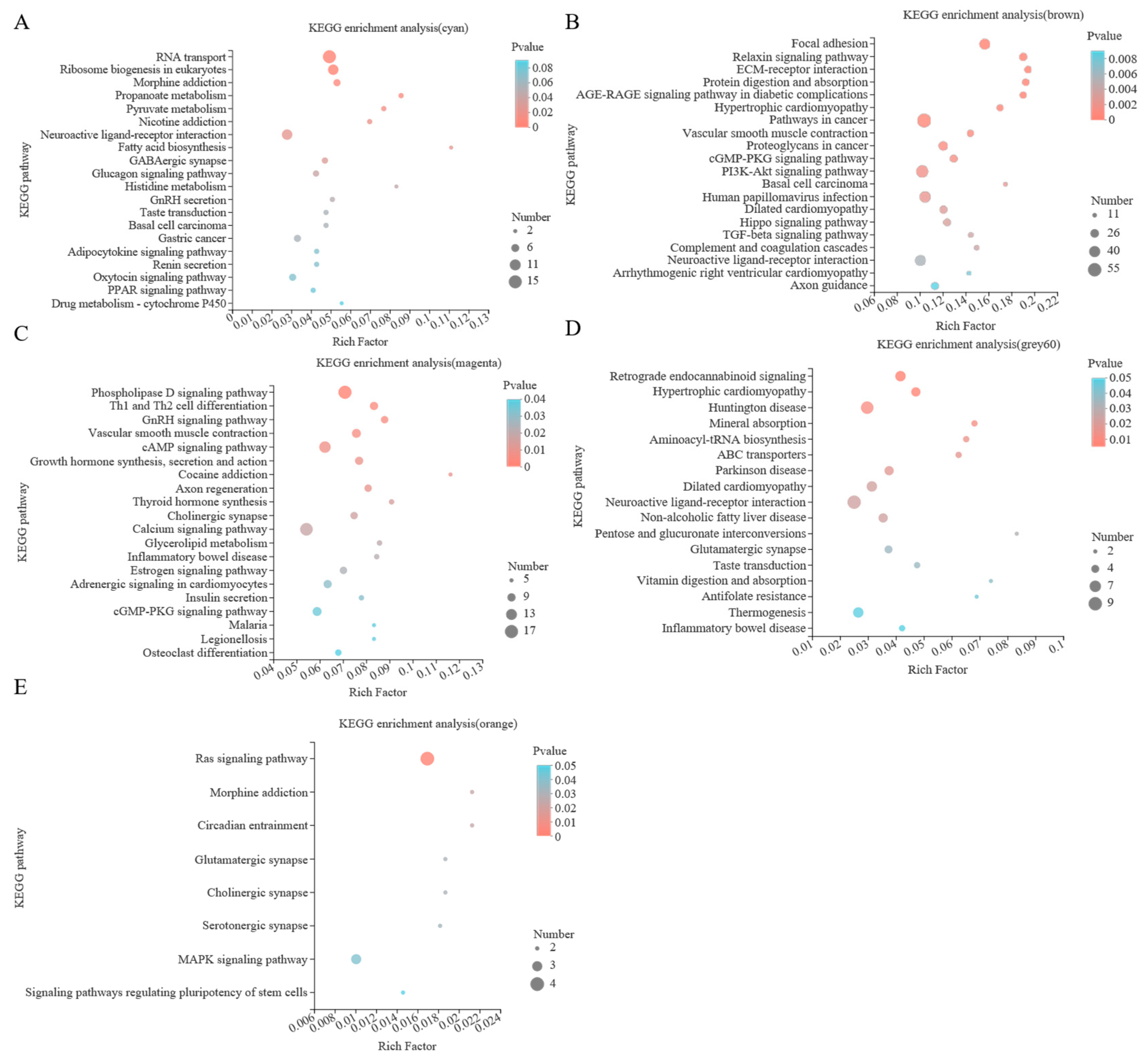
3.4.3. Enrichment Analysis of Module Gene KEGG
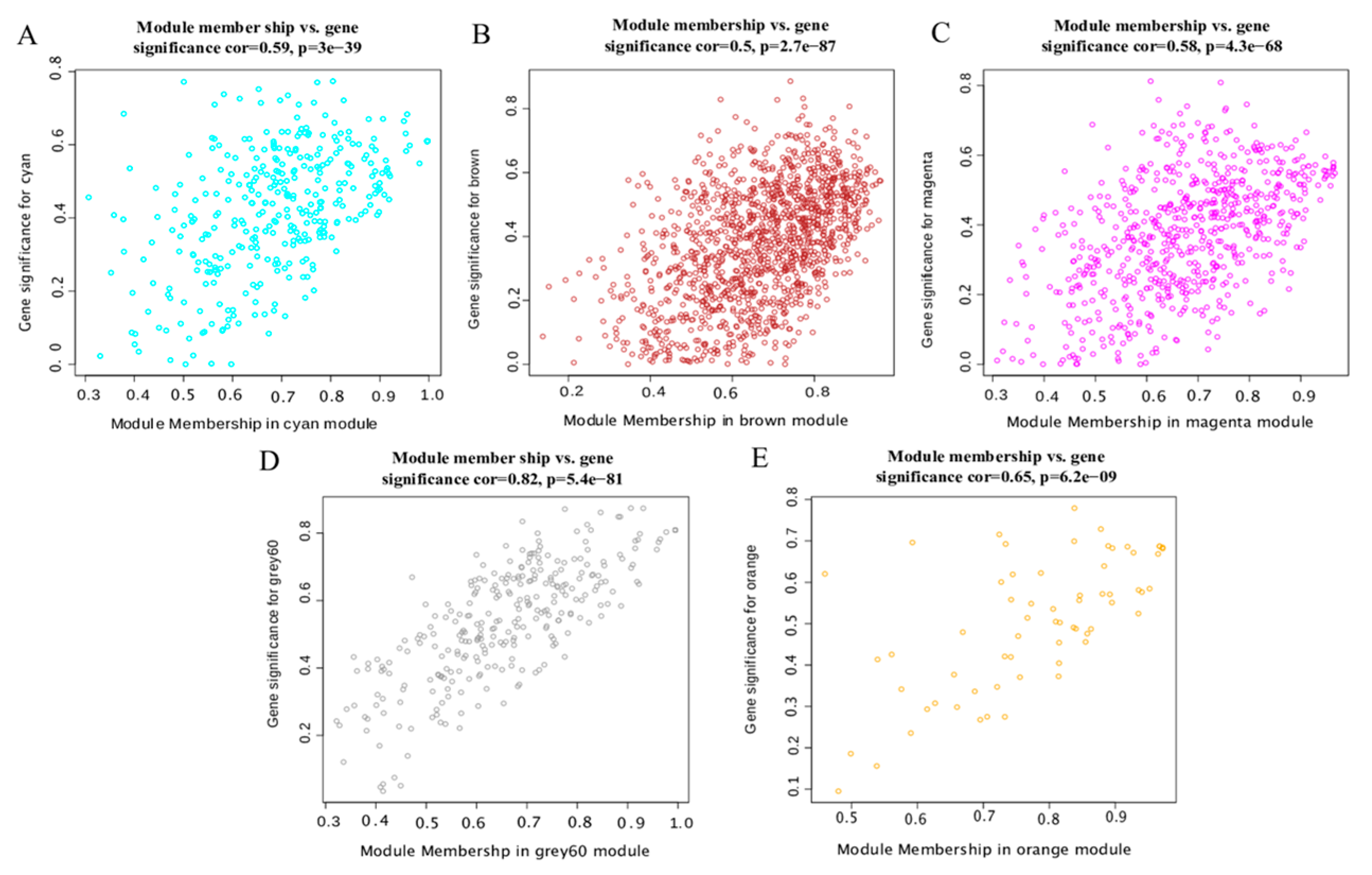
3.4.4. Hub Gene Screening of Lipid Metabolism-Related Modules
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Izuddin, W.I.; Awad, E.A.; Idrus, Z.; Samsudin, A.A.; Mustapha, N.M. Dietary supplementation of postbiotics mitigates adverse impacts of heat stress on antioxidant enzyme activity, total antioxidant, lipid peroxidation, physiological stress indicators, lipid profile and meat quality in broilers. Animals 2020, 10, 982. [Google Scholar] [CrossRef]
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Izuddin, W.I.; Zulkifli, I.; Samsudin, A.A.; Mustapha, N.M. Supplementation of postbiotic RI11 improves antioxidant enzyme activity, upregulated gut barrier genes, and reduced cytokine, acute phase protein, and heat shock protein 70 gene expression levels in heat-stressed broilers. Poult. Sci. 2021, 100, 100908. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S. Effect of heat stress on broiler performance and nutrition regulation. Chin. J. Anim. Husb. Vet. Med. 2020, 11, 175–176. [Google Scholar] [CrossRef]
- Xu, Y.; Ji, F.; Chen, X.; Liu, Y.; Mao, Y.; Su, S. Effect of heat stress on poultry health. Poult. Sci. 2021, 2, 34–41. [Google Scholar]
- Bertocchi, M.; Sirri, F.; Palumbo, O.; Luise, D.; Maiorano, G.; Bosi, P.; Trevisi, P. Exploring differential transcriptome between jejunal and cecal tissue of broiler chickens. Animals 2019, 5, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Lu, Z.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Effects of chronic heat exposure on growth performance, intestinal epithelial histology, appetite-related hormones and genes expression in broilers. J. Sci. Food Agric. 2018, 98, 4471–4478. [Google Scholar] [CrossRef]
- Al-Khalaifah, H.S. Benefits of probiotics and/or prebiotics for antibiotic-reduced poultry. Poult. Sci. 2018, 97, 3807–3815. [Google Scholar] [CrossRef]
- Best, A.A.; Porter, A.L.; Fraley, S.M.; Fraley, G.S. Characterization of gut microbiome dynamics in developing pekin ducks and impact of management system. Front. Microbiol. 2016, 7, 2125. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Jia, H.; Wang, Y.; Zhang, R.; Liu, X. Effect of Healthy tribeneficial bacteria on lipid metabolism in broilers based on transcriptome sequencing. Chin. J. Anim. Vet. Sci. 2022, 53, 1154–1164. [Google Scholar] [CrossRef]
- Shi, Z. Carotid artery bloodletting in chickens. Heilongjiang Anim. Sci. Vet. Med. 1988, 9, 33. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Jacobs, J.A.; Murugesan, G.R.; Cheng, H.W. Effect of dietary synbiotic supplement on behavioral patterns and growth performance of broiler chickens reared under heat stress. Poult. Sci. 2018, 4, 1101–1108. [Google Scholar] [CrossRef]
- St-Pierre, N.R.; Cobanov, B.; Schnitkey, G. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 2003, 86, E52–E77. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Yu, Q.; He, Y.; Hu, R.; Xia, S.; He, J. Dietary resveratrol supplementation inhibits heat stress-induced high-activated innate immunity and inflammatory response in spleen of yellow-feather broilers. Poult. Sci. 2019, 98, 6378–6387. [Google Scholar] [CrossRef]
- Lu, Z.; He, X.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Chronic Heat stress impairs the quality of breast-muscle meat in broilers by affecting redox status and energy-substance metabolism. J. Agric. Food Chem. 2017, 51, 11251–11258. [Google Scholar] [CrossRef]
- Wang, X.; Wei, L.; He, Z.; Liu, D. Effects of Bacillus Subtilis and Lactobacillus on growth performance and liver and kidney function of broilers under heat stress. J. Anhui Sci. Technol. Univ. 2019, 6, 23–28. [Google Scholar] [CrossRef]
- Wang, L.; Gong, L. Effects of probiotics complex on growth performance, intestinal morphology and immunity of broilers under continuous heat stress. China Feed 2021, 18, 25–28. [Google Scholar] [CrossRef]
- Moustafa, E.S.; Alsanie, W.F.; Gaber, A.; Kamel, N.N.; Alaqil, A.A.; Abbas, A.O. Blue-Green Algae (Spirulina platensis) alleviates the negative impact of heat stress on broiler production performance and redox status. Animals 2021, 5, 1243. [Google Scholar] [CrossRef]
- Jin, L.Z.; Ho, Y.W.; Abdullah, N.; Jalaludin, S. Digestive and bacterial enzyme activities in broilers fed diets supplemented with Lactobacillus cultures. Poult. Sci. 2000, 6, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Cramer, T.A.; Kim, H.W.; Chao, Y.; Wang, W.; Cheng, H.W.; Kim, Y.H.B. Effects of probiotic (Bacillus subtilis) supplementation on meat quality characteristics of breast muscle from broilers exposed to chronic heat stress. Poult. Sci. 2018, 97, 3358–3368. [Google Scholar] [CrossRef]
- Mehr, M.A.; Shargh, M.S.; Dastar, B.; Hassani, S.; Akbari, M.R. Effect of different levels of protein and protexin on broiler performance. Int. J. Poult. Sci. 2007, 6, 573–577. [Google Scholar] [CrossRef] [Green Version]
- Anjum, M.I.; Khan, A.G.; Azim, A.; Afzal, M. Effect of dietary supplementation of multi-strain probiotic on broiler growth performance. Pak. Vet. J. 2005, 25, 25–29. [Google Scholar]
- Ravangard, A.H.; Houshmand, M.; Khajavi, M.; Naghiha, R. Performance and cecal bacteria counts of broilers fed low protein diets with and without a combination of probiotic and prebiotic. Braz. J. Poult. Sci. 2017, 19, 75–82. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Zaki, R.S.; Negm, E.A.; Mahmoud, M.A.; Cheng, H.W. Effects of dietary supplementation of a probiotic (Bacillus subtilis) on bone mass and meat quality of broiler chickens. Poult. Sci. 2021, 100, 100906. [Google Scholar] [CrossRef]
- Tang, X.; Liu, X.; Liu, H. Effects of Dietary Probiotic (Bacillus subtilis) Supplementation on carcass traits, meat quality, amino acid, and fatty acid profile of broiler chickens. Front. Vet. Sci. 2021, 8, 767802. [Google Scholar] [CrossRef]
- Bai, K.; Huang, Q.; Zhang, J.; He, J.; Zhang, L.; Wang, T. Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poult. Sci. 2017, 96, 74–82. [Google Scholar] [CrossRef]
- Emami, N.K.; Jung, U.; Voy, B.; Dridi, S. Radical response: Effects of heat stress-induced oxidative stress on lipid metabolism in the avian liver. Antioxidants 2020, 10, 35. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, Y.; Du, M.; Zhou, Y. Protective effects of dietary synbiotic supplementation on meat quality and oxidative status in broilers under heat stress. Environ. Sci. Pollut. Res. Int. 2021, 28, 30197–30206. [Google Scholar] [CrossRef]
- Khan, A.Z.; Kumbhar, S.; Liu, Y.; Hamid, M.; Pan, C.; Nido, S.A.; Parveen, F.; Huang, K. Dietary supplementation of selenium-enriched probiotics enhances meat quality of broiler chickens (Gallus gallus domesticus) raised under high ambient temperature. Biol. Trace Elem. Res. 2018, 182, 328–338. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, Y.; Guo, S.; Tan, J. Effects of Clostridium butyricum and Enterococcus faecium on growth performance, lipid metabolism, and cecal microbiota of broiler chickens. Appl. Microbiol. Biotechnol. 2013, 97, 6477–6488. [Google Scholar] [CrossRef]
- Yang, S.; Li, M.; Xia, M.; Shang, Y.; Chen, Y.; Lu, B.; Huang, Y.; Chen, W. Effects of different feed restriction methods on growth performance, slaughter performance and skeletal traits of Arbor Acres Chickens. Chin. J. Anim. Nutr. 2017, 29, 3341–3351. [Google Scholar] [CrossRef]
- Kers, J.G.; Velkers, F.C.; Fischer, E.A.J.; Hermes, G.D.A.; Stegeman, J.A.; Smidt, H. Host and environmental factors affecting the intestinal microbiota in chickens. Front. Microbiol. 2018, 9, 235. [Google Scholar] [CrossRef] [Green Version]
- Macpherson, A.J.; Geuking, M.B.; McCoy, K.D. Homeland security: IgA immunity at the frontiers of the body. Trends Immunol. 2012, 33, 160–167. [Google Scholar] [CrossRef]
- Macpherson, A.J.; McCoy, K.D.; Johansen, F.E.; Brandtzaeg, P. The immune geography of IgA induction and function. Mucosal Immunol. 2008, 1, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Chen, J. Effects of tea physiological and biochemical index in serum of heat-stressed broilers. J. Luoyang Norm. Univ. 2021, 40, 10–14. [Google Scholar] [CrossRef]
- Yazhini, P.; Visha, P.; Selvaraj, P.; Vasanthakumar, P.; Chandran, V. Dietary encapsulated probiotic effect on broiler serum biochemical parameters. Vet. World 2018, 11, 1344–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilliland, S.E. Acidophilus milk products: A review of potential benefits to consumers. J. Dairy Sci. 1989, 72, 2483–2494. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, Y. The probiotic Lactobacillus acidophilus reduces cholesterol absorption through the down-regulation of Niemann-Pick C1-like 1 in Caco-2 cells. Br. J. Nutr. 2010, 103, 473–478. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.; He, L.; Zhang, R.; Li, C.; Zhang, L.; Zhu, J. Research progress on physiological function of Bifidobacterium. Sci. Technol. Food Ind. 2013, 34, 353–358. [Google Scholar] [CrossRef]
- Panda, A.K.; Rao, S.V.R.; Raju, M.V.L.N.; Sharma, S.R. Dietary supplementation of Lactobacillus Sporogenes on performance and serum biochemico–lipid profile of broiler chickens. J. Poult. Sci. 2006, 43, 235–240. [Google Scholar] [CrossRef] [Green Version]
- Piray, A.; Foroutanifar, S. Chromium supplementation on the growth performance, carcass traits, blood constituents, and immune competence of broiler chickens under heat stress: A systematic review and dose–response meta-analysis. Biol. Trace Elem. Res. 2022, 200, 2876–2888. [Google Scholar] [CrossRef]
- Xiao, F.; Ao, D.; Zhou, B.; Spears, J.W.; Lin, X.; Huang, Y. Effects of supplemental chromium propionate on serum lipids, carcass traits, and meat quality of heat-stressed broilers. Biol. Trace Elem. Res. 2017, 176, 401–406. [Google Scholar] [CrossRef]
- He, S.; Zhao, S.; Dai, S.; Liu, D.; Bokhari, S.G. Effects of dietary betaine on growth performance, fat deposition and serum lipids in broilers subjected to chronic heat stress. Anim. Sci. J. 2015, 86, 897–903. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Z.; Lin, Q.; Yang, Y.; Hang, Y.; Zhou, X.; Wu, C.; Xie, Z. Nuciferine reduced fat deposition by controlling triglyceride and cholesterol concentration in broiler chickens. Poult. Sci. 2020, 12, 7101–7108. [Google Scholar] [CrossRef]
- Romon, M.; Thomas-Desrousseaux, P.; Beuscart, R.; Fossati, P.; Sezille, G.; Jaillard, J. Insuline et métabolisme des lipoprotéines [Insulin and the metabolism of lipoproteins]. Ann. Endocrinol. 1983, 1, 77–81. [Google Scholar]
- Sharif-Askari, F.S.; Sharif-Askari, N.S.; Halwani, R.; Abusnana, S.; Hamoudi, R.; Sulaiman, N. Low Vitamin D serum level is associated with HDL-C dyslipidemia and increased serum thrombomodulin levels of insulin-resistant individuals. Diabetes Metab. Syndr. Obes. 2020, 13, 1599–1607. [Google Scholar] [CrossRef]
- Mahmoud, U.T.; Abdel-Mohsein, H.S.; Mahmoud, M.A.M.; Amen, O.A.; Hassan, R.I.M.; Abd-El-Malek, A.M.; Rageb, S.M.M.; Waly, H.S.A.; Othman, A.A.; Osman, M.A. Effect of zinc oxide nanoparticles on broilers’ performance and health status. Trop. Anim. Health Prod. 2020, 4, 2043–2054. [Google Scholar] [CrossRef]
- Sui, G.; Jia, L.; Song, N.; Min, D.; Chen, S.; Wu, Y.; Yang, G. Aberrant expression of HDL-bound microRNA induced by a high-fat diet in a pig model: Implications in the pathogenesis of dyslipidaemia. BMC Cardiovasc. Disord. 2021, 1, 280. [Google Scholar] [CrossRef]
- Chatree, S.; Thongmaen, N.; Tantivejkul, K.; Sitticharoon, C.; Vucenik, I. Role of inositols and inositol phosphates in energy metabolism. Molecules 2020, 25, 5079. [Google Scholar] [CrossRef]
- Resnyk, C.W.; Carré, W.; Wang, X.; Porter, T.E.; Simon, J.; Bihan-Duval, E.L.; Duclos, M.J.; Aggrey, S.E.; Cogburn, L.A. Transcriptional analysis of abdominal fat in chickens divergently selected on bodyweight at two ages reveals novel mechanisms controlling adiposity: Validating visceral adipose tissue as a dynamic endocrine and metabolic organ. BMC Genom. 2017, 18, 626. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, X.; Han, K.; Zhang, G.; Wang, J.; Xie, K.; Xue, Q. Genome-wide analysis of lncRNA and mRNA expression during differentiation of abdominal preadipocytes in the chicken. G3 (Bethesda) 2017, 3, 953–966. [Google Scholar] [CrossRef] [Green Version]
- Trius-Soler, M.; Vilas-Franquesa, A.; Tresserra-Rimbau, A.; Sasot, G.; Storniolo, C.E.; Estruch, R.; Lamuela-Raventós, R.M. Effects of the non-alcoholic fraction of beer on abdominal fat, osteoporosis, and body hydration in women. Molecules 2020, 25, 3910. [Google Scholar] [CrossRef]
- Li, J.; Yuan, J.; Miao, Z.; Song, Z.; Yang, Y.; Tian, W.; Guo, Y. Effect of dietary nutrient density on small intestinal phosphate transport and bone mineralization of broilers during the growing period. PLoS ONE 2016, 11, e0153859. [Google Scholar] [CrossRef] [Green Version]
- Meng, H.C.; Matthan, N.R.; Angellotti, E.; Pittas, A.G.; Lichtenstein, A.H. Exploring the effect of vitamin D3 supplementation on surrogate biomarkers of cholesterol absorption and endogenous synthesis in patients with type 2 diabetes—Randomized controlled trial. Am. J. Clin. Nutr. 2020, 112, 538–547. [Google Scholar] [CrossRef]
- Khundmiri, S.J.; Murray, R.D.; Lederer, E. PTH and vitamin D. Compr. Physiol. 2016, 2, 561–601. [Google Scholar] [CrossRef]
- Pokhrel, S.; Giri, N.; Pokhrel, R.; Pardhe, B.D.; Lamichhane, A.; Chaudhary, A.; Bhatt, M.P. Vitamin D deficiency and cardiovascular risk in type 2 diabetes population. Open Life Sci. 2021, 1, 464–474. [Google Scholar] [CrossRef]
- Yari, F.A.; Shabani, P.; Karami, S.; Sarmadi, N.; Poustchi, H.; Bandegi, A.R. Circulating levels of FAM19A5 are inversely associated with subclinical atherosclerosis in non-alcoholic fatty liver disease. BMC Endocr. Disord. 2021, 1, 153. [Google Scholar] [CrossRef]
- Lee, Y.B.; Hwang, H.J.; Kim, J.A.; Hwang, S.Y.; Roh, E.; Hong, S.H.; Choi, K.M.; Baik, S.H.; Yoo, H.J. Association of serum FAM19A5 with metabolic and vascular risk factors in human subjects with or without type 2 diabetes. Diabetes Vasc. Dis. Res. 2019, 16, 530–538. [Google Scholar] [CrossRef]
- Volf, J.; Polansky, O.; Varmuzova, K.; Gerzova, L.; Sekelova, Z.; Faldynova, M.; Babak, V.; Medvecky, M.; Smith, A.L.; Kaspers, B.; et al. Transient and prolonged response of chicken cecum mucosa to colonization with different gut microbiota. PLoS ONE 2016, 11, e0163932. [Google Scholar] [CrossRef]
- Divoux, A.; Erdos, E.; Whytock, K.; Timothy, F.O.; Smith, S.R. Transcriptional and DNA methylation signatures of subcutaneous adipose tissue and adipose-derived stem cells in PCOS Women. Cells 2022, 11, 848. [Google Scholar] [CrossRef]
- Pérez-Vázquez, V.; Guzmán-Flores, J.M.; Mares-Álvarez, D.; Hernández-Ortiz, M.; Macías-Cervantes, M.H.; Ramírez-Emiliano, J.; Encarnación-Guevara, S. Differential proteomic analysis of the pancreas of diabetic db/db mice reveals the proteins involved in the development of complications of diabetes mellitus. Int. J. Mol. Sci. 2014, 15, 9579–9593. [Google Scholar] [CrossRef] [Green Version]
- Resnyk, C.W.; Chen, C.M.; Huang, H.; Wu, C.H.; Simon, J.; Bihan-Duval, E.L.; Duclos, M.J.; Cogburn, L.A. RNA-Seq analysis of abdominal fat in genetically fat and lean chickens highlights a divergence in expression of genes controlling adiposity, hemostasis, and lipid metabolism. PLoS ONE 2015, 10, e0139549. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.; Chang, G.; Chen, R.; Sheng, Z.; Dai, A.; Zhai, F.; Li, J.; Xia, M.; Hua, D.; Xu, L.; et al. Identification of key genes in the response to salmonella enterica enteritidis, salmonella enterica pullorum, and poly(I:C) in chicken spleen and caecum. BioMed Res. Int. 2014, 2014, 154946. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Hua, G.; Han, D.; Zheng, X.; Dong, X.; Wang, S.; Long, J.; Zheng, Z.; Wang, A.; Wang, J.; et al. An EAV-HP insertion in the promoter region of SLCO1B3 has pleiotropic effects on chicken liver metabolism based on the transcriptome and proteome analysis. Sci. Rep. 2021, 11, 7571. [Google Scholar] [CrossRef]
- Tummala, H.; Fleming, S.; Hocking, P.M.; Wehner, D.; Naseem, Z.; Ali, M.; Inglehearn, C.F.; Zhelev, N.; Lester, D.H. The D153del mutation in GNB3 gene causes tissue specific signalling patterns and an abnormal renal morphology in Rge chickens. PLoS ONE 2011, 6, e21156. [Google Scholar] [CrossRef] [Green Version]
- Ozdemir, A.C.; Wynn, G.M.; Vester, A.; Weitzmann, M.N.; Neigh, G.N.; Srinivasan, S.; Rudd, M.K. GNB3 overexpression causes obesity and metabolic syndrome. PLoS ONE 2017, 12, e0188763. [Google Scholar] [CrossRef] [Green Version]
- Domanski, M.J.; Tian, X.; Wu, C.O.; Reis, J.P.; Dey, A.K.; Gu, Y.; Zhao, L.; Bae, S.; Liu, K.; Hasan, A.A.; et al. Time course of LDL cholesterol exposure and cardiovascular disease event risk. J. Am. Coll. Cardiol. 2020, 13, 1507–1516. [Google Scholar] [CrossRef]
- Méndez-Giménez, L.; Ezquerro, S.; Silva, I.V.D.; Soveral, G.; Frühbeck, G.; Rodríguez, A. Pancreatic aquaporin-7: A novel target for anti-diabetic drugs? Front. Chem. 2018, 6, 99. [Google Scholar] [CrossRef] [Green Version]



| Item | 1–21 Days | 22–42 Days |
|---|---|---|
| Diet composition, % | ||
| Corn | 56.49 | 61.42 |
| Soybean oil | 2.22 | 3.00 |
| Soybean meal | 30.24 | 25.30 |
| Cotton seed meal | 5.00 | 5.00 |
| Fishmeal | 2.43 | 1.98 |
| CaHCO3 | 1.60 | 1.39 |
| Limestone | 1.16 | 1.10 |
| Methionine | 0.15 | 0.05 |
| NaCl | 0.30 | 0.35 |
| Choline | 0.19 | 0.19 |
| Premix 1 | 0.22 | 0.22 |
| Nutrient, % 2 | ||
| ME (MJ·kg−1) | 12.12 | 12.54 |
| Crude protein | 21.00 | 19.00 |
| Lysine | 1.12 | 0.98 |
| Methionine + Cystine | 0.84 | 0.68 |
| Calcium | 1.00 | 0.90 |
| Available phosphorus | 0.30 | 0.30 |
| Item 1 | Control | Experiment 1 | Experiment 2 | Experiment 3 | p-Value |
|---|---|---|---|---|---|
| Body Weight, g | 2911.243 ± 10.092 b | 3121.163 ± 24.104 a | 2658.173 ± 39.407 c | 2894.857 ± 82.152 b | 0.001 |
| ADG, g/brid | 89.010 ± 0.967 b | 106.317 ± 1.971 a | 73.463 ± 5.239 c | 92.624 ± 4.528 b | 0.002 |
| ADFI, g/brid | 168.630 ± 1.649 | 168.780 ± 3.296 | 151.144 ± 2.975 | 167.542 ± 15.013 | 0.372 |
| FCR, g/g | 1.895 ± 0.039 ab | 1.590 ± 0.059 c | 2.073 ± 0.105 a | 1.802 ± 0.077 bc | 0.011 |
| Dressed Weight, g | 2436.667 ± 46.369 ab | 2656.333 ± 24.694 a | 2297.667 ± 58.373 b | 2622.000 ± 161.970 a | 0.071 |
| Eviscerated Weight, g | 1899.333 ± 52.922 ab | 2008.000 ± 58.141 a | 1835.667 ± 28.109 b | 1988.000 ± 47.438 ab | 0.110 |
| Half-eviscerated Weight, g | 2153.787 ± 18.068 b | 2598.653 ± 188.262 a | 2038.098 ± 16.619 b | 2420.005 ± 159.501 ab | 0.047 |
| Item 1 | Control | Experiment 1 | Experiment 2 | Experiment 3 | p-Value |
|---|---|---|---|---|---|
| Dripping loss, % | 4.352 ± 0.274 | 3.866 ± 0.532 | 4.614 ± 0.287 | 4.254 ± 0.315 | 0.571 |
| Crude moisture, % | 74.939 ± 0.251 | 75.479 ± 0.802 | 74.441 ± 0.443 | 74.741 ± 0.644 | 0.644 |
| Cooking loss, % | 11.840 ± 0.817 | 11.190 ± 0.418 | 12.243 ± 0.392 | 11.248 ± 0.822 | 0.629 |
| Cooked meat rate, % | 62.112 ± 0.488 | 63.095 ± 0.318 | 61.936 ± 0.398 | 62.563 ± 0.444 | 0.277 |
| Shear force, N | 25.783 ± 1.444 | 23.208 ± 0.848 | 26.132 ± 1.276 | 24.611 ± 1.421 | 0.410 |
| IMF, % | 2.577 ± 0.430 | 2.978 ± 0.521 | 2.355 ± 0.550 | 3.669 ± 0.557 | 0.336 |
| AF rate, % | 2.333 ± 0.229 | 1.978 ± 0.152 | 2.163 ± 0.270 | 2.114 ± 0.297 | 0.781 |
| Item 1 | Control group | Experiment 1 | Experiment 2 | Experiment 3 | p-Value |
|---|---|---|---|---|---|
| TG, mmol/L | 0.325 ± 0.008 b | 0.317 ± 0.006 b | 0.428 ± 0.028 a | 0.257 ± 0.022 c | 0.001 |
| TC, mmol/L | 4.213 ± 0.107 | 4.163 ± 0.197 | 4.236 ± 0.108 | 4.289 ± 0.201 | 0.955 |
| HDL-C, mmol/L | 2.751 ± 0.019 b | 2.846 ± 0.096 ab | 2.698 ± 0.075 b | 3.171 ± 0.207 a | 0.067 |
| LDL-C, mmol/L | 1.387 ± 0.049 | 1.280 ± 0.140 | 1.545 ± 0.191 | 1.310 ± 0.212 | 0.659 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Liu, X.; Jia, H. WGCNA Analysis of Important Modules and Hub Genes of Compound Probiotics Regulating Lipid Metabolism in Heat-Stressed Broilers. Animals 2022, 12, 2644. https://doi.org/10.3390/ani12192644
Zhang L, Liu X, Jia H. WGCNA Analysis of Important Modules and Hub Genes of Compound Probiotics Regulating Lipid Metabolism in Heat-Stressed Broilers. Animals. 2022; 12(19):2644. https://doi.org/10.3390/ani12192644
Chicago/Turabian StyleZhang, Lihuan, Xuan Liu, and Hao Jia. 2022. "WGCNA Analysis of Important Modules and Hub Genes of Compound Probiotics Regulating Lipid Metabolism in Heat-Stressed Broilers" Animals 12, no. 19: 2644. https://doi.org/10.3390/ani12192644






