Value Ranges and Clinical Comparisons of Serum DHEA-S, IL-6, and TNF-α in Western Lowland Gorillas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects, Sample Collection, and Assessment of Health Status
2.2. Enzyme Immunoassays
2.3. Quantitative Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, M.P.; Arheart, K.L.; Cray, C. Reference Intervals, Longitudinal Analyses, and Index of Individuality of Commonly Measured Laboratory Variables in Captive Bald Eagles (Haliaeetus leucocephalus). J. Avian Med. Surg. 2014, 28, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Horn, P.S.; Pesce, A.J. Reference Intervals: An Update. Clin. Chim. Acta 2003, 334, 5–23. [Google Scholar] [CrossRef]
- Concordet, D.; Geffré, A.; Braun, J.P.; Trumel, C. A New Approach for the Determination of Reference Intervals from Hospital-Based Data. Clin. Chim. Acta 2009, 405, 43–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedrichs, K.R.; Harr, K.E.; Freeman, K.P.; Szladovits, B.; Walton, R.M.; Barnhart, K.F.; Blanco-Chavez, J. American Society for Veterinary Clinical Pathology ASVCP Reference Interval Guidelines: Determination of de Novo Reference Intervals in Veterinary Species and Other Related Topics. Vet. Clin. Pathol. 2012, 41, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Geffré, A.; Friedrichs, K.; Harr, K.; Concordet, D.; Trumel, C.; Braun, J.-P. Reference Values: A Review. Vet. Clin. Pathol. 2009, 38, 288–298. [Google Scholar] [CrossRef] [Green Version]
- Hill, S.P.; Broom, D.M. Measuring Zoo Animal Welfare: Theory and Practice. Zoo Biol. 2009, 28, 531–544. [Google Scholar] [CrossRef]
- Wolfensohn, S.; Shotton, J.; Bowley, H.; Davies, S.; Thompson, S.; Justice, W.S.M. Assessment of Welfare in Zoo Animals: Towards Optimum Quality of Life. Animals 2018, 8, 110. [Google Scholar] [CrossRef] [Green Version]
- Edwards, K.L.; Edes, A.N.; Brown, J.L. Stress, Well-Being and Reproductive Success. In Reproductive Sciences in Animal Conservation; Comizzoli, P., Brown, J.L., Holt, W.V., Eds.; Advances in Experimental Medicine and Biology; Springer Nature: Berlin/Heidelberg, Germany, 2019; Volume 1200, pp. 91–162. [Google Scholar]
- McEwen, B.S.; Seeman, T.E. Protective and Damaging Effects of Mediators of Stress: Elaborating and Testing the Concepts of Allostasis and Allostatic Load. Ann. N. Y. Acad. Sci. 1999, 896, 30–47. [Google Scholar] [CrossRef]
- Kamin, H.S.; Kertes, D.A. Cortisol and DHEA in Development and Psychopathology. Horm. Behav. 2017, 89, 69–85. [Google Scholar] [CrossRef]
- Whitham, J.C.; Bryant, J.L.; Miller, L.J. Beyond Glucocorticoids: Integrating Dehydroepiandrosterone (DHEA) into Animal Welfare Research. Animals 2020, 10, 1381. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, T.G.; Edwards, L. Chronic Stress and the HPA Axis. Standard 2010, 9, 1–12. [Google Scholar]
- Karlamangla, A.S.; Singer, B.H.; McEwen, B.S.; Rowe, J.W.; Seeman, T.E. Allostatic Load as a Predictor of Functional Decline: MacArthur Studies of Successful Aging. J. Clin. Epidemiol. 2002, 55, 696–710. [Google Scholar] [CrossRef]
- Lowenstine, L.J.; McManamon, R.; Terio, K.A. Comparative Pathology of Aging Great Apes: Bonobos, Chimpanzees, Gorillas, and Orangutans. Vet. Pathol. 2016, 53, 250–276. [Google Scholar] [CrossRef] [PubMed]
- Murphy, H.W.; Danforth, M.D.; Clyde, V.L. The Great Ape Heart Project. Int. Zoo Yearb. 2018, 52, 103–112. [Google Scholar] [CrossRef]
- Cockrem, J.F. Individual Variation in Glucocorticoid Stress Responses in Animals. Gen. Comp. Endocrinol. 2013, 181, 45–58. [Google Scholar] [CrossRef]
- MacDougall-Shackleton, S.A.; Bonier, F.; Romero, L.M.; Moore, I.T. Glucocorticoids and “Stress” Are Not Synonymous. Integr. Org. Biol. 2019, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEwen, B.S. What Is the Confusion with Cortisol? Chronic Stress 2019, 3, 2470547019833647. [Google Scholar] [CrossRef]
- Edes, A.N.; Wolfe, B.A.; Crews, D.E. Assessing Stress in Zoo-Housed Western Lowland Gorillas (Gorilla Gorilla Gorilla) Using Allostatic Load. Int. J. Primatol. 2016, 37, 241–259. [Google Scholar] [CrossRef]
- Edes, A.N.; Wolfe, B.A.; Crews, D.E. The First Multi-Zoo Application of an Allostatic Load Index to Western Lowland Gorillas (Gorilla Gorilla Gorilla). Gen. Comp. Endocrinol. 2018, 266, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, R.S.C.; Mendonça, R.S.; Bercovitch, F.B.; Huffman, M.A. Developmental Changes in the Endocrine Stress Response in Orangutans (Pongo pygmaeus). J. Comp. Physiol. B 2019, 189, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, R.S.C.; Huffman, M.A.; Mouri, K.; Shimizu, K.; Bercovitch, F.B. Dead or Alive? Predicting Fetal Loss in Japanese Macaques (Macaca fuscata) by Fecal Metabolites. Anim. Reprod. Sci. 2016, 175, 33–38. [Google Scholar] [CrossRef]
- Prall, S.P.; Ambu, L.; Nathan, S.; Alsisto, S.; Ramirez, D.; Muehlenbein, M.P. Androgens and Innate Immunity in Rehabilitated Semi-Captive Orangutans (Pongo pygmaeus morio) from Malaysian borneo. Am. J. Primatol. 2015, 77, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, C.; Montillo, M.; Prandi, A.; Mkupasi, E.M.; Ngowi, H.A.; Johansen, M.V. Hair Cortisol and Dehydroepiandrosterone Concentrations in Naturally Taenia Solium Infected Pigs in Tanzania. Gen. Comp. Endocrinol. 2017, 246, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Kindt, T.J.; Osborne, B.A.; Goldsby, R.A. (Eds.) Kuby Immunology, 6th ed.; W. H. Freeman Company: New York, NY, USA, 2006. [Google Scholar]
- De Guise, S.; Levin, M.; Gebhard, E.; Jasperse, L.; Burdett Hart, L.; Smith, C.R.; Venn-Watson, S.; Townsend, F.; Wells, R.; Balmer, B.; et al. Changes in Immune Functions in Bottlenose Dolphins in the Northern Gulf of Mexico Associated with the Deepwater Horizon Oil Spill. Endanger. Species Res. 2017, 33, 291–303. [Google Scholar] [CrossRef] [Green Version]
- Pai, J.K.; Pischon, T.; Ma, J.; Manson, J.E.; Hankinson, S.E.; Joshipura, K.; Curhan, G.C.; Rifai, N.; Cannuscio, C.C.; Stampfer, M.J.; et al. Inflammatory Markers and the Risk of Coronary Heart Disease in Men and Women. N. Engl. J. Med. 2004, 351, 2599–2610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landolfi, J.A.; Schultz, S.A.; Mikota, S.K.; Terio, K.A. Development and Validation of Cytokine Quantitative, Real Time RT-PCR Assays for Characterization of Asian Elephant Immune Responses. Vet. Immunol. Immunopathol. 2009, 131, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Miller, A.H. When Not Enough Is Too Much: The Role of Insufficient Glucocorticoid Signaling in the Pathophysiology of Stress-Related Disorders. Am. J. Psychiatry 2003, 160, 1554–1565. [Google Scholar] [CrossRef]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The Metabolic Syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Grosse, L.; Ambrée, O.; Jörgens, S.; Jawahar, M.C.; Singhal, G.; Stacey, D.; Arolt, V.; Baune, B.T. Cytokine Levels in Major Depression Are Related to Childhood Trauma but not to Recent Stressors. Psychoneuroendocrinology 2016, 73, 24–31. [Google Scholar] [CrossRef]
- Mauss, D.; Li, J.; Schmidt, B.; Angerer, P.; Jarczok, M.N. Measuring Allostatic Load in the Workforce: A Systematic Review. Ind. Health 2015, 53, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Harris, T.B.; Ferucci, L.; Tracy, R.P.; Corti, M.C.; Wacholder, S.; Ettinger, W.H.; Heimovitz, H.; Cohen, H.J.; Wallace, R. Associations of Elevated Interleukin-6 and C-Reactive Protein Levels with Mortality in the Elderly. Am. J. Med. 1999, 106, 506–512. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and Atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation and Cardiovascular Disease Mechanisms. Am. J. Clin. Nutr. 2006, 83, 456S–460S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golia, E.; Limongelli, G.; Natale, F.; Fimiani, F.; Maddaloni, V.; Pariggiano, I.; Bianchi, R.; Crisci, M.; D’Acierno, L.; Giordano, R.; et al. Inflammation and Cardiovascular Disease: From Pathogenesis to Therapeutic Target. Curr. Atheroscler. Rep. 2014, 16, 435. [Google Scholar] [CrossRef]
- Lutgendorf, S.K.; Anderson, B.; Sorosky, J.I.; Buller, R.E.; Lubaroff, D.M. Interleukin-6 and Use of Social Support in Gynecologic Cancer Patients. Int. J. Behav. Med. 2000, 7, 127–142. [Google Scholar] [CrossRef]
- Costanzo, E.S.; Lutgendorf, S.K.; Sood, A.K.; Anderson, B.; Sorosky, J.; Lubaroff, D.M. Psychosocial Factors and Interleukin-6 among Women with Advanced Ovarian Cancer. Cancer 2005, 104, 305–313. [Google Scholar] [CrossRef]
- Kronfol, Z. Behavioral Effects of Cytokines: A Psychiatrist’s Perspective. In Cytokines: Stress and Immunity; Plotnikoff, N.P., Faith, R.E., Murgo, A.J., Good, R.A., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 1–16. [Google Scholar]
- Heikkilä, K.; Harris, R.; Lowe, G.; Rumley, A.; Yarnell, J.; Gallacher, J.; Ben-Shlomo, Y.; Ebrahim, S.; Lawlor, D.A. Associations of Circulating C-Reactive Protein and Interleukin-6 with Cancer Risk: Findings from Two Prospective Cohorts and a Meta-Analysis. Cancer Causes Control. 2009, 20, 15–26. [Google Scholar] [CrossRef]
- Stetler, C.; Murali, R.; Chen, E.; Miller, G.E. Stress, Immunity, and Disease. In Handbook of Stress Medicine and Health; Cooper, C.L., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 131–154. [Google Scholar]
- Hawkley, L.C.; Bosch, J.A.; Engeland, C.G.; Marucha, P.T.; Cacioppo, J.T. Loneliness, Dysphoria, Stress, and Immunity: A Role for Cytokines. In Cytokines: Stress and Immunity; Plotnikoff, N.P., Faith, R.E., Murgo, A.J., Good, R.A., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 67–85. [Google Scholar]
- Kiecolt-Glaser, J.K.; Preacher, K.J.; MacCallum, R.C.; Atkinson, C.; Malarkey, W.B.; Glaser, R. Chronic Stress and Age-Related Increases in the Proinflammatory Cytokine IL-6. Proc. Natl. Acad. Sci. USA 2003, 100, 9090–9095. [Google Scholar] [CrossRef] [Green Version]
- Murali, R.; Hanson, M.D.; Chen, E. Psychological Stress and Its Relationship to Cytokines and Inflammatory Disease. In Cytokines: Stress and Immunity; Plotnikoff, N.P., Faith, R.E., Murgo, A.J., Good, R.A., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 29–49. [Google Scholar]
- Edes, A.N.; Brand, C.M. Age, Sex, and Inflammatory Markers Predict Chronic Conditions, Cardiac Disease, and Mortality among Captive Western Lowland Gorillas (Gorilla Gorilla Gorilla). Primates 2021, 62, 931–943. [Google Scholar] [CrossRef]
- Beineke, A.; Siebert, U.; Müller, G.; Baumgärtner, W. Increased Blood Interleukin-10 MRNA Levels in Diseased Free-Ranging Harbor porpoises (Phocoena phocoena). Vet. Immunol. Immunopathol. 2007, 115, 100–106. [Google Scholar] [CrossRef]
- Fonfara, S.; Kakuschke, A.; Rosenberger, T.; Siebert, U.; Prange, A. Cytokine and Acute Phase Protein Expression in Blood Samples of Harbour Seal Pups. Mar. Biol. 2008, 155, 337–345. [Google Scholar] [CrossRef]
- Edwards, K.L.; Miller, M.A.; Siegal-Willott, J.; Brown, J.L. Serum Health Biomarkers in African and Asian Elephants: Value Ranges and Clinical Values Indicative of the Immune Response. Animals 2020, 10, 1756. [Google Scholar] [CrossRef] [PubMed]
- Franklin, A.D.; Crosier, A.E.; Vansandt, L.M.; Mattson, E.; Xiao, Z. Induction of Cytokine Production in Cheetah (Acinonyx jubatus) Peripheral Blood Mononuclear Cells and Validation of Feline-Specific Cytokine Assays for Analysis of Cheetah Serum. J. Zoo Wildl. Med. 2015, 46, 306–313. [Google Scholar] [CrossRef]
- Sabbi, K.H.; Muller, M.N.; Machanda, Z.P.; Otali, E.; Fox, S.A.; Wrangham, R.W.; Emery Thompson, M. Human-like Adrenal Development in Wild Chimpanzees: A Longitudinal Study of Urinary Dehydroepiandrosterone-Sulfate and Cortisol. Am. J. Primatol. 2020, 82, e23064. [Google Scholar] [CrossRef]
- Orentreich, N.; Brind, J.L.; Rizer, R.L.; Vogelman, J.H. Age Changes and Sex Differences in Serum Dehydroepiandrosterone Sulfate Concentrations throughout Adulthood. J. Clin. Endocrinol. Metab. 1984, 59, 551–555. [Google Scholar] [CrossRef]
- Harder, J.D. Reproduction and Hormones. In The Wildlife Techniques Manual; Silvy, N.J., Ed.; John Hopkins University Press: Baltimore, MD, USA, 2012; Volume 1, pp. 502–525. [Google Scholar]
- Tworoger, S.S.; Hankinson, S.E. Collection, Processing, and Storage of Biological Samples in Epidemiological Studies: Sex Hormones, Carotenoids, Inflammatory Markers, and Proteomics as Examples. CEBP Focus Biorepository Biospecimen Sci. 2006, 15, 1578–1581. [Google Scholar]
- Finnegan, D. Referenceintervals: Reference Intervals. version 1.2.0. 2020. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Dai, B.; Scheipl, F.; Grothendieck, G.; Green, P.; et al. Lme4: Linear Mixed-Effects Models Using “Eigen” and S4; The R Project for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Edes, A.N. Dehydroepiandrosterone-Sulfate (DHEA-S), Sex, and Age in Zoo-Housed Western Lowland Gorillas (Gorilla Gorilla Gorilla). Primates 2017, 58, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, R.M.; Sterner, K.N.; Wildman, D.E. Adrenal Androgen Production in Catarrhine Primates and the Evolution of Adrenarche. Am. J. Phys. Anthropol. 2012, 147, 389–400. [Google Scholar] [CrossRef] [Green Version]
- Straub, R.H.; Schuld, A.; Mullington, J.; Haack, M.; Schölmerich, J.; Pollmächer, T. The Endotoxin-Induced Increase of Cytokines Is Followed by an Increase of Cortisol Relative to Dehydroepiandrosterone (DHEA) in Healthy Male Subjects. J. Endocrinol. 2002, 175, 467–474. [Google Scholar] [CrossRef] [Green Version]
- Maggio, M.; Guralnik, J.M.; Longo, D.L.; Ferrucci, L. Interleukin-6 in Aging and Chronic Disease: A Magnificent Pathway. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 575–584. [Google Scholar] [CrossRef]
- Du, C.; Khalil, M.W.; Sriram, S. Administration of Dehydroepiandrosterone Suppresses Experimental Allergic Encephalomyelitis in SJL/J Mice. J. Immunol. 2001, 167, 7094–7101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberbeck, R.; Dahlweid, M.; Koch, R.; van Griensven, M.; Emmendörfer, A.; Tscherne, H.; Pape, H.C. Dehydroepiandrosterone Decreases Mortality Rate and Improves Cellular Immune Function during Polymicrobial Sepsis. Crit. Care Med. 2001, 29, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.; Thusu, K.; Abdel-Rahman, E.; Prabhala, A.; Tomani, M.; Dandona, P. Elevated Serum Levels of Tumor Necrosis Factor Alpha in Normal-Weight Women with Polycystic Ovary Syndrome. Metabolism 1999, 48, 437–441. [Google Scholar] [CrossRef]
- Szczeklik, K.; Owczarek, D.; Pytko-Polończyk, J.; Kęsek, B.; Mach, T.H. Proinflammatory Cytokines in the Saliva of Patients with Active and Non-Active Crohn’s Disease. Pol. Arch. Med. Wewn. 2012, 122, 200–208. [Google Scholar]
- Barton, M.H.; Collatos, C. Tumor Necrosis Factor and Interleukin-6 Activity and Endotoxin Concentration in Peritoneal Fluid and Blood of Horses with Acute Abdominal Disease. J. Vet. Intern. Med. 1999, 13, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Puzianowska-Kuźnicka, M.; Owczarz, M.; Wieczorowska-Tobis, K.; Nadrowski, P.; Chudek, J.; Slusarczyk, P.; Skalska, A.; Jonas, M.; Franek, E.; Mossakowska, M. Interleukin-6 and C-Reactive Protein, Successful Aging, and Mortality: The PolSenior Study. Immun. Ageing 2016, 13, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Kock, M.; Loix, S.; Lavand’homme, P. Ketamine and Peripheral Inflammation. CNS Neurosci. Ther. 2013, 19, 403–410. [Google Scholar] [CrossRef]
- Walker, A.J.; Foley, B.M.; Sutor, S.L.; McGillivray, J.A.; Frye, M.A.; Tye, S.J. Peripheral Proinflammatory Markers Associated with Ketamine Response in a Preclinical Model of Antidepressant-Resistance. Behav. Brain Res. 2015, 293, 198–202. [Google Scholar] [CrossRef]

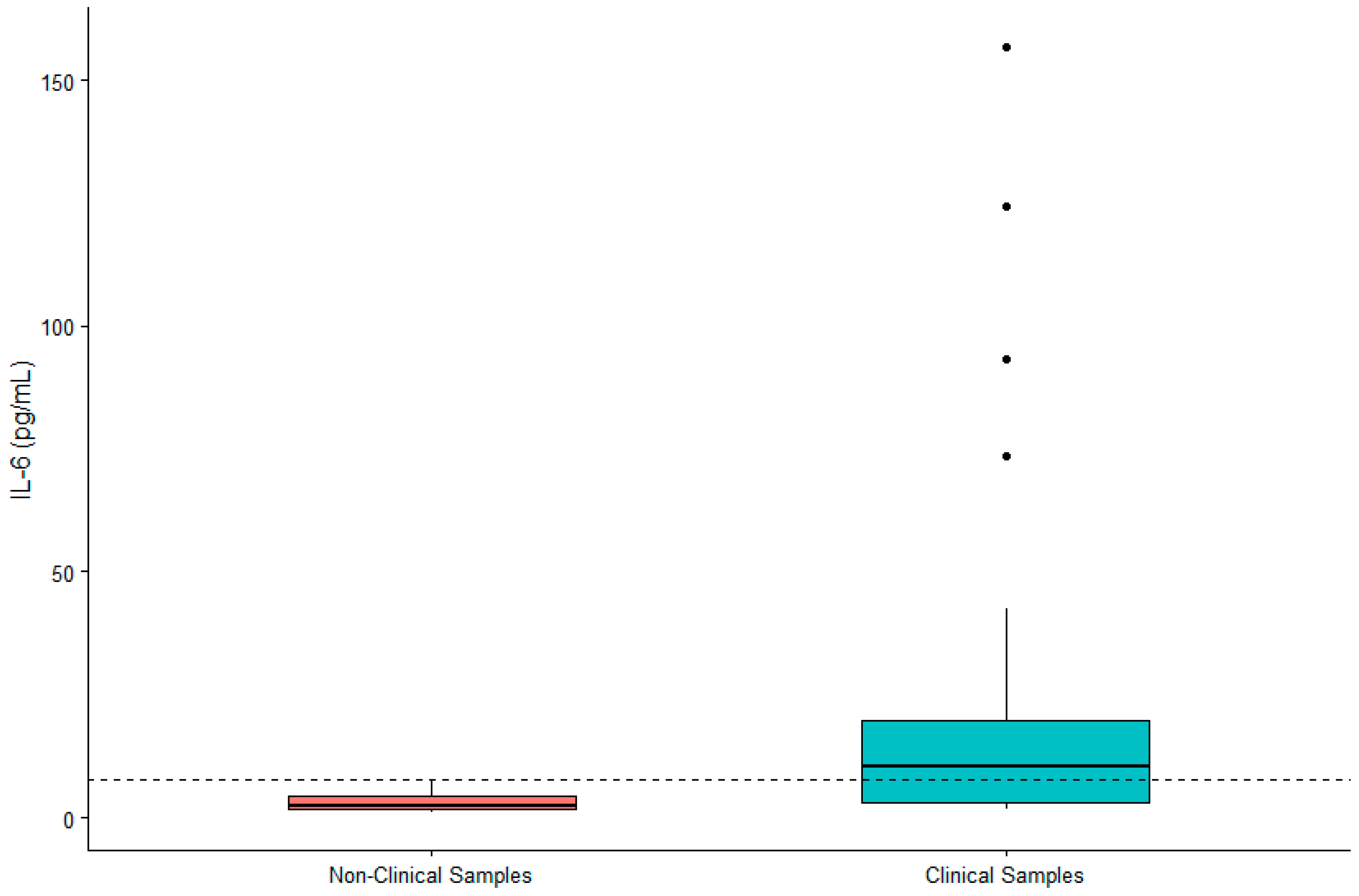
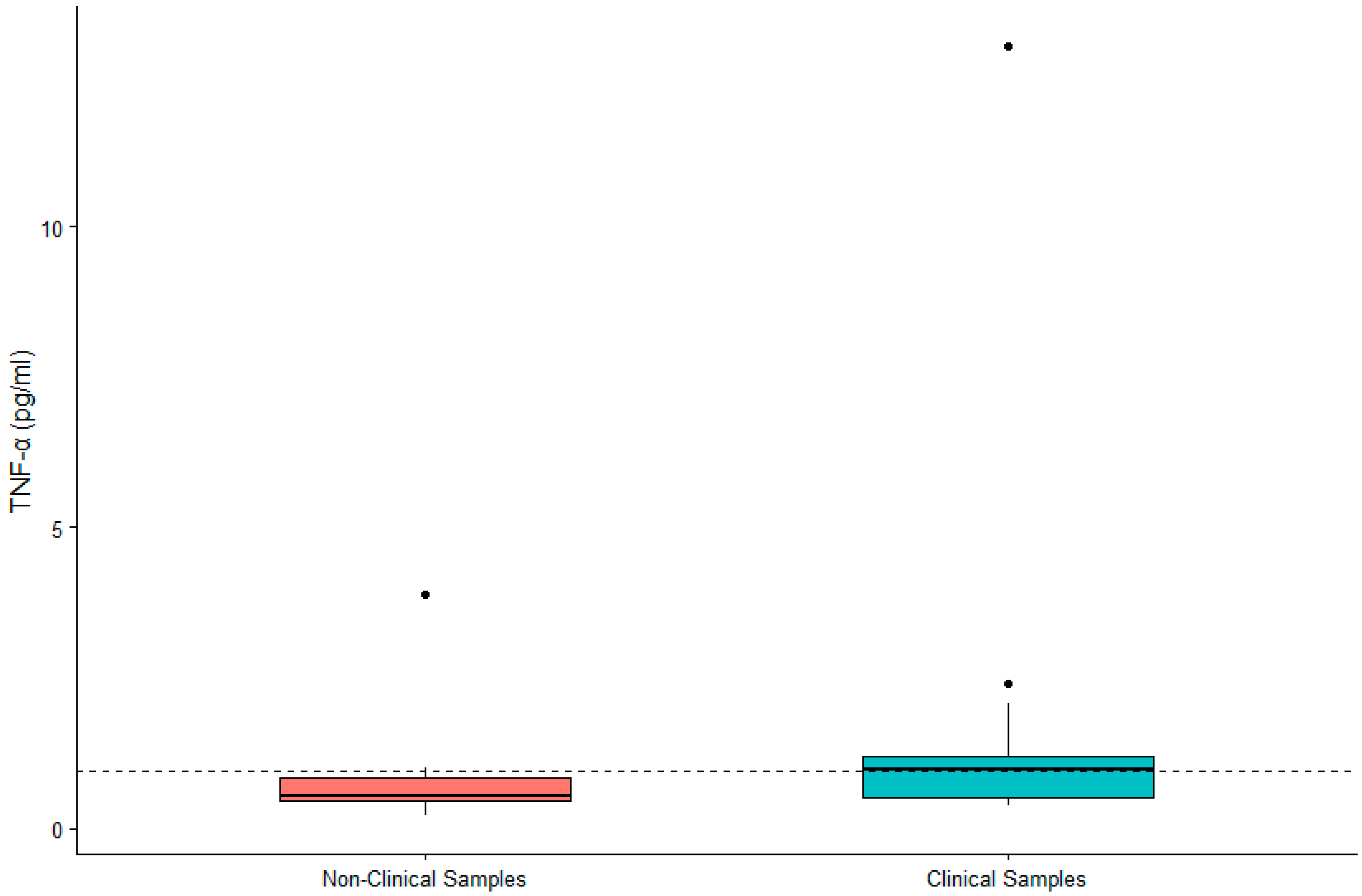
| Biomarker | Mean | SD | Median | Minimum | Maximum | N a | Value Range | Lower CI b | Upper CI |
|---|---|---|---|---|---|---|---|---|---|
| DHEA-S (µg/dL) | 31.23 | 18.70 | 28.60 | 15.00 | 123.50 | 55 | 0.13–54.84 | 0.01–8.49 | 49.38–61.58 |
| IL-6 (pg/mL) | 3.73 | 3.12 | 2.44 | 0.49 | 15.20 | 55 | 0.04–7.65 | 6.27–8.96 | |
| TNF-α (pg/mL) | 0.59 | 0.68 | 0.44 | 0.11 | 3.90 | 55 | 0.05–0.95 | 0.80–1.05 | |
| Males only (n = 30) | |||||||||
| DHEA-S (µg/dL) | 32.91 | 14.75 | 32.65 | 15.00 | 69.00 | 28 | 4.43–55.35 | 0.01–10.19 | 49.12–62.45 |
| IL-6 (pg/mL) | 4.46 | 3.60 | 2.89 | 1.03 | 15.20 | 28 | 0.04–8.82 | 7.31–10.97 | |
| TNF-α (pg/mL) | 0.44 | 0.28 | 0.38 | 0.11 | 1.01 | 27 | 0.05–0.81 | 0.67–0.95 | |
| Females only (n = 27) | |||||||||
| DHEA-S (µg/dL) | 29.37 | 22.44 | 21.20 | 15.00 | 123.50 | 26 | 0.01–49.04 | 0.01–0.07 | 39.23–59.06 |
| IL-6 (pg/mL) | 2.92 | 2.29 | 1.99 | 0.49 | 9.72 | 25 | 0.04–5.42 | 4.06–6.82 | |
| TNF-α (pg/mL) | 0.77 | 0.92 | 0.47 | 0.11 | 3.90 | 25 | 0.05–0.95 | 0.73–1.08 | |
| Description | Age | Sex | # of Months from Routine Sample | DHEA-S (µg/dL) | IL-6 (pg/mL) | TNF-α (pg/mL) |
|---|---|---|---|---|---|---|
| Active clinical symptoms | ||||||
| Normal parturition, healthy baby, maternal neglect | 14 | F | 37 | 87.70 | 19.78 | 2.08 |
| Root canal | 15 | F | 19 | 44.33 | 4.59 | 0.91 |
| Root canal, finger laceration; known mild cardiac disease | 22 | M | 32 | 35.81 | 10.44 | 0.98 |
| Root canal | 24 | F | 30 | 26.51 | 5.47 | 0.66 |
| Significant dental disease; multiple dental extractions | 28 | F | 82 | 26.22 | 2.71 | 0.80 |
| Constipation, lethargic, decreased appetite 3 month duration | 23 | M | 8 * | 19.20 | 1.86 | 1.00 |
| Preshipment and biannual exam, chronic loose stools; history of intestinal resection for Balantidium enteritis | 11 | F | 98 * | 15.00 | 10.43 | 0.40 |
| Colonoscopy for diarrhea; known cardiac disease | 27 | M | 10 | 15.00 | 1.63 | 0.37 |
| Distended abdomen, mild exercise intolerance, diagnosed with right-sided congestive heart failure | 43 | F | 68 | 82.71 | 3.04 | 1.19 |
| Weight gain despite dietary reduction, slightly distended abdomen; known cardiomyopathy and hypothyroid | 26 | M | 15 * | 60.60 | 19.69 | 0.38 |
| Recheck bloodwork (leukopenia); known cardiac disease | 30 | M | 47 | 15.00 | 1.63 | 0.37 |
| Acute onset stiffness, mild exercise intolerance | 40 | F | 39 | 200.43 | 124.22 | 13.00 |
| Right leg lameness (significant coxofemoral arthritis) | 39 | F | 27 * | 15.5 | 5.63 | 0.53 |
| Non-healing abscess on shoulder; known hypothyroid | 19 | F | 33 | 15.00 | 2.03 | 0.39 |
| Deep laceration to left forearm; known hypothyroid | 20 | F | 48 | 15.00 | 11.10 | 0.58 |
| Right forelimb/hindlimb lameness, fractured right clavicle and radius; known hip arthritis and hypothyroid | 48 | F | 14 | 15.00 | 10.89 | 1.16 |
| Chronic weight loss, intermittent cough non-responsive to antibiotics, diagnosed with congestive heart failure; 10 days prior to death | 23 | M | 35 | 23.48 | 156.88 | 1.80 |
| Samples collected day of death | ||||||
| Immobilized for cardiac resynchronization therapy; died | 23 | M | 35 | 8.75 | 73.66 | 1.77 |
| Coughing for one month, chronic stiffness; known cardiac disease; acutely collapsed during social introduction | 34 | M | 36 | 48.70 | 42.32 | 1.13 |
| Euthanized, diagnosed with right-sided congestive heart failure December 2010 | 43 | F | 71 | 158.21 | 93.32 | 2.41 |
| Immobilized for lethargy and increased respirations; did not recover; preliminary necropsy results show mild gastritis, small bowel enteritis, possible pancreatitis, and pleural effusion/pulmonary edema (possibly from CPR) | 50 | F | 28 | 23.80 | 14.2 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edes, A.N.; Zimmerman, D.; Jourdan, B.; Brown, J.L.; Edwards, K.L. Value Ranges and Clinical Comparisons of Serum DHEA-S, IL-6, and TNF-α in Western Lowland Gorillas. Animals 2022, 12, 2705. https://doi.org/10.3390/ani12192705
Edes AN, Zimmerman D, Jourdan B, Brown JL, Edwards KL. Value Ranges and Clinical Comparisons of Serum DHEA-S, IL-6, and TNF-α in Western Lowland Gorillas. Animals. 2022; 12(19):2705. https://doi.org/10.3390/ani12192705
Chicago/Turabian StyleEdes, Ashley N., Dawn Zimmerman, Balbine Jourdan, Janine L. Brown, and Katie L. Edwards. 2022. "Value Ranges and Clinical Comparisons of Serum DHEA-S, IL-6, and TNF-α in Western Lowland Gorillas" Animals 12, no. 19: 2705. https://doi.org/10.3390/ani12192705





