Evaluation of Four Clinical Metrology Instruments for the Assessment of Osteoarthritis in Dogs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
| Measure | SI | Deviation | Pedometer | HVAS | PIS | PSS | LOAD | COI | Stiffness | Function | Gait | QOL | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
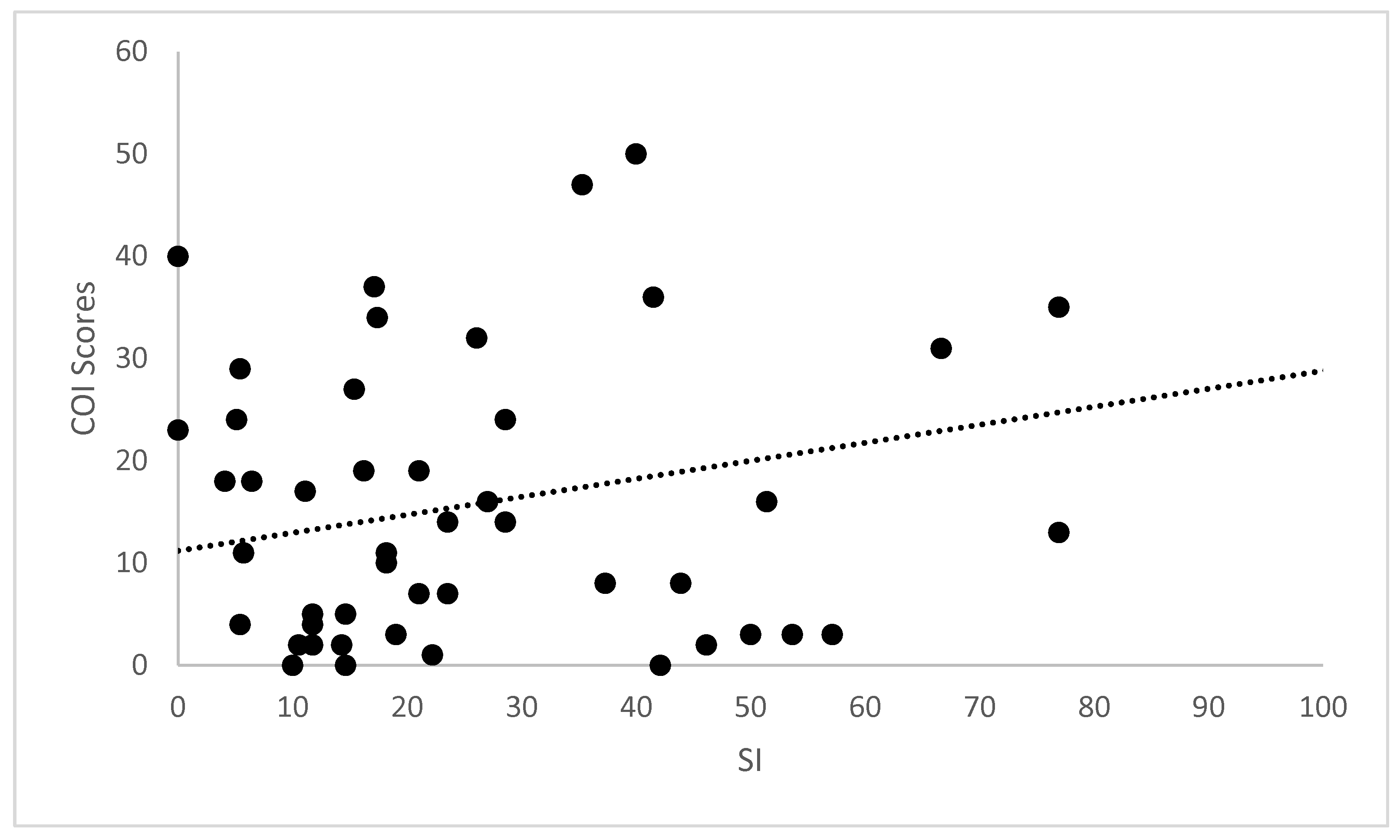
| SI | rs | 1.00 | 0.072 | −0.040 | −0.203 | 0.036 | 0.196 | 0.300 | 0.324 | 0.026 | 0.367 | 0.324 | 0.177 |
| Sig. | 0.617 | 0.823 | 0.161 | 0.300 | 0.178 | 0.036 * | 0.023 * | 0.318 | 0.010 * | 0.023 * | 0.224 | ||
| Deviation | rs | 0.072 | 1.00 | −0.200 | −0.151 | 0.198 | 0.210 | 0.169 | 0.240 | 0.180 | 0.233 | 0.229 | 0.265 |
| Sig. | 0.617 | 0.256 | 0.200 | 0.174 | 0.147 | 0.245 | 0.096 | 0.216 | 0.107 | 0.114 | 0.066 | ||
| Pedometer | rs | −0.040 | −0.200 | 1.00 | 0.326 | −0.323 | −0.323 | −0.425 | −0.523 | −0.531 | −0.499 | −0.427 | −0.530 |
| Sig. | 0.823 | 0.256 | 0.060 | −0.063 | 0.078 | 0.012 * | 0.002 * | 0.000 * | 0.003 * | 0.012 * | 00.01 * | ||
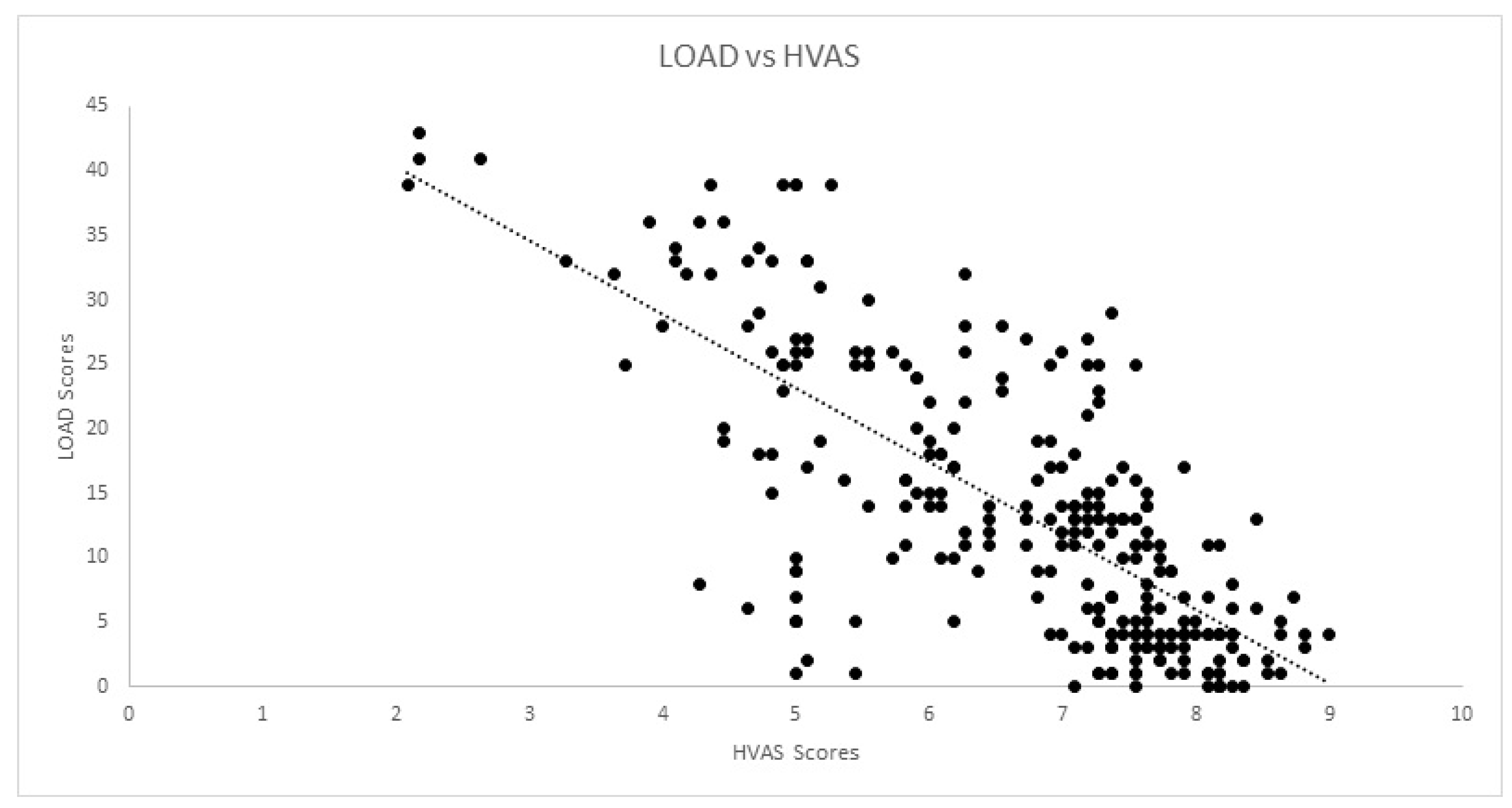
| HVAS | rs | −0.203 | −0.151 | 0.326 | 1.00 | −0.806 | −0.775 | −0.698 | −0.643 | −0.640 | −0.578 | −0.577 | −0.652 |
| Sig. | 0.161 | 0.200 | 0.060 | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | ||
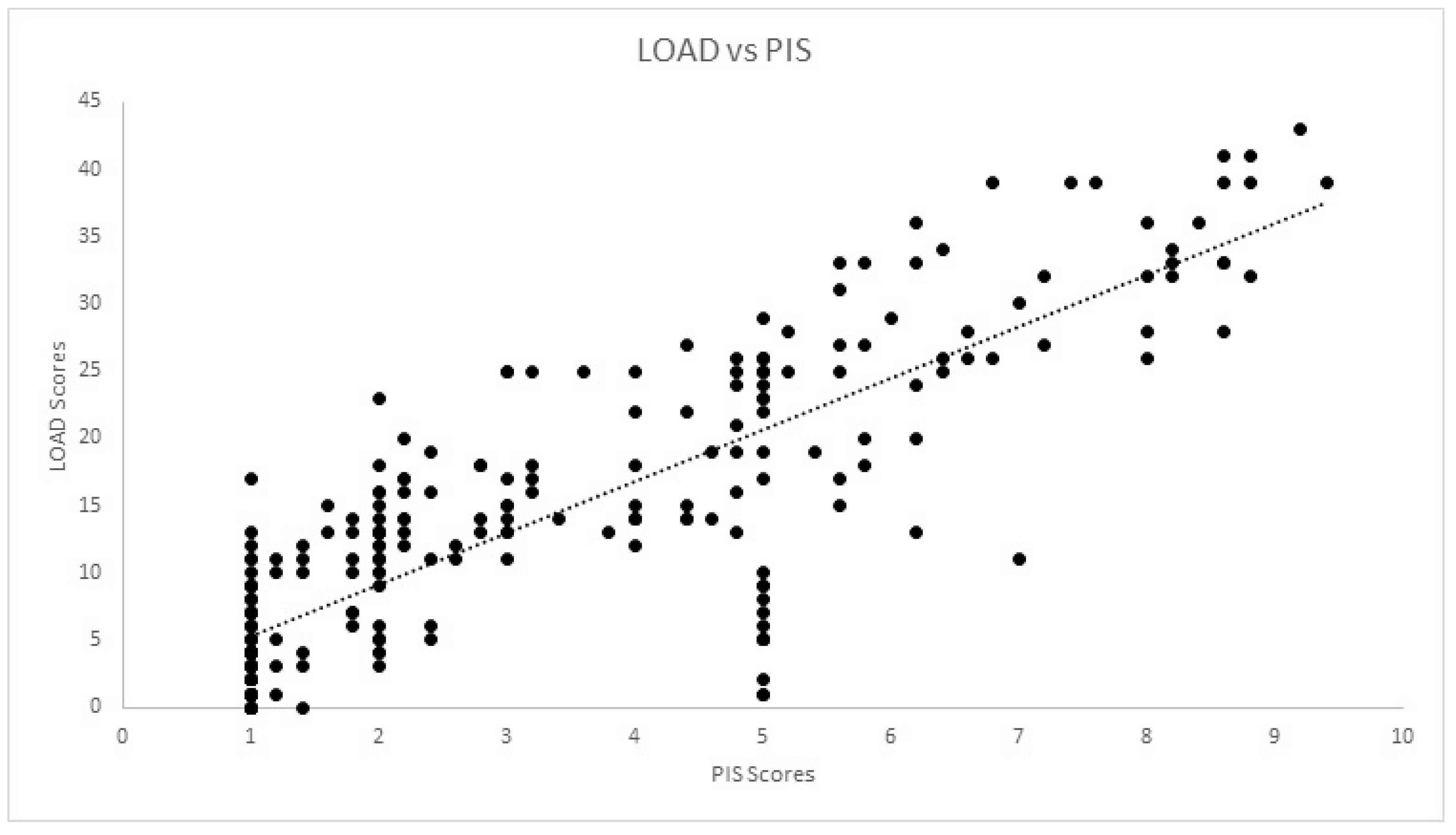
| PIS | rs | 0.036 | 0.198 | −0.323 | −0.806 | 1.00 | 0.966 | 0.831 | 0.842 | 0.808 | 0.774 | 0.793 | 60.801 |
| Sig. | 0.300 | 0.174 | −0.063 | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | ||
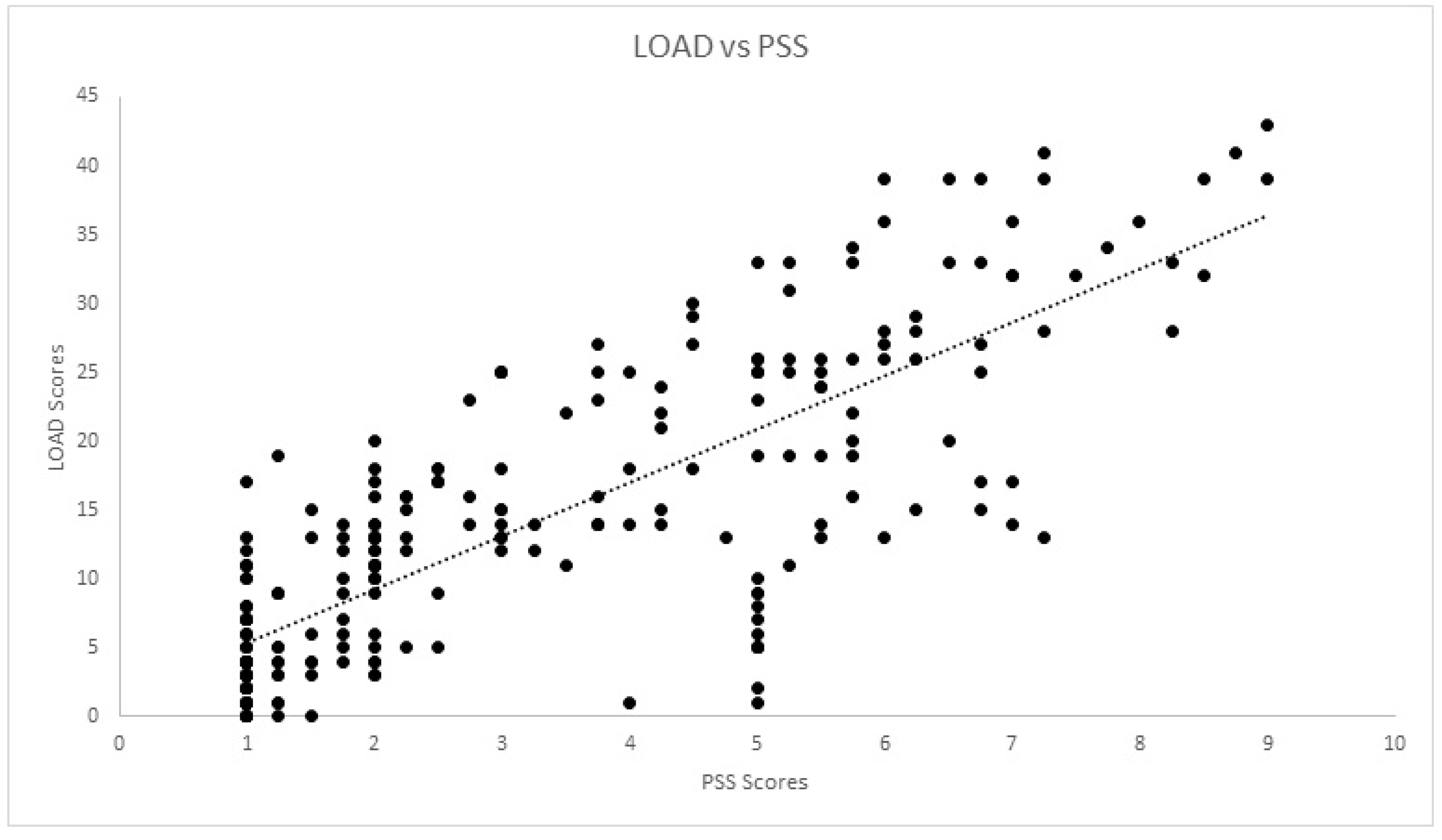
| PSS | rs | 0.196 | 0.210 | −0.323 | −0.775 | 0.966 | 1.00 | 0.784 | 0.811 | 0.772 | 0.722 | 0.770 | 0.800 |
| Sig. | 0.178 | 0.147 | 0.078 | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | ||
| LOAD | rs | 0.300 | 0.169 | −0.425 | −0.698 | 0.831 | 0.784 | 1.00 | 0.936 | 0.918 | 0.910 | 0.854 | 0.848 |
| Sig. | 0.036 | 0.245 | 0.012 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | ||
| COI | rs | 0.324 | 0.240 | −0.523 | −0.643 | 0.842 | 0.811 | 0.936 | 1.00 | 0.964 | 0.937 | 0.954 | 0.905 |
| Sig. | 0.023 * | 0.096 | 0.002 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | ||
| Stiffness | rs | 0.026 | 0.180 | −0.531 | −0.640 | 0.808 | 0.772 | 0.918 | 0.964 | 1.00 | 0.885 | 0.907 | 0.836 |
| Sig. | 0.318 | 0.216 | 0.001 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | ||
| Function | rs | 0.367 | 0.233 | −0.499 | −0.578 | 0.774 | 0.722 | 0.910 | 0.937 | 0.885 | 1.00 | 0.830 | 0.810 |
| Sig. | 0.010 * | 0.107 | 0.003 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | ||
| Gait | rs | 0.324 | 0.229 | −0.427 | −0.577 | 0.793 | 0.770 | 0.854 | 0.954 | 0.907 | 0.830 | 1.00 | 0.809 |
| Sig. | 0.023 * | 0.114 | 0.012 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | ||
| QOL | rs | 0.177 | 0.265 | −0.530 | −0.652 | 0.801 | 0.800 | 0.848 | 0.905 | 0.836 | 0.810 | 0.809 | 1.00 |
| Sig. | 0.224 | 0.066 | 0.01 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * |
| Measure | SI | Deviation | Pedometer | HVAS | PIS | PSS | LOAD | COI | Stiffness | Function | Gait | QOL | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SI | rs | 1.00 | 0.261 | −0.040 | −0.152 | 0.196 | 0.130 | 0.230 | 0.211 | 0.177 | 0.227 | 0.195 | 0.202 |
| Sig. | 0.000 * | 0.823 | 0.013 * | 0.001 * | 0.033 * | 0.000 * | 0.001 * | 0.004 * | 0.000 * | 0.001 * | 0.001 * | ||
| Deviation | rs | 0.261 | 1.00 | −0.200 | −0.127 | 0.141 | 0.112 | 0.133 | 0.174 | 0.129 | 0.156 | 0.191 | 0.179 |
| Sig. | 0.000 * | 0.256 | 0.038 * | 0.021 * | 0.068 | 0.030 * | 0.004 * | 0.035 * | 0.010 * | 0.002 * | 0.003 * | ||
| Pedometer | rs | −0.040 | −0.200 | 1.00 | 0.063 | −0.147 | −0.118 | −0.168 | −0.183 | −0.167 | −0.260 | −0.135 | −0.122 |
| Sig. | 0.823 | 0.256 | 0.412 | 0.055 | 0.125 | 0.028 * | 0.017 * | 0.029 * | 0.001 * | 0.078 | 0.111 | ||
| HVAS | rs | −0.152 | −0.127 | 0.063 | 1.00 | −0.830 | −0.814 | −0.736 | −0.716 | −0.695 | −0.057 | −0.677 | −0.732 |
| Sig. | 0.013 * | 0.038 * | 0.412 | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.355 | 0.000 * | 0.000 * | ||
| PIS | rs | 0.196 | 0.141 | −0.147 | −0.830 | 1.00 | 0.956 | 0.844 | 0.853 | 0.828 | 0.805 | 0.808 | 0.813 |
| Sig. | 0.001 * | 0.021 * | 0.055 | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 | 0.000 * | ||
| PSS | rs | 0.130 | 0.112 | −0.118 | −0.814 | 0.956 | 1.00 | 0.807 | 0.817 | 0.792 | 0.755 | 0.783 | 0.785 |
| Sig. | 0.033 * | 0.068 | 0.125 | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | ||
| LOAD | rs | 0.230 | 0.133 | −0.168 | −0.736 | 0.844 | 0.807 | 1.00 | 0.954 | 0.928 | 0.919 | 0.912 | 0.863 |
| Sig. | 0.000 * | 0.030 * | 0.028 * | 0.000 * | 0.000 * | * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | ||
| COI | rs | 0.211 | 0.174 | −0.183 | −0.716 | 0.853 | 0.817 | 0.954 | 1.00 | 0.966 | 0.952 | 0.965 | 0.916 |
| Sig. | 0.001 * | 0.004 * | 0.017 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | ||
| Stiffness | rs | 0.177 | 0.129 | −0.167 | −0.695 | 0.828 | 0.792 | 0.928 | 0.966 | 1.00 | 0.906 | 0.919 | 0.847 |
| Sig. | 0.004 * | 0.035 * | 0.029 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | ||
| Function | rs | 0.227 | 0.156 | −0.260 | −0.643 | 0.805 | 0.755 | 0.919 | 0.952 | 0.906 | 1.00 | 0.872 | 0.836 |
| Sig. | 0.000 * | 0.010 * | 0.001 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | ||
| Gait | rs | 0.195 | 0.191 | −0.135 | −0.677 | 0.808 | 0.783 | 0.912 | 0.965 | 0.919 | 0.872 | 1.00 | 0.965 |
| Sig. | 0.001 * | 0.002 * | 0.078 | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | ||
| QOL | rs | 0.202 | 0.179 | −0.122 | −0.732 | 0.813 | 0.785 | 0.863 | 0.916 | 0.847 | 0.836 | 0.965 | 1.00 |
| Sig. | 0.001 * | 0.003 * | 0.111 | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * |

4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venable, R.O.; Stoker, A.M.; Cook, C.R.; Cockrell, M.K.; Cook, J.L. Examination of synovial fluid hyaluronan quantity and quality in stifle joints of dogs with osteoarthritis. Am. J. Vet. Res. 2008, 69, 1569–1573. [Google Scholar] [CrossRef] [PubMed]
- Van Weeren, P.R. General Anatomy and Physiology of Joints. In Joint Disease in the Horse; WB Saunders: Philadelphia, PA, USA, 2015; pp. 1–24. [Google Scholar]
- Cuervo, B.; Chicharro, D.; Del Romero, A.; Damia, E.; Carrillo, J.; Sopena, J.; Peláez, P.; Miguel, L.; Vilar, J.; Rubio, M. Objective and subjective evaluation of plasma rich in growth factors therapy for the treatment of osteoarthritis in dogs. Osteoarthr. Cartil. 2019, 27, S482. [Google Scholar] [CrossRef] [Green Version]
- Meeson, R.L.; Todhunter, R.J.; Blunn, G.; Nuki, G.; Pitsillides, A.A. Spontaneous dog osteoarthritis—A One Medicine vision. Nat. Rev. Rheumatol. 2019, 15, 273–287. [Google Scholar] [CrossRef]
- Pascual-Garrido, C.; Guilak, F.; Rai, M.F.; Harris, M.D.; Lopez, M.J.; Todhunter, R.J.; Clohisy, J.C. Canine hip dysplasia: A natural animal model for human developmental dysplasia of the hip. J. Orthop. Res. 2018, 36, 1807–1817. [Google Scholar] [CrossRef] [Green Version]
- Gregory, M.H.; Capito, N.; Kuroki, K.; Stoker, A.M.; Cook, J.L.; Sherman, S.L. A Review of Translational Animal Models for Knee Osteoarthritis. Arthritis 2012, 2012, 764621. [Google Scholar] [CrossRef]
- Marijnissen, A.C.A.; van Roermund, P.M.; TeKoppele, J.M.; Bijlsma, J.W.J.; Lafeber, F.P.J.G. The canine “groove” model, compared with the ACLT model of osteoarthritis. Osteoarthr. Cartil. 2002, 10, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Moreau, M.; Pelletier, J.P.; Lussier, B.; d’Anjou, M.A.; Blond, L.; Pelletier, J.M.; del Castillo, J.R.; Troncy, E. A Posteriori Comparison of Natural and Surgical Destabilization Models of Canine Osteoarthritis. BioMed Res. Int. 2013, 2013, 180453. [Google Scholar] [CrossRef] [Green Version]
- McCoy, A.M. Animal models of osteoarthritis: Comparisons and key considerations. Vet. Pathol. 2015, 52, 803–818. [Google Scholar] [CrossRef]
- Piel, M.J.; Kroin, J.S.; Van Wijnen, A.J.; Kc, R.; Im, H.J. Pain assessment in animal models of osteoarthritis. Gene 2014, 537, 184–188. [Google Scholar] [CrossRef]
- Reid, J.; Nolan, A.M.; Scott, E.M. Measuring pain in dogs and cats using structured behavioural observation. Vet. J. 2018, 236, 72–79. [Google Scholar] [CrossRef]
- National Clinical Guideline Centre. Osteoarthritis: Care and Management in Adults; National Institute for Health and Care Excellence: London, UK, 2014. [Google Scholar]
- Cimino Brown, D. What can we learn from osteoarthritis pain in companion animals? Clin. Exp. Rheumatol. 2017, 35 (Suppl. S1), 53–58. [Google Scholar]
- Stadig, S.; Lascelles, B.D.X.; Nyman, G.; Bergh, A. Evaluation and comparison of pain questionnaires for clinical screening of osteoarthritis in cats. Vet. Rec. 2019, 185, 757. [Google Scholar] [CrossRef] [Green Version]
- Gruen, M.E.; Griffith, E.H.; Thomson, A.E.; Simpson, W.; Lascelles, B.D.X. Criterion Validation Testing of Clinical Metrology Instruments for Measuring Degenerative Joint Disease Associated Mobility Impairment in Cats. PLoS ONE 2015, 10, e0131839. [Google Scholar] [CrossRef]
- Lascelles, B.D.X.; Brown, D.C.; Maixner, W.; Mogil, J.S. Spontaneous painful disease in companion animals can facilitate the development of chronic pain therapies for humans. Osteoarthr. Cartil. 2018, 26, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Hercock, C.A.; Pinchbeck, G.; Giejda, A.; Clegg, P.D.; Innes, J.F. Validation of a client-based clinical metrology instrument for the evaluation of canine elbow osteoarthritis. J. Small Anim. Pract. 2009, 50, 266–271. [Google Scholar] [CrossRef]
- Walton, M.B.; Cowderoy, E.; Lascelles, D.; Innes, J.F. Evaluation of construct and criterion validity for the ‘Liverpool Osteoarthritis in Dogs’ (LOAD) clinical metrology instrument and comparison to two other instruments. PLoS ONE 2013, 8, e58125. [Google Scholar] [CrossRef]
- Walton, B.; Cox, T.; Innes, J. ‘How do I know my animal got better?’—Measuring outcomes in small animal orthopaedics. Practice 2018, 40, 42–50. [Google Scholar] [CrossRef]
- Upchurch, D.A.; Renberg, W.C.; Roush, J.K.; Milliken, G.A.; Weiss, M.L. Effects of administration of adipose-derived stromal vascular fraction and platelet-rich plasma to dogs with osteoarthritis of the hip joints. Am. J. Vet. Res. 2016, 77, 940–951. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.C. The Canine Orthopedic Index. Step 2: Psychometric testing. Vet. Surg. 2014, 43, 241–246. [Google Scholar] [CrossRef]
- Hudson, J.T.; Slater, M.R.; Taylor, L.; Scott, H.M.; Kerwin, S.C. Assessing repeatability and validity of a visual analogue scale questionnaire for use in assessing pain and lameness in dogs. Am. J. Vet. Res. 2004, 65, 1634–1643. [Google Scholar] [CrossRef]
- Moreau, M.; Lussier, B.; Ballaz, L.; Troncy, E. Kinetic measurements of gait for osteoarthritis research in dogs and cats. Can. Vet. J. 2014, 55, 1057–1065. [Google Scholar]
- Madore, E.; Huneault, L.; Moreau, M.; Dupuis, J. Comparison of trot kinetics between dogs with stifle or hip arthrosis. Vet. Comp. Orthop. Traumatol. 2007, 2, 102–107. [Google Scholar] [CrossRef]
- Nordquist, B.; Fischer, J.; Kim, S.Y.; Stover, S.M.; Garcia-Nolen, T.; Hayashi, K.; Liu, J.; Kapatkin, A.S. Effects of trial repetition, limb side, intraday and inter-week variation on vertical and craniocaudal ground reaction forces in clinically normal Labrador Retrievers. Vet. Comp. Orthop. Traumatol. 2011, 24, 435–444. [Google Scholar] [CrossRef] [Green Version]
- Lascelles, B.D.X.; Roe, S.C.; Smith, E.; Reynolds, L.; Markham, J.; Marcellin-Little, D.; Bergh, M.S.; Budsberg, S.C. Evaluation of a pressure walkway system for measurement of vertical limb forces in clinically normal dogs. Am. J. Vet. Res. 2006, 67, 277–282. [Google Scholar] [CrossRef]
- Seibert, R.; Marcellin-Little, D.J.; Roe, S.C.; DePuy, V.; Lascelles, B.D.X. Comparison of Body Weight Distribution, Peak Vertical Force, and Vertical Impulse as Measures of Hip Joint Pain and Efficacy of Total Hip Replacement. Vet. Surg. 2012, 41, 443–447. [Google Scholar] [CrossRef]
- Clough, W.; Canapp, S.; Taboada, L.; Dycus, D.; Leasure, C. Sensitivity and specificity of a weight distribution platform for the detection of objective lameness and orthopaedic disease. Vet. Comp. Orthop. Traumatol. 2018, 31, 391–395. [Google Scholar] [CrossRef]
- Clough, W.; Canapp, S. Assessing clinical relevance of weight distribution as measured on a stance analyzer through comparison with lameness determined on a pressure sensitive walkway and clinical diagnosis. Vet. Comp. Orthop. Traumatol. 2018, 31 (Suppl. S2), A1–A25. [Google Scholar] [CrossRef]
- Lascelles, B.; Freire, M.; Roe, S.; DePuy, V.; Smith, E.; Marcellin-Little, D. Evaluation of functional outcome after BFX total hip replacement using a pressure sensitive walkway. Vet. Surg. 2010, 39, 71–77. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Williams, J.E.; Reis, J.P.; Pluto, D. Utility of Pedometers for Assessing Physical Activity. Sport. Med. 2002, 32, 795–808. [Google Scholar] [CrossRef]
- Brown, D.C. The Canine Orthopedic Index. Step 1: Devising the Items. Vet. Surg. 2014, 43, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Beraud, R.; Moreau, M.; Lussier, B. Effect of exercise on kinetic gait analysis of dogs afflicted by osteoarthritis. Vet. Comp. Orthop. Traumatol. 2010, 23, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.B.; Spierenburg, M.; Ihle, S.L.; Tudor-Locke, C. Use of pedometers to measure physical activity in dogs. J. Am. Vet. Med. Assoc. 2005, 226, 2010–2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volstad, N.; Sandberg, G.; Robb, S.; Budsberg, S. The evaluation of limb symmetry indices using ground reaction forces collected with one or two force plates in healthy dogs. Vet. Comp. Orthop. Traumatol. 2017, 30, 54–58. [Google Scholar] [CrossRef]
- Venator, K.; Frye, C.W.; Gamble, L.-J.; Wakshlag, J.J. Assessment of a Single Intra-Articular Stifle Injection of Pure Platelet Rich Plasma on Symmetry Indices in Dogs with Unilateral or Bilateral Stifle Osteoarthritis from Long-Term Medically Managed Cranial Cruciate Ligament Disease. Vet. Med. Res. Rep. 2020, 11, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Klinck, M.P.; Mogil, J.S.; Moreau, M.; Lascelles, B.D.X.; Flecknell, P.A.; Poitte, T.; Troncy, E. Translational pain assessment. Pain 2017, 158, 1633–1646. [Google Scholar] [CrossRef]
- Flecknell, P. Analgesia from a veterinary perspective. Br. J. Anaesth. 2008, 101, 121–124. [Google Scholar] [CrossRef] [Green Version]
- Bosscher, G.; Tomas, A.; Roe, S.; Marcellin-Little, D.; Lascelles, B.D. Repeatability and accuracy testing of a weight distribution platform and comparison to a pressure sensitive walkway to assess static weight distribution. Vet. Comp. Orthop. Traumatol. 2017, 30, 160–164. [Google Scholar] [CrossRef]
- Oosterlinck, M.; Bosmans, T.; Gasthuys, F.; Polis, I.; Van Ryssen, B.; Dewulf, J.; Pille, F. Accuracy of pressure plate kinetic asymmetry indices and their correlation with visual gait assessment scores in lame and nonlame dogs. Am. J. Vet. Res. 2011, 72, 820–825. [Google Scholar] [CrossRef]
- Brown, D.C.; Boston, R.C.; Coyne, J.C.; Farrar, J.T. Development and psychometric testing of an instrument designed to measure chronic pain in dogs with osteoarthritis. Am. J. Vet. Res. 2007, 68, 631–637. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.C.; Boston, R.C.; Coyne, J.C.; Farrar, J.T. Ability of the canine brief pain inventory to detect response to treatment in dogs with osteoarthritis. J. Am. Vet. Med. Assoc. 2008, 233, 1278–1283. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.C.; Boston, R.C.; Farrar, J.T. Comparison of Force Plate Gait Analysis and Owner Assessment of Pain Using the Canine Brief Pain Inventory in Dogs with Osteoarthritis. J. Vet. Intern. Med. 2013, 27, 22–30. [Google Scholar] [CrossRef]
- Webster, R.P.; Anderson, G.I.; Gearing, D.P. Canine Brief Pain Inventory scores for dogs with osteoarthritis before and after administration of a monoclonal antibody against nerve growth factor. Am. J. Vet. Res. 2014, 75, 532–535. [Google Scholar] [CrossRef] [Green Version]
- Essner, A.; Zetterberg, L.; Hellström, K.; Gustås, P.; Högberg, H.; Sjöström, R. Psychometric evaluation of the canine brief pain inventory in a Swedish sample of dogs with pain related to osteoarthritis. Acta Vet. Scand. 2017, 59, 44. [Google Scholar] [CrossRef] [Green Version]
- Quinn, M.; Keuler, N.; Lu, Y.; Faria, M.; Muir, P.; Markel, M. Evaluation of Agreement Between Numerical Rating Scales, Visual Analogue Scoring Scales, and Force Plate Gait Analysis in Dogs. Vet. Surg. 2007, 36, 360–367. [Google Scholar] [CrossRef]
- Eskander, B.S.; Barbar, M.; Evans, R.B.; Enomoto, M.; Lascelles, B.D.X.; Conzemius, M.G. Correlation of activity data in normal dogs to distance traveled. Can. J. Vet. Res. 2020, 84, 44–51. [Google Scholar]
- Tavakol, M.; Dennick, R. Making sense of Cronbach’s alpha. Int. J. Med. Educ. 2011, 2, 53–55. [Google Scholar] [CrossRef]
- Ragetly, G.R.; Massey, L.; Brown, D.C. Initial psychometric testing and validation of the French version of the Canine Brief Pain Inventory. Vet. Anaesth. Analg. 2019, 46, 667–672. [Google Scholar] [CrossRef]
- Taber, K.S. The Use of Cronbach’s Alpha When Developing and Reporting Research Instruments in Science Education. Res. Sci. Educ. 2018, 48, 1273–1296. [Google Scholar] [CrossRef] [Green Version]
- Muller, C.; Gaines, B.; Gruen, M.; Case, B.; Arrufat, K.; Innes, J.; Lascelles, B.D.X. Evaluation of clinical metrology instrument in dogs with osteoarthritis. J. Vet. Intern. Med. 2016, 30, 836–846. [Google Scholar] [CrossRef] [Green Version]
- Hielm-Björkman, A.K.; Kapatkin, A.S.; Rita, H.J. Reliability and validity of a visual analogue scale used by owners to measure chronic pain attributable to osteoarthritis in their dogs. Am. J. Vet. Res. 2011, 72, 601–607. [Google Scholar] [CrossRef]
- Horstman, C.L.; Conzemius, M.G.; Evans, R.; Gordon, W.J. Assessing the Efficacy of Perioperative Oral Carprofen after Cranial Cruciate Surgery Using Noninvasive, Objective Pressure Platform Gait Analysis. Vet. Surg. 2004, 33, 286–292. [Google Scholar] [CrossRef]
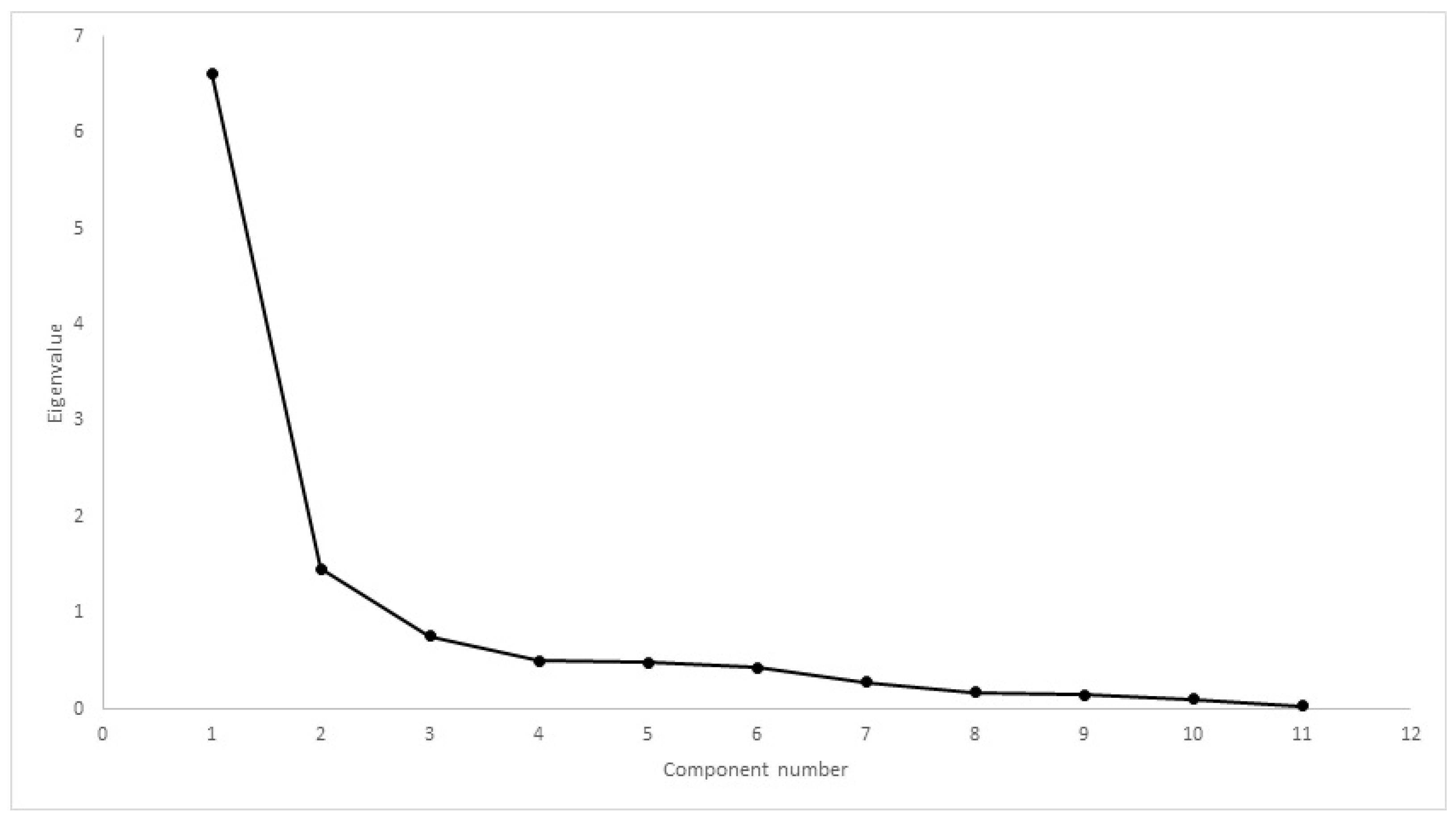
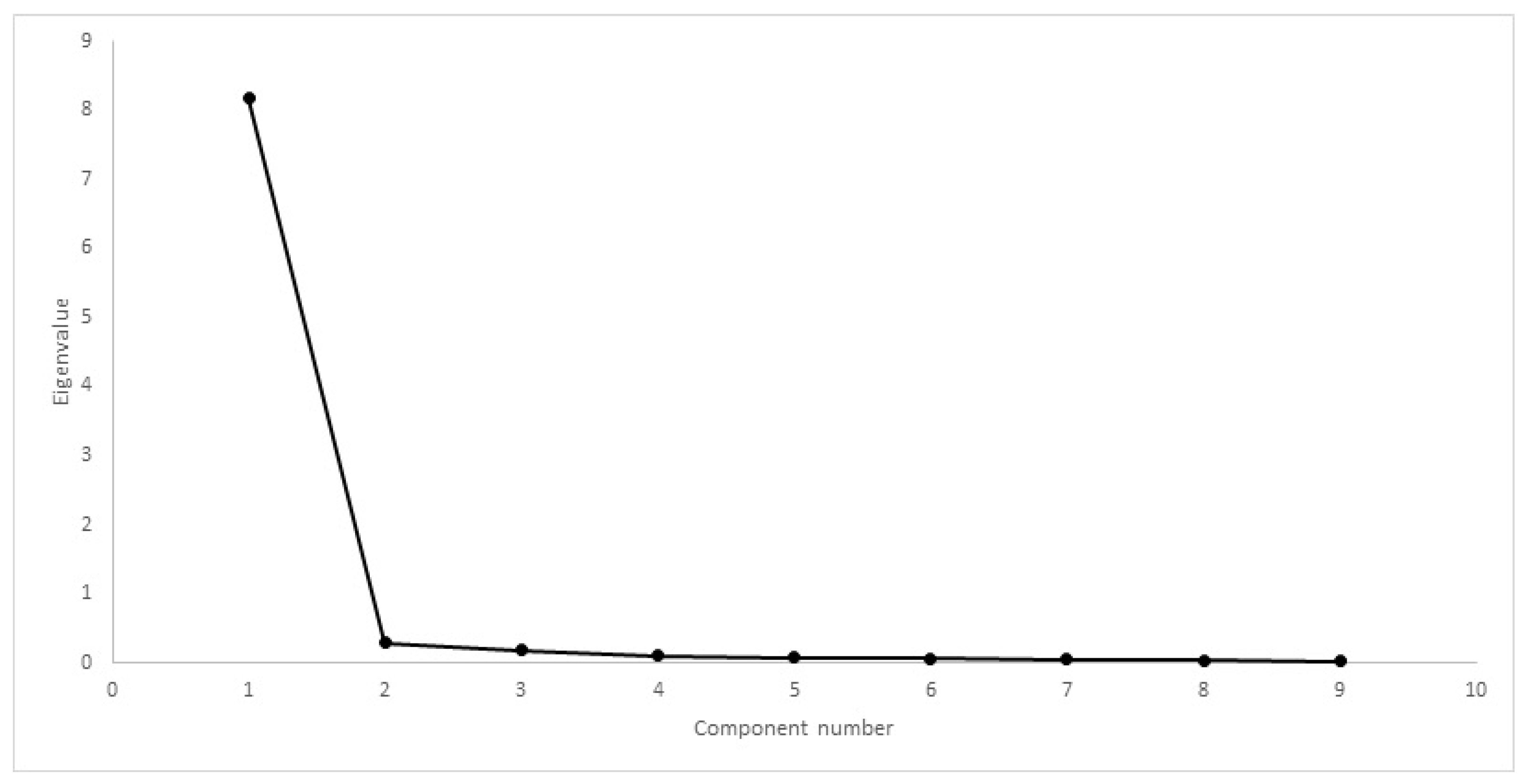
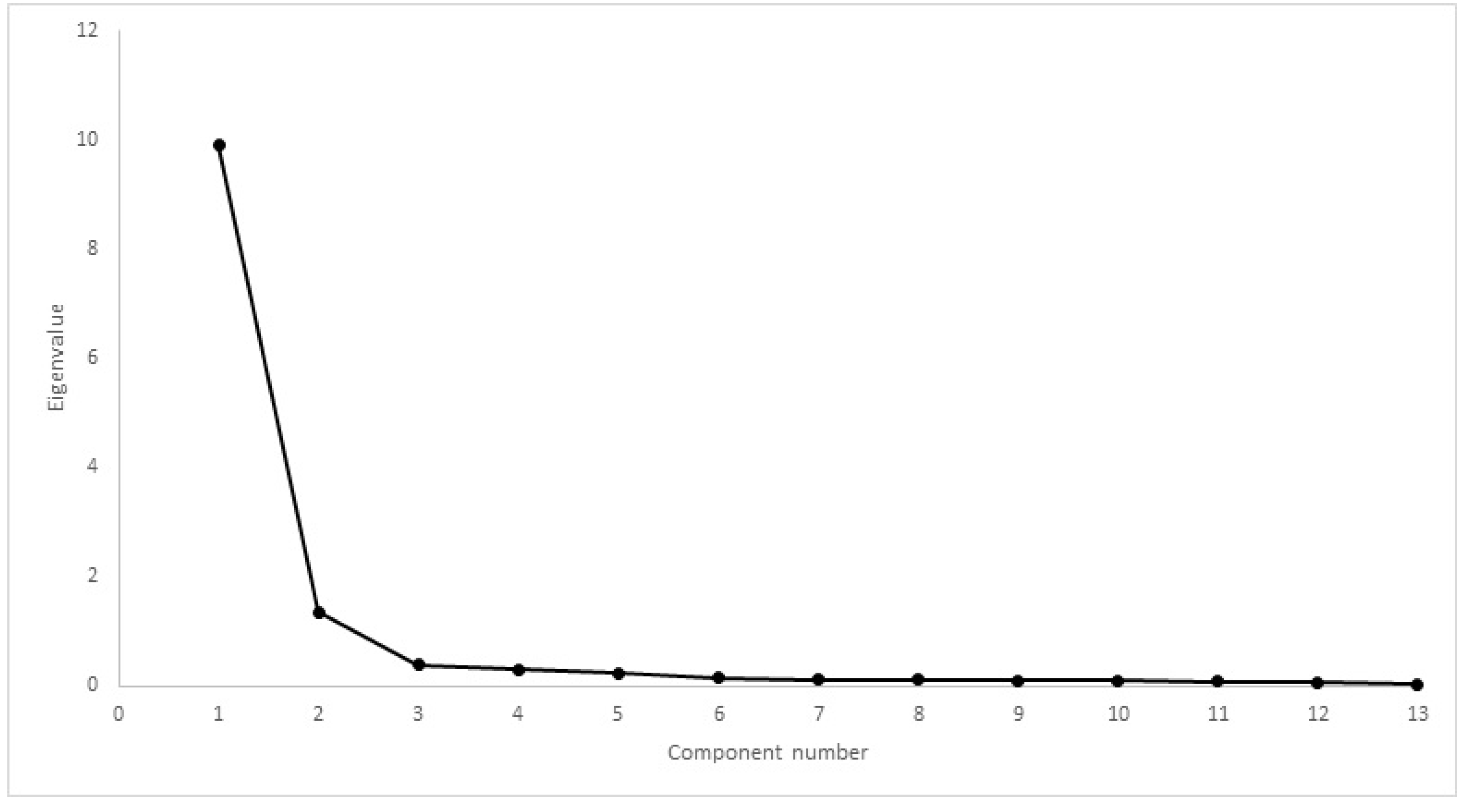
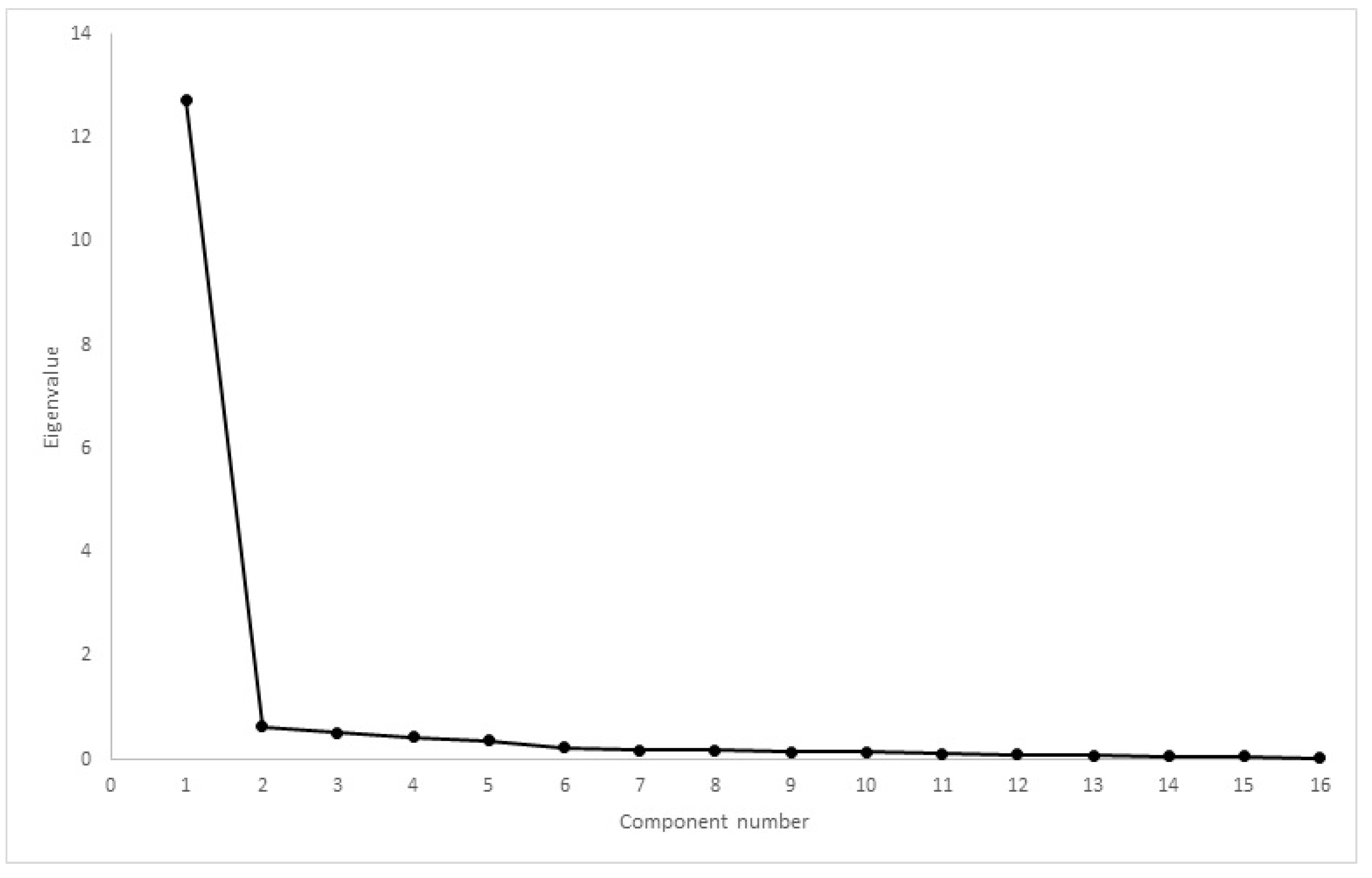
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Mobility impairment, as described by the trainer and detected by the assisting veterinarian; | Suspected or diagnosed neurological/musculoskeletal disorder other than hip OA; |
| Bodyweight ≥ 15 kg; | Documented or suspected presence of concomitant disease; |
| Age ≥ 1 year; | Receiving any other drugs; |
| Radiographic evidence of bilateral OA of the hip joint; | Results of routine blood testing outside normal limits. |
| Not to be on any medication or nutritional supplements for six weeks or more. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, J.C.; Santos, A.; Jorge, P.; Lavrador, C.; Carreira, L.M. Evaluation of Four Clinical Metrology Instruments for the Assessment of Osteoarthritis in Dogs. Animals 2022, 12, 2808. https://doi.org/10.3390/ani12202808
Alves JC, Santos A, Jorge P, Lavrador C, Carreira LM. Evaluation of Four Clinical Metrology Instruments for the Assessment of Osteoarthritis in Dogs. Animals. 2022; 12(20):2808. https://doi.org/10.3390/ani12202808
Chicago/Turabian StyleAlves, João C., Ana Santos, Patrícia Jorge, Catarina Lavrador, and Luís Miguel Carreira. 2022. "Evaluation of Four Clinical Metrology Instruments for the Assessment of Osteoarthritis in Dogs" Animals 12, no. 20: 2808. https://doi.org/10.3390/ani12202808







