High Intake of Sodium Chloride for 28 Days Causes No Effect on Serum FGF23 Concentrations in Cats
Abstract
:Simple Summary
Abstract
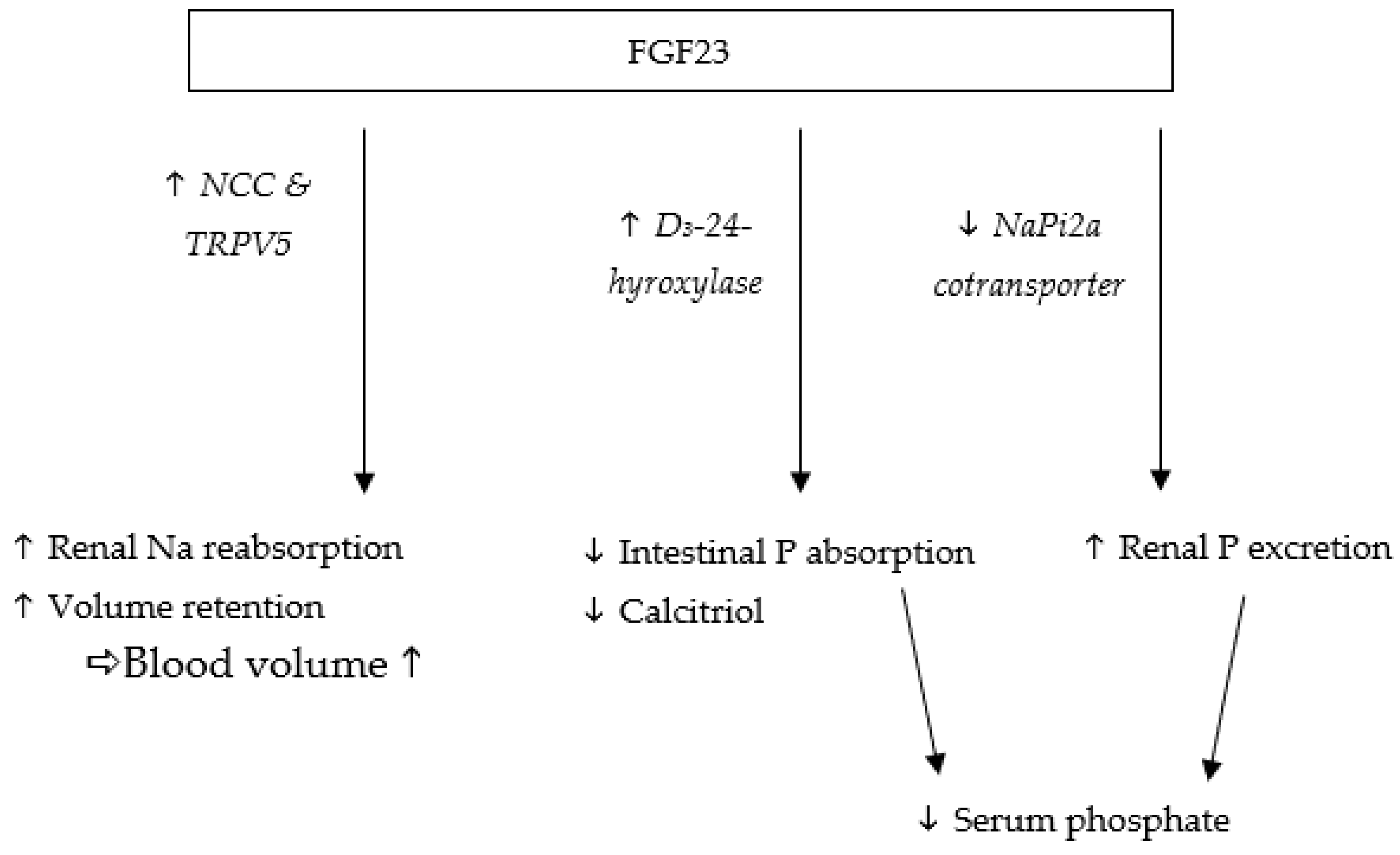
1. Introduction
2. Animals, Materials, and Methods
2.1. Housing
2.2. Diets
2.3. Sample Collection and Storage
2.4. Laboratory Analyses
2.5. Calculations and Statistical Analysis
3. Results
3.1. Nutrient Intake and Balance
3.2. Blood Parameters
3.3. Urine Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoshiko, Y.; Wang, H.; Minamizaki, T.; Ijuin, C.; Yamamoto, R.; Suemune, S.; Kozai, K.; Tanne, K.; Aubin, J.E.; Maeda, N. Mineralized tissue cells are a principal source of FGF23. Bone 2007, 40, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Noonan, M.L.; White, K.E. FGF23 Synthesis and Activity. Curr. Mol. Biol. Rep. 2019, 5, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Erben, R.G. Update on FGF23 and Klotho signaling. Mol. Cell Endocrinol. 2016, 432, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Gattineni, J.; Bates, C.; Twombley, K.; Dwarakanath, V.; Robinson, M.L.; Goetz, R.; Mohammadi, M.; Baum, M. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am. J. Physiol.-Ren. Physiol. 2009, 297, F282–F291. [Google Scholar] [CrossRef] [Green Version]
- Erben, R.G. Physiological Actions of Fibroblast Growth Factor-23. Front. Endocrinol. 2018, 9, 267. [Google Scholar] [CrossRef] [PubMed]
- Steffen, C.; Kienzle, E.; Dobenecker, B. Oral Intake of Some Inorganic Phosphates for 28 Days Increases FGF-23 Serum Concentrations also in Cats; ESVCN: Vila Real, Portugal, 2021. [Google Scholar]
- Shimada, T.; Hasegawa, H.; Yamazaki, Y.; Muto, T.; Hino, R.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Fukumoto, S.; Yamashita, T. FGF-23 Is a Potent Regulator of Vitamin D Metabolism and Phosphate Homeostasis. J. Bone Miner. Res. 2004, 19, 429–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, M.B.; Watanuki, M.; Kim, S.; Shevde, N.K.; Pike, J.W. The Human Transient Receptor Potential Vanilloid Type 6 Distal Promoter Contains Multiple Vitamin D Receptor Binding Sites that Mediate Activation by 1,25-Dihydroxyvitamin D3 in Intestinal Cells. Mol. Endocrinol. 2006, 20, 1447–1461. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Landowski, C.P.; Hediger, M.A. Mechanisms and regulation of epithelial Ca2+ absorption in health and disease. Annu. Rev. Physiol. 2008, 70, 257–271. [Google Scholar] [CrossRef]
- Ichikawa, S.; Imel, E.A.; Kreiter, M.L.; Yu, X.; Mackenzie, D.S.; Sorenson, A.H.; Goetz, R.; Mohammadi, M.; White, K.E.; Econs, M.J. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J. Clin. Investig. 2007, 117, 2684–2691. [Google Scholar] [CrossRef] [Green Version]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef]
- de Borst, M.H.; Vervloet, M.G.; ter Wee, P.M.; Navis, G. Cross Talk Between the Renin-Angiotensin-Aldosterone System and Vitamin D-FGF-23-klotho in Chronic Kidney Disease. J. Am. Soc. Nephrol. 2011, 22, 1603–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitani, H.; Ishizaka, N.; Aizawa, T.; Ohno, M.; Usui, S.; Suzuki, T.; Amaki, T.; Mori, I.; Nakamura, Y.; Sato, M.; et al. In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension 2002, 39, 838–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurpas, A.; Supeł, K.; Idzikowska, K.; Zielińska, M. FGF23: A Review of Its Role in Mineral Metabolism and Renal and Cardiovascular Disease. Dis. Markers 2021, 2021, 8821292. [Google Scholar] [CrossRef] [PubMed]
- Isakova, T.; Wahl, P.; Vargas, G.S.; Gutiérrez, O.M.; Scialla, J.; Xie, H.; Appleby, D.; Nessel, L.; Bellovich, K.; Chen, J.; et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011, 79, 1370–1378. [Google Scholar] [CrossRef] [Green Version]
- Block, G.; Port, F.K. Calcium phosphate metabolism and cardiovascular disease in patients with chronic kidney disease. Semin. Dial. 2003, 16, 140–147. [Google Scholar] [CrossRef]
- Geddes, R.; Finch, N.; Elliott, J.; Syme, H. Fibroblast Growth Factor 23 in Feline Chronic Kidney Disease. J. Vet. Intern. Med./Am. Coll. Vet. Intern. Med. 2013, 27, 234–241. [Google Scholar] [CrossRef]
- Hardcastle, M.R.; Dittmer, K.E. Fibroblast Growth Factor 23: A New Dimension to Diseases of Calcium-Phosphorus Metabolism. Vet. Pathol. 2015, 52, 770–784. [Google Scholar] [CrossRef]
- Harjes, L.M.; Parker, V.J.; Dembek, K.; Young, G.S.; Giovaninni, L.H.; Kogika, M.M.; Chew, D.J.; Toribio, R.E. Fibroblast Growth Factor-23 Concentration in Dogs with Chronic Kidney Disease. J. Vet. Intern. Med. 2017, 31, 784–790. [Google Scholar] [CrossRef]
- Kestenbaum, B.; Sampson, J.N.; Rudser, K.D.; Patterson, D.J.; Seliger, S.L.; Young, B.; Sherrard, D.J.; Andress, D.L. Serum phosphate levels and mortality risk among people with chronic kidney disease. J. Am. Soc. Nephrol. 2005, 16, 520–528. [Google Scholar] [CrossRef] [Green Version]
- Voormolen, N.; Noordzij, M.; Grootendorst, D.C.; Beetz, I.; Sijpkens, Y.W.; van Manen, J.G.; Boeschoten, E.W.; Huisman, R.M.; Krediet, R.T.; Dekker, F.W. High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol. Dial. Transpl. 2007, 22, 2909–2916. [Google Scholar] [CrossRef]
- King, J.N.; Tasker, S.; Gunn-Moore, D.A.; Strehlau, G. Prognostic factors in cats with chronic kidney disease. J. Vet. Intern. Med. 2007, 21, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Boyd, L.M.; Langston, C.; Thompson, K.; Zivin, K.; Imanishi, M. Survival in Cats with Naturally Occurring Chronic Kidney Disease (2000–2002). J. Vet. Intern. Med. 2008, 22, 1111–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrukhova, O.; Slavic, S.; Smorodchenko, A.; Zeitz, U.; Shalhoub, V.; Lanske, B.; Pohl, E.E.; Erben, R.G. FGF23 regulates renal sodium handling and blood pressure. EMBO Mol. Med. 2014, 6, 744–759. [Google Scholar] [CrossRef] [PubMed]
- Fliser, D.; Kollerits, B.; Neyer, U.; Ankerst, D.P.; Lhotta, K.; Lingenhel, A.; Ritz, E.; Kronenberg, F. Fibroblast Growth Factor 23 (FGF23) Predicts Progression of Chronic Kidney Disease: The Mild to Moderate Kidney Disease (MMKD) Study. J. Am. Soc. Nephrol. 2007, 18, 2600–2608. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, O.; Isakova, T.; Rhee, E.; Shah, A.; Holmes, J.; Collerone, G.; Jüppner, H.; Wolf, M. Fibroblast Growth Factor-23 Mitigates Hyperphosphatemia but Accentuates Calcitriol Deficiency in Chronic Kidney Disease. J. Am. Soc. Nephrol. 2005, 16, 2205–2215. [Google Scholar] [CrossRef]
- Isakova, T.; Gutierrez, O.; Shah, A.; Castaldo, L.; Holmes, J.; Lee, H.; Wolf, M. Postprandial Mineral Metabolism and Secondary Hyperparathyroidism in Early CKD. J. Am. Soc. Nephrol. 2008, 19, 615–623. [Google Scholar] [CrossRef] [Green Version]
- Mente, A.; O’Donnell, M.J.; Rangarajan, S.; McQueen, M.J.; Poirier, P.; Wielgosz, A.; Morrison, H.; Li, W.; Wang, X.; Di, C.; et al. Association of Urinary Sodium and Potassium Excretion with Blood Pressure. N. Engl. J. Med. 2014, 371, 601–611. [Google Scholar] [CrossRef] [Green Version]
- Intersalt Cooperative Research, G. Intersalt: An International Study Of Electrolyte Excretion And Blood Pressure. Results For 24 Hour Urinary Sodium And Potassium Excretion. BMJ Br. Med. J. 1988, 297, 319–328. [Google Scholar]
- Ferguson, J.F.; Aden, L.A.; Barbaro, N.R.; Van Beusecum, J.P.; Xiao, L.; Simmons, A.J.; Warden, C.; Pasic, L.; Himmel, L.E.; Washington, M.K.; et al. High dietary salt-induced dendritic cell activation underlies microbial dysbiosis-associated hypertension. JCI Insight 2019, 5, e126241. [Google Scholar] [CrossRef] [Green Version]
- Lifton, R.P.; Gharavi, A.G.; Geller, D.S. Molecular Mechanisms of Human Hypertension. Cell 2001, 104, 545–556. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Umbach, A.T.; Chen, H.; Yan, J.; Fakhri, H.; Fajol, A.; Salker, M.S.; Spichtig, D.; Daryadel, A.; Wagner, C.A.; et al. Up-regulation of FGF23 release by aldosterone. Biochem. Biophys. Res. Commun. 2016, 470, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-W.; Wang, Y.; Chu, C.; Mu, J.-J. Effect of Salt Intervention on Serum Levels of Fibroblast Growth Factor 23 (FGF23) in Chinese Adults: An Intervention Study. Med. Sci. Monit. 2018, 24, 1948–1954. [Google Scholar] [CrossRef] [Green Version]
- Erben, R.G.; Andrukhova, O. FGF23-Klotho signaling axis in the kidney. Bone 2017, 100, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Executive summary: Heart disease and stroke statistics--2013 update: A report from the American Heart Association. Circulation 2013, 127, 143–152. [Google Scholar] [CrossRef]
- Brazy, P.C.; Stead, W.W.; Fitzwilliam, J.F. Progression of renal insufficiency: Role of blood pressure. Kidney Int. 1989, 35, 670–674. [Google Scholar] [CrossRef] [Green Version]
- Segura, J.; Campo, C.; Gil, P.; Roldán, C.; Vigil, L.; Rodicio, J.L.; Ruilope, L.M. Development Of Chronic Kidney Disease and Cardiovascular Prognosis in Essential Hypertensive Patients. J. Am. Soc. Nephrol. 2004, 15, 1616–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinberger, M.H.; Fineberg, N.S.; Fineberg, S.E.; Weinberger, M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 2001, 37, 429–432. [Google Scholar] [CrossRef] [Green Version]
- Lev-Ran, A.; Porta, M. Salt and hypertension: A phylogenetic perspective. Diabetes Metab. Res. Rev. 2005, 21, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.J.; Foley, R.N.; Gilbertson, D.T.; Chen, S.-C. United States Renal Data System public health surveillance of chronic kidney disease and end-stage renal disease. Kidney Int. Suppl. (2011) 2015, 5, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Bidani, A.K.; Griffin, K.A. Long-term renal consequences of hypertension for normal and diseased kidneys. Curr. Opin. Nephrol. Hypertens. 2002, 11, 73–80. [Google Scholar] [CrossRef]
- Faul, C.; Amaral, A.P.; Oskouei, B.; Hu, M.-C.; Sloan, A.; Isakova, T.; Gutiérrez, O.M.; Aguillon-Prada, R.; Lincoln, J.; Hare, J.M.; et al. FGF23 induces left ventricular hypertrophy. J. Clin. Investig. 2011, 121, 4393–4408. [Google Scholar] [CrossRef] [PubMed]
- Grabner, A.; Amaral, A.P.; Schramm, K.; Singh, S.; Sloan, A.; Yanucil, C.; Li, J.; Shehadeh, L.A.; Hare, J.M.; David, V.; et al. Activation of Cardiac Fibroblast Growth Factor Receptor 4 Causes Left Ventricular Hypertrophy. Cell Metab. 2015, 22, 1020–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humalda, J.K.; Lambers Heerspink, H.J.; Kwakernaak, A.J.; Slagman, M.C.J.; Waanders, F.; Vervloet, M.G.; Ter Wee, P.M.; Navis, G.; de Borst, M.H. Fibroblast Growth Factor 23 and the Antiproteinuric Response to Dietary Sodium Restriction During Renin-Angiotensin-Aldosterone System Blockade. Am. J. Kidney Dis. 2015, 65, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Freundlich, M.; Gamba, G.; Rodriguez-Iturbe, B. Fibroblast growth factor 23—Klotho and hypertension: Experimental and clinical mechanisms. Pediatr. Nephrol. 2021, 36, 3007–3022. [Google Scholar] [CrossRef] [PubMed]
- Bijsmans, E.S.; Jepson, R.E.; Chang, Y.M.; Syme, H.M.; Elliott, J. Changes in systolic blood pressure over time in healthy cats and cats with chronic kidney disease. J. Vet. Intern. Med. 2015, 29, 855–861. [Google Scholar] [CrossRef]
- Kobayashi, D.L.; Peterson, M.E.; Graves, T.K.; Lesser, M.; Nichols, C.E. Hypertension in cats with chronic renal failure or hyperthyroidism. J. Vet. Intern. Med. 1990, 4, 58–62. [Google Scholar] [CrossRef]
- Brown, C.A.; Elliott, J.; Schmiedt, C.W.; Brown, S.A. Chronic Kidney Disease in Aged Cats: Clinical Features, Morphology, and Proposed Pathogeneses. Vet. Pathol. 2016, 53, 309–326. [Google Scholar] [CrossRef]
- Christina, L.; Marino, B.D.X.L.; Vaden, S.L.; Gruen, M.E.; Marks1, S.L. The prevalence and classification of chronic kidney disease in cats randomly selected within four age groups and in cats recruited for degenerative joint disease studies. J. Feline Med. Surg. 2014, 16, 465–472. [Google Scholar]
- Chen, H.; Dunaevich, A.; Apfelbaum, N.; Kuzi, S.; Mazaki-Tovi, M.; Aroch, I.; Segev, G. Acute on chronic kidney disease in cats: Etiology, clinical and clinicopathologic findings, prognostic markers, and outcome. J. Vet. Intern. Med. 2020, 34, 1496–1506. [Google Scholar] [CrossRef]
- Chetboul, V.; Reynolds, B.S.; Trehiou-Sechi, E.; Nguyen, P.; Concordet, D.; Sampedrano, C.C.; Testault, I.; Elliott, J.; Abadie, J.; Biourge, V.; et al. Cardiovascular effects of dietary salt intake in aged healthy cats: A 2-year prospective randomized, blinded, and controlled study. PLoS ONE 2014, 9, e97862. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Ishizawa, K.; Li, J.; Fujii, W.; Nemoto, Y.; Yamazaki, O.; Tamura, Y.; Miura, Y.; Nie, X.; Abe, R.; et al. Urinary phosphate-containing nanoparticle contributes to inflammation and kidney injury in a salt-sensitive hypertension rat model. Commun. Biol. 2020, 3, 575. [Google Scholar] [CrossRef] [PubMed]
- Dobenecker, B.; Hertel-Bohnke, P.; Webel, A.; Kienzle, E. Renal phosphorus excretion in adult healthy cats after the intake of high phosphorus diets with either calcium monophosphate or sodium monophosphate. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Herbst, S.D.B. Effects of dietary phosphates from organic and inorganic sources on parameters of phosphorus homeostasis in healthy adult dogs. PLoS ONE 2021, 16, e0246950. [Google Scholar] [CrossRef]
- Dobenecker, B.; Kienzle, E.; Siedler, S. The Source Matters-Effects of High Phosphate Intake from Eight Different Sources in Dogs. Animals 2021, 11, 3456. [Google Scholar] [CrossRef] [PubMed]
- FEDIAF. Nutritional Guidelines For Complete and Complementary Pet Food for Cats and Dogs; European Pet FoodIndustry Federation: Brussels, Belgium, 2020. [Google Scholar]
- Gericke, S.; Kurmies, B. Colorimetrische Bestimmung der Phosphorsäure mit Vanadat-Molybdat. Fresenius’ Z. Anal. Chem. 1952, 137, 15–22. [Google Scholar] [CrossRef]
- Janßen, E.M.Y.; Rieß, P.; Seifert, D. Nassaufschluss unter Druck. VDLufa Methodenbuch III 6.Erg. 2006, 10, 1–4. [Google Scholar]
- Block, G.A.; Port, F.K. Re-evaluation of risks associated with hyperphosphatemia and hyperparathyroidism in dialysis patients: Recommendations for a change in management. Am. J. Kidney Dis. 2000, 35, 1226–1237. [Google Scholar] [CrossRef]
- Jackson, H.A.; Barber, P.J. Resolution of metastatic calcification in the paws of a cat with successful dietary management of renal hyperparathyroidism. J. Small Anim. Pract. 1998, 39, 495–497. [Google Scholar] [CrossRef]
- Coltherd, J.C.; Staunton, R.; Colyer, A.; Thomas, G.; Gilham, M.; Logan, D.W.; Butterwick, R.; Watson, P. Not all forms of dietary phosphorus are equal: An evaluation of postprandial phosphorus concentrations in the plasma of the cat. Br. J. Nutr. 2019, 121, 270–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarafidis, P.A.; Li, S.; Chen, S.-C.; Collins, A.J.; Brown, W.W.; Klag, M.J.; Bakris, G.L. Hypertension Awareness, Treatment, and Control in Chronic Kidney Disease. Am. J. Med. 2008, 121, 332–340. [Google Scholar] [CrossRef]
- Cowgill, L.D.; Kallet, A.J. Systemic hypertension. Curr. Vet. Ther. Phila. WB Saunders 1986, 32, 360–366. [Google Scholar]
- Wehner, A.; Hartmann, K.; Hirschberger, J. Associations between proteinuria, systemic hypertension and glomerular filtration rate in dogs with renal and non-renal diseases. Vet. Rec. 2008, 162, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Humalda, J.K.; Yeung, S.M.H.; Geleijnse, J.M.; Gijsbers, L.; Riphagen, I.J.; Hoorn, E.J.; Rotmans, J.I.; Vogt, L.; Navis, G.; Bakker, S.J.L.; et al. Effects of Potassium or Sodium Supplementation on Mineral Homeostasis: A Controlled Dietary Intervention Study. J. Clin. Endocrinol. Metab. 2020, 105, e3246–e3256. [Google Scholar] [CrossRef] [PubMed]
- Eckberg, K.; Kramer, H.; Wolf, M.; Durazo-Arvizu, R.; Tayo, B.; Luke, A.; Cooper, R. Impact of westernization on fibroblast growth factor 23 levels among individuals of African ancestry. Nephrol. Dial. Transpl. 2015, 30, 630–635. [Google Scholar] [CrossRef]
- Yeung, S.M.H.; Hoorn, E.J.; Rotmans, J.I.; Gansevoort, R.T.; Bakker, S.J.L.; Vogt, L.; de Borst, M.H. Urinary Potassium Excretion, Fibroblast Growth Factor 23, and Incident Hypertension in the General Population-Based PREVEND Cohort. Nutrients 2021, 13, 4532. [Google Scholar] [CrossRef]
- Humalda, J.K.; Keyzer, C.A.; Binnenmars, S.H.; Kwakernaak, A.J.; Slagman, M.C.J.; Laverman, G.D.; Bakker, S.J.L.; de Borst, M.H.; Navis, G.J. Concordance of dietary sodium intake and concomitant phosphate load: Implications for sodium interventions. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 689–696. [Google Scholar] [CrossRef]
- Yin, Y.; Huang, C.; Wu, X.; Li, T.; Huang, R.; Kang, P.; Hu, Q.; Chu, W.; Kong, X. Nutrient digestibility response to graded dietary levels of sodium chloride in weanling pigs. J. Sci. Food Agric. 2008, 88, 940–944. [Google Scholar] [CrossRef]
- Kaup, S.M.; Greger, J.L. Effect of various chloride salts on the utilization of phosphorus, calcium, and magnesium. J. Nutr. Biochem. 1990, 1, 542–548. [Google Scholar] [CrossRef]
- Dobenecker, B.; Webel, A.; Reese, S.; Kienzle, E. Effect of a high phosphorus diet on indicators of renal health in cats. J. Feline Med. Surg 2018, 20, 339–343. [Google Scholar] [CrossRef]
- Böswald, L.; Herbst, S.; Dobenecker, B. Influence of Phosphorus Source on the Difference between Dietary and Faecal Ca/P Ratio and Serum Phosphorus Levels in Dogs; ESVCN: Munich, Germany, 2020. [Google Scholar]
- Böswald, L.; Herbst, S.; Schaschl, C.; Kienzle, E.; Dobenecker, B. Inorganic P Salts Disrupt the Strong Linear Correlation between Faecal Calcium and Phosphorus Excretion in Dogs but Not in Cats; ESVCN: Munich, Germany, 2020. [Google Scholar]
- Dobenecker, B. Phosphate intake with complete food and diets for chronic kidney disease available on the German market. Tierärztliche Prax. Kleintiere Heimtiere 2021, 49, 247–254. [Google Scholar] [CrossRef]
- Nguyen, P.; Reynolds, B.; Zentek, J.; Paßlack, N.; Leray, V. Sodium in feline nutrition. J. Anim. Physiol. Anim. Nutr. 2017, 101, 403–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spurgeon-Pechman, K.R.; Donohoe, D.L.; Mattson, D.L.; Lund, H.; James, L.; Basile, D.P. Recovery from acute renal failure predisposes hypertension and secondary renal disease in response to elevated sodium. Am. J. Physiol. -Ren. Physiol. 2007, 293, F269–F278. [Google Scholar] [CrossRef] [PubMed]

| CON | HNaCl | ||
|---|---|---|---|
| Ingredients basal diet | % | Beef (Heart, Steak) 72 Rice 24 Cellulose 1 Rapeseed oil 3 | |
| DM | g/kg | 431 | |
| GE | MJ/kg DM | 27 | |
| Crude protein | g/kg DM | 439 | |
| Crude fat | 362 | ||
| Crude fibre | 35 | ||
| Crude ash | 16 | ||
| NfE | 77 | ||
| Ca | 4.6 ± 0.2 | ||
| P | 3.5 ± 0.1 | ||
| K | 8 | 18 | |
| Mg | 0.75 ± 0.2 | ||
| Na | 1.4 | 12.1 | |
| Cl | 6.1 | 29.1 | |
| Ca/P | - | 1.3/1 | |
| Vit. D3 | IU/kg DM | 428 ± 30 | |
| Nutrient | Diet | Intake | Faecal Excretion | aD | Renal Excretion | Retention |
|---|---|---|---|---|---|---|
| DM [g] | CON | 13 ± 2 | 1.3 ± 0.3 | 90 ± 2 | - | - |
| HNaCl | 12 ± 1 *** | 1.2 ± 0.5 | 90 ± 3 | - | - | |
| Ca [mg] | CON | 64 ± 4 | 58 ± 11 | 10 ± 17 | 0.4 ± 0.1 | 6 ± 11 |
| HNaCl | 55 ± 4 *** | 47 ± 16 * | 16 ± 26 | 0.4 ± 0.1 | 8 ± 14 | |
| P [mg] | CON | 49 ± 3 | 19 ± 4 | 60 ± 9 | 14 ± 5 | 15 ± 4 |
| HNaCl | 42 ± 3 *** | 13 ± 4 ** | 69 ± 10 * | 16 ± 4 | 13 ± 4 | |
| K [mg] | CON | 107 ± 6 | 3 ± 1 | 97 ± 1 | 92 ± 14 | 12 ± 11 |
| HNaCl | 218 ± 14 *** | 2 ± 1 | 99 ± 1 *** | 174 ± 32 *** | 42 ± 21 *** | |
| Mg [mg] | CON | 12 ± 1 | 6 ± 1 | 46 ± 10 | 2 ± 1 | 4 ± 2 |
| HNaCl | 8 ± 1 *** | 5 ± 1 ** | 43 ± 17 | 1 ± 1 | 2 ± 1** | |
| Na [mg] | CON | 19 ± 1 | 3 ± 1 | 86 ± 5 | 29 ± 4 | −12 ± 4 |
| HNaCl | 149 ± 10 *** | 3 ± 1 | 98 ± 1 *** | 136 ± 26 *** | 10 ± 19 *** | |
| Cl [mg] | CON | 83 ± 5 | 3 ± 1 | 96 ± 2 | 94 ± 12 | −14 ± 10 |
| HNaCl | 376 ± 52 *** | 3 ± 1 | 99 ± 0 *** | 346 ± 67 *** | 27 ± 71* |
| Serum | Time Point | CON | HNaCl | Reference Range |
|---|---|---|---|---|
| FGF23 [pg/mL] | Pre-prandial | 202 ± 53 | 183 ± 23 | - |
| Postprandial | 142 ± 22 # | 146 ± 25 # | ||
| Creatinine [mmol/L] | Pre-prandial | 0.14 ± 0.01 | 0.14 ± 0.02 | 0.08–0.2 |
| Postprandial | 0.15 ± 0.01 # | 0.15 ± 0.02 | ||
| Ca [mmol/L] | Pre-prandial | 2.3 ± 0.1 | 2.5 ± 0.2 ** | 2.2–2.9 |
| Postprandial | 2.2 ± 0.2 # | 2.3 ± 0.1 # | ||
| P [mmol/L] | Pre-prandial | 1.8 ± 0.2 | 1.9 ± 0.3 | 0.8–2.2 |
| Postprandial | 1.4 ± 0.1 # | 1.6 ± 0.2 # | ||
| sCaxP [mg2/dL2] | Pre-prandial | 52 ± 6 | 60 ± 10 | <55/<70 Δ |
| Postprandial | 39 ± 3 # | 44 ± 8 # | ||
| K [mmol/L] | Pre-prandial | 4.1 ± 0.3 | 5.7 ± 1.0 *** | 3.3–5.8 |
| Postprandial | 4.0 ± 0.1 | 5.4 ± 0.5 *** | ||
| Na [mmol/L] | Pre-prandial | 156 ± 4 | 154 ± 9 | 147–159 |
| Postprandial | 157 ± 2 | 146 ± 4 #*** |
| Urine | CON | HNaCl | Reference Range |
|---|---|---|---|
| Creatinine [mmol/L] | 32 ± 4 | 20 ± 2 *** | - |
| USG [mg/mL] | 1060 ± 2 | 1052 ± 5 *** | 1001–1085 |
| Volume [mL/kg BW/d] | 14 ± 3 | 22 ± 4 *** | <50 |
| Na [g/L] | 2.0 ± 0.3 | 6.2 ± 0.5 *** | - |
| Cl [g/L] | 6.6 ± 0.7 | 15.8 ± 1.3 *** | - |
| K [g/L] | 6.5 ± 0.7 | 8.0 ± 0.6 *** | - |
| P [g/L] | 1.0 ± 0.2 | 0.7 ± 0.1 ** | - |
| P/Crea [mmol/L] | 1.0 ± 0.3 | 1.2 ± 0.2 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steffen, C.; Kienzle, E.; Dobenecker, B. High Intake of Sodium Chloride for 28 Days Causes No Effect on Serum FGF23 Concentrations in Cats. Animals 2022, 12, 3195. https://doi.org/10.3390/ani12223195
Steffen C, Kienzle E, Dobenecker B. High Intake of Sodium Chloride for 28 Days Causes No Effect on Serum FGF23 Concentrations in Cats. Animals. 2022; 12(22):3195. https://doi.org/10.3390/ani12223195
Chicago/Turabian StyleSteffen, Carla, Ellen Kienzle, and Britta Dobenecker. 2022. "High Intake of Sodium Chloride for 28 Days Causes No Effect on Serum FGF23 Concentrations in Cats" Animals 12, no. 22: 3195. https://doi.org/10.3390/ani12223195





