Equine Encephalosis Virus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Etiology
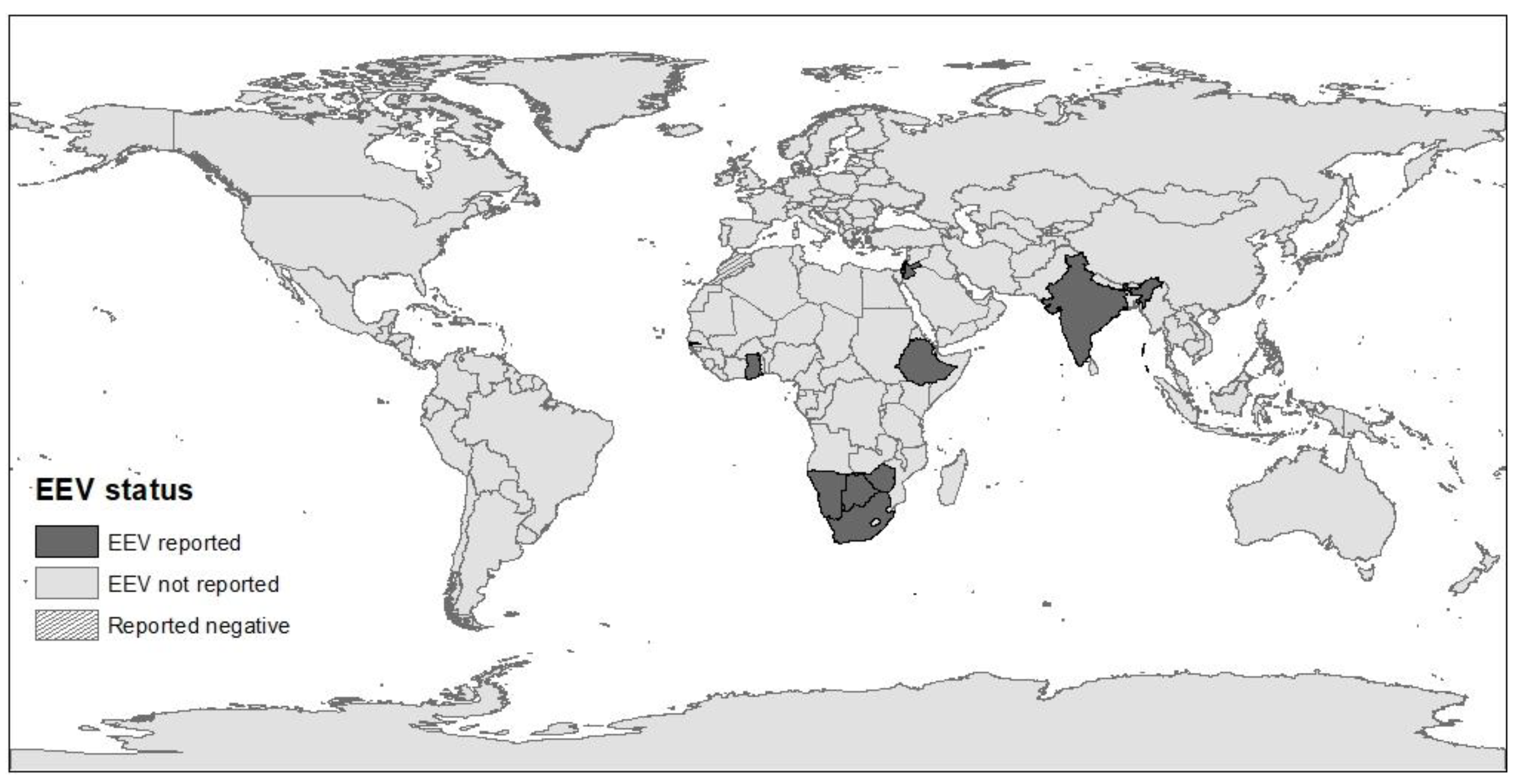
3. Epidemiology
4. Clinical Disease
5. Treatment, Prevention, and Control
6. Diagnosis
7. The Israeli Perspective
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Theiler, A. Notes on a fever in horses simulating horse-sickness. Transvaal. Agric. J. 1910, 8, 581–586. [Google Scholar]
- Erasmus, B.; Adelaar, T.; Smit, J.; Lecatsas, G.; Toms, T. The isolation and characterization of equine encephalosis virus. Bull. Off. Int. Epizoot. 1970, 74, 781–789. [Google Scholar]
- Attoui, H.; Jaafar, F.M. Zoonotic and emerging orbivirus infections. Rev. Sci. Tech. 2015, 34, 353–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhama, K.; Pawaiya, R.; Karthik, K.; Chakrabort, S.; Tiwari, R.; Verma, A. Equine encephalosis virus (EEV): A Review. Asian J. Anim. Vet. Adv. 2014, 9, 123–133. [Google Scholar] [CrossRef]
- Paweska, J.T.; Venter, G.J. Vector competence of Culicoides species and the seroprevalence of homologous neutralizing antibody in horses for six serotypes of equine encephalosis virus (EEV) in South Africa. Med. Vet. Èntomol. 2004, 18, 398–407. [Google Scholar] [CrossRef]
- Aharonson-Raz, K.; Steinman, A.; Kavkovsky, A.; Bumbarov, V.; Berlin, D.; Lichter-Peled, A.; Berke, O.; Klement, E. Analysis of the Association of Climate, Weather and Herd Immunity with the Spread of Equine Encephalosis Virus in Horses in Israel. Transbound. Emerg. Dis. 2015, 64, 593–602. [Google Scholar] [CrossRef]
- MacLachlan, N.J.; Guthrie, A.J. Re-emergence of bluetongue, African horse sickness, and other Orbivirus diseases. Vet. Res. 2010, 41, 35. [Google Scholar] [CrossRef] [Green Version]
- Gordon, S.J.; Bolwell, C.; Rogers, C.W.; Musuka, G.; Kelly, P.; Guthrie, A.; Mellor, P.S.; Hamblin, C. The sero-prevalence and sero-incidence of African horse sickness and equine encephalosis in selected horse and donkey populations in Zimbabwe. Onderstepoort J. Vet. Res. 2017, 84, 5. [Google Scholar] [CrossRef]
- Mildenberg, Z.; Westcott, D.; Bellaiche, M.; Dastjerdi, A.; Steinbach, F.; Drew, T. Equine Encephalosis Virus in Israel. Transbound. Emerg. Dis. 2009, 56, 291. [Google Scholar] [CrossRef]
- Oura, C.A.L.; Batten, C.A.; Ivens, P.A.S.; Balcha, M.; Alhassan, A.; Gizaw, D.; Elharrak, M.; Jallow, D.B.; Sahle, M.; Maan, N.; et al. Equine encephalosis virus: Evidence for circulation beyond southern Africa. Epidemiol. Infect. 2012, 140, 1982–1986. [Google Scholar] [CrossRef] [Green Version]
- Yadav, P.D.; Albarino, C.G.; Nyayanit, D.A.; Guerrero, L.; Jenks, M.H.; Sarkale, P.; Nichol, S.T.; Mourya, D.T. Equine Encephalosis Virus in India. Emerg. Infect. Dis. 2018, 24, 898–901. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.A.J.; López, E.P.M.; De Vos, C.J.; Faverjon, C. Quantitative analysis of the probability of introducing equine encephalosis virus (EEV) into The Netherlands. Prev. Vet. Med. 2016, 131, 48–59. [Google Scholar] [CrossRef] [PubMed]
- King, A.M.; Adams, M.J.; Carstens, E.B.; Lefkowitz, E.J. Virus taxonomy. In Ninth report of the International Committee on Taxonomy of Viruses; Elsevier: London, UK, 2012. [Google Scholar]
- Howell, P.G.; Groenewald, D.; Visage, C.W.; Bosman, A.-M.; Coetzer, J.A.W.; Guthrie, A.J. The classification of seven serotypes of equine encephalosis virus and the prevalence of homologous antibody in horses in South Africa. Onderstepoort J. Vet. Res. 2002, 69, 79–93. [Google Scholar] [PubMed]
- Gould, A.R.; Hyatt, A.D. The orbivirus genus. Diversity, structure, replication and phylogenetic relationships. Comp. Immunol. Microbiol. Infect. Dis. 1994, 17, 163–188. [Google Scholar] [CrossRef]
- Dennis, S.J.; Meyers, A.E.; Hitzeroth, I.I.; Rybicki, E.P. African Horse Sickness: A Review of Current Understanding and Vaccine Development. Viruses 2019, 11, 844. [Google Scholar] [CrossRef] [Green Version]
- Huismans, H.; Van Staden, V.; Fick, W.C.; Van Niekerk, M.; Meiring, T.L. A comparison of different orbivirus proteins that could affect virulence and pathogenesis. Vet. Ital. 2010, 40, 417–425. [Google Scholar]
- Viljoen, G.J.; Huismans, H. The Characterization of Equine Encephalosis Virus and the Development of Genomic Probes. J. Gen. Virol. 1989, 70, 2007–2015. [Google Scholar] [CrossRef]
- Maan, S.; Belaganahalli, M.N.; Maan, N.S.; Potgieter, A.C.; Mertens, P.P.C. Quantitative RT-PCR assays for identification and typing of the Equine encephalosis virus. Braz. J. Microbiol. 2019, 50, 287–296. [Google Scholar] [CrossRef]
- Van Niekerk, M.; Freeman, M.; Paweska, J.T.; Howell, P.G.; Guthrie, A.J.; Potgieter, A.C.; Van Staden, V.; Huismans, H. Variation in the NS3 gene and protein in South African isolates of bluetongue and equine encephalosis viruses. J. Gen. Virol. 2003, 84, 581–590. [Google Scholar] [CrossRef]
- Lecatsas, G.; Erasmus, B.J.; Els, H.J. Electron microscopic studies on equine encephalosis virus. Onderstepoort J. Vet. Res. 1973, 40, 53–57. [Google Scholar]
- Howell, P.G.; Nurton, J.P.; Nel, D.; Lourens, C.W.; Guthrie, A.J. Prevalence of serotype specific antibody to equine encephalosis virus in Thoroughbred yearlings South Africa (1999–2004). Onderstepoort J. Vet. Res. 2008, 75, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.C.; Venter, G.J.; Mellor, P.S.; Paweska, J.T.; Woolhouse, M.E.J. Transmission patterns of African horse sickness and equine encephalosis viruses in South African donkeys. Epidemiol. Infect. 2002, 128, 265–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnard, B.J.; Paweska, J.T. Prevalence of antibodies against some equine viruses in zebra (Zebra burchelli) in the Kruger National Park, 1991–1992. Onderstepoort J. Vet. Res. 1993, 60, 175–179. [Google Scholar] [PubMed]
- Barnard, B.J. Antibodies against some viruses of domestic animals in southern African wild animals. Onderstepoort J. Vet. Res. 1997, 64, 95–110. [Google Scholar] [PubMed]
- Williams, R.; Du Plessis, D.H.; Van Wyngaardt, W. Group-Reactive ELISAs for Detecting Antibodies to African Horsesickness and Equine Encephalosis Viruses in Horse, Donkey, and Zebra Sera. J. Vet. Diagn. Investig. 1993, 5, 3–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aharonson-Raz, K.; Steinman, A.; Bumbarov, V.; Maan, S.; Maan, N.S.; Nomikou, K.; Batten, C.; Potgieter, C.; Gottlieb, Y.; Mertens, P.; et al. Isolation and Phylogenetic Grouping of Equine Encephalosis Virus in Israel. Emerg. Infect. Dis. 2011, 17, 1883–1886. [Google Scholar] [CrossRef] [PubMed]
- Wescott, D.G.; Mildenberg, Z.; Bellaiche, M.; McGowan, S.L.; Grierson, S.S.; Choudhury, B.; Steinbach, F. Evidence for the Circulation of Equine Encephalosis Virus in Israel since 2001. PLoS ONE 2013, 8, e70532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tirosh-Levy, S.; Gelman, B.; Zivotofsky, D.; Quraan, L.; Khinich, E.; Nasereddin, A.; Abdeen, Z.; Steinman, A. Seroprevalence and risk factor analysis for exposure to equine encephalosis virus in Israel, Palestine and Jordan. Vet. Med. Sci. 2017, 3, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Grewar, J.D.; Thompson, P.N.; Lourens, C.W.; Guthrie, A.J. Equine encephalosis in Thoroughbred foals on a South African stud farm. Onderstepoort J. Vet. Res. 2015, 82, 4. [Google Scholar] [CrossRef]
- Snyman, J.; Venter, G.J.; Venter, M. An Investigation of Culicoides (Diptera: Ceratopogonidae) as Potential Vectors of Med-ically and Veterinary Important Arboviruses in South Africa. Viruses 2021, 13, 1978. [Google Scholar] [CrossRef]
- Snyman, J.; Koekemoer, O.; van Schalkwyk, A.; van Vuren, P.J.; Snyman, L.; Williams, J.; Venter, M. Epidemiology and Genomic Analysis of Equine Encephalosis Virus Detected in Horses with Clinical Signs in South Africa, 2010–2017. Viruses 2021, 13, 398. [Google Scholar] [CrossRef] [PubMed]
- Venter, G.J.; Groenewald, D.; Venter, E.; Hermanides, K.G.; Howell, P.G. A comparison of the vector competence of the biting midges, Culicoides (Avaritia) bolitinos and C. (A.) imicola, for the Bryanston serotype of equine encephalosis virus. Med. Vet. Èntomol. 2002, 16, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Venter, G.J.; Groenewald, D.M.; Paweska, J.T.; Venter, E.; Howell, P.G. Vector competence of selected South African Culicoides species for the Bryanston serotype of equine encephalosis virus. Med. Vet. Èntomol. 1999, 13, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Purse, B.V.; Carpenter, S.; Venter, G.J.; Bellis, G.; Mullens, B.A. Bionomics of Temperate and Tropical Culicoides Midges: Knowledge Gaps and Consequences for Transmission of Culicoides-Borne Viruses. Annu. Rev. Èntomol. 2015, 60, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Purse, B.V.; Mellor, P.S.; Rogers, D.J.; Samuel, A.R.; Mertens, P.; Baylis, M. Climate change and the recent emergence of bluetongue in Europe. Nat. Rev. Microbiol. 2005, 3, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Venter, G.J.; Koekemoer, J.J.O.; Paweska, J.T. Investigations on outbreaks of African horse sickness in the surveillance zone in South Africa. Rev. Sci. Tech. 2006, 25, 1097–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathogwa, N.; Quan, M.; Smit, J.; Lourens, C.; Guthrie, A.; Van Vuuren, M. Development of a real time polymerase chain reaction assay for equine encephalosis virus. J. Virol. Methods 2014, 195, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Mellor, P.S.; Boorman, J.; Baylis, M. Culicoides Biting Midges: Their Role as Arbovirus Vectors. Annu. Rev. Èntomol. 2000, 45, 307–340. [Google Scholar] [CrossRef]
- Baylis, M.; Mellor, P.S.; Meiswinkel, R. Horse sickness and ENSO in South Africa. Nature 1999, 397, 574. [Google Scholar] [CrossRef]
- Calzolari, M.; Albieri, A. Could drought conditions trigger Schmallenberg virus and other arboviruses circulation? Int. J. Health Geogr. 2013, 12, 7. [Google Scholar] [CrossRef] [Green Version]
- Leta, S.; Fetene, E.; Mulatu, T.; Amenu, K.; Jaleta, M.B.; Beyene, T.J.; Negussie, H.; Revie, C.W. Modeling the global distribution of Culicoides imicola: An Ensemble approach. Sci. Rep. 2019, 9, 14187. [Google Scholar] [CrossRef]
- Faverjon, C.; Leblond, A.; Lecollinet, S.; Bødker, R.; De Koeijer, A.A.; Fischer, E. Comparative Risk Analysis of TwoCulicoides-Borne Diseases in Horses: Equine Encephalosis More Likely to Enter France than African Horse Sickness. Transbound. Emerg. Dis. 2016, 64, 1825–1836. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, U.; Herholz, C.; Schwermer, H.; Hofmann, M.; Griot, C. African horse sickness and equine encephalosis: Must Switzerland get prepared? Schweiz. Arch. Tierh. 2010, 152, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Harrup, L.; Miranda, M.; Carpenter, S. Advances in control techniques for Culicoides and future prospects. Vet. Ital. 2016, 52, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Crafford, J.; Guthrie, A.; Van Vuuren, M.; Mertens, P.; Burroughs, J.; Howell, P.; Batten, C.; Hamblin, C. A competitive ELISA for the detection of group-specific antibody to equine encephalosis virus. J. Virol. Methods 2011, 174, 60–64. [Google Scholar] [CrossRef] [Green Version]
- Crafford, J.; Guthrie, A.; Van Vuuren, M.; Mertens, P.; Burroughs, J.; Howell, P.; Hamblin, C. A group-specific, indirect sandwich ELISA for the detection of equine encephalosis virus antigen. J. Virol. Methods 2003, 112, 129–135. [Google Scholar] [CrossRef]
- Venter, E.; Viljoen, G.; Nel, L.; Huismans, H.; Van Dijk, A. A comparison of different genomic probes in the detection of virus-specified RNA in Orbivirus-infected cells. J. Virol. Methods 1991, 32, 171–180. [Google Scholar] [CrossRef]
- Quan, M.; van Vuuren, M.; Howell, P.G.; Groenewald, D.; Guthrie, A.J. Molecular epidemiology of the African horse sickness virus S10 gene. J. Gen. Virol. 2008, 89, 1159–1168. [Google Scholar] [CrossRef]
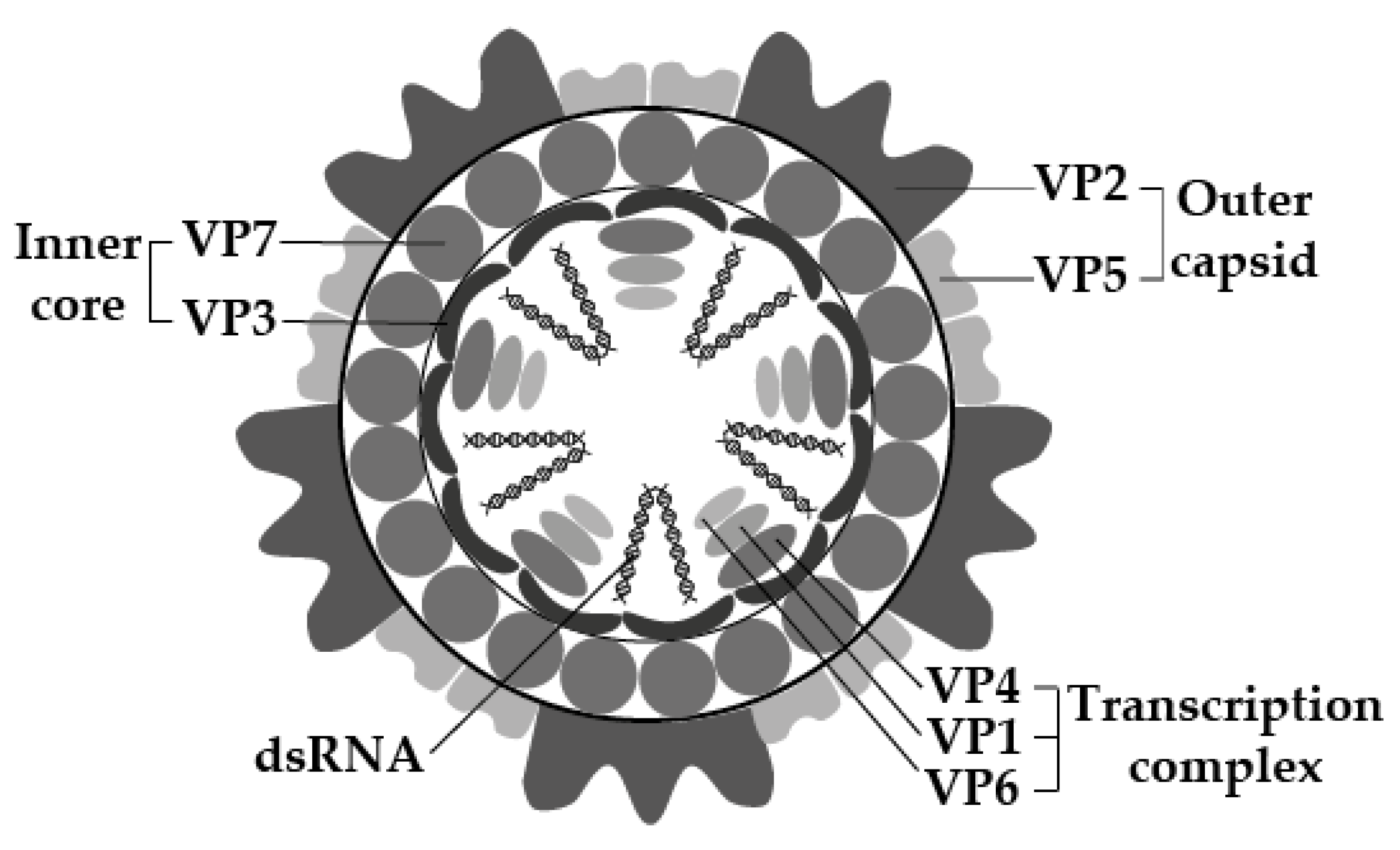
| Genome Segment | Protein | Function |
|---|---|---|
| Seg-1 | VP1 | RNA-dependent RNA polymerase |
| Seg-2 | VP2 | Protein of the outer layer of the outer capsid, involves in cell attachment, most variable, determines serotype |
| Seg-3 | VP3 | Innermost protein capsid shell |
| Seg-4 | VP4 | Capping enzyme |
| Seg-5 | NS1 | Forms tubules of unknown function |
| Seg-6 | VP5 | Inner layer of outer capsid, involves in cell penetration |
| Seg-7 | VP7 | Protein of the outer core surface, involves in cell entry, immunodominant |
| Seg-8 | NS2 | Inclusion body matrix protein |
| Seg-9 | VP6/VP6A | Helicase |
| Seg-10 | NS3/NS3A | Membrane protein, involves in cell exit, variable |
| Culicoides spp. | EEV Serotype Survival |
|---|---|
| C. imicola | EEV-1 >> 4 > 2,5 > 3 > 6 |
| C. bolitinos | EEV-2 > 1 >> 4 > 6 |
| C. leucostictus | EEV-1 > 2 |
| C. magnus | EEV-1 |
| C. zuluensis | EEV-2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirosh-Levy, S.; Steinman, A. Equine Encephalosis Virus. Animals 2022, 12, 337. https://doi.org/10.3390/ani12030337
Tirosh-Levy S, Steinman A. Equine Encephalosis Virus. Animals. 2022; 12(3):337. https://doi.org/10.3390/ani12030337
Chicago/Turabian StyleTirosh-Levy, Sharon, and Amir Steinman. 2022. "Equine Encephalosis Virus" Animals 12, no. 3: 337. https://doi.org/10.3390/ani12030337







