Supplementing a Phytogenic Feed Additive Modulates the Risk of Subacute Rumen Acidosis, Rumen Fermentation and Systemic Inflammation in Cattle Fed Acidogenic Diets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Design, Treatments and Management
2.2. Feed Sampling and Chemical Analysis
2.3. Measurements of Ruminal pH and Monitoring of SARA
2.4. Collection of Ruminal and Reticular Fluid and Analysis
2.5. Blood Sampling and Analysis of Systemic Health Biomarkers
2.6. Statistical Analysis
3. Results
3.1. Ruminal pH and SARA
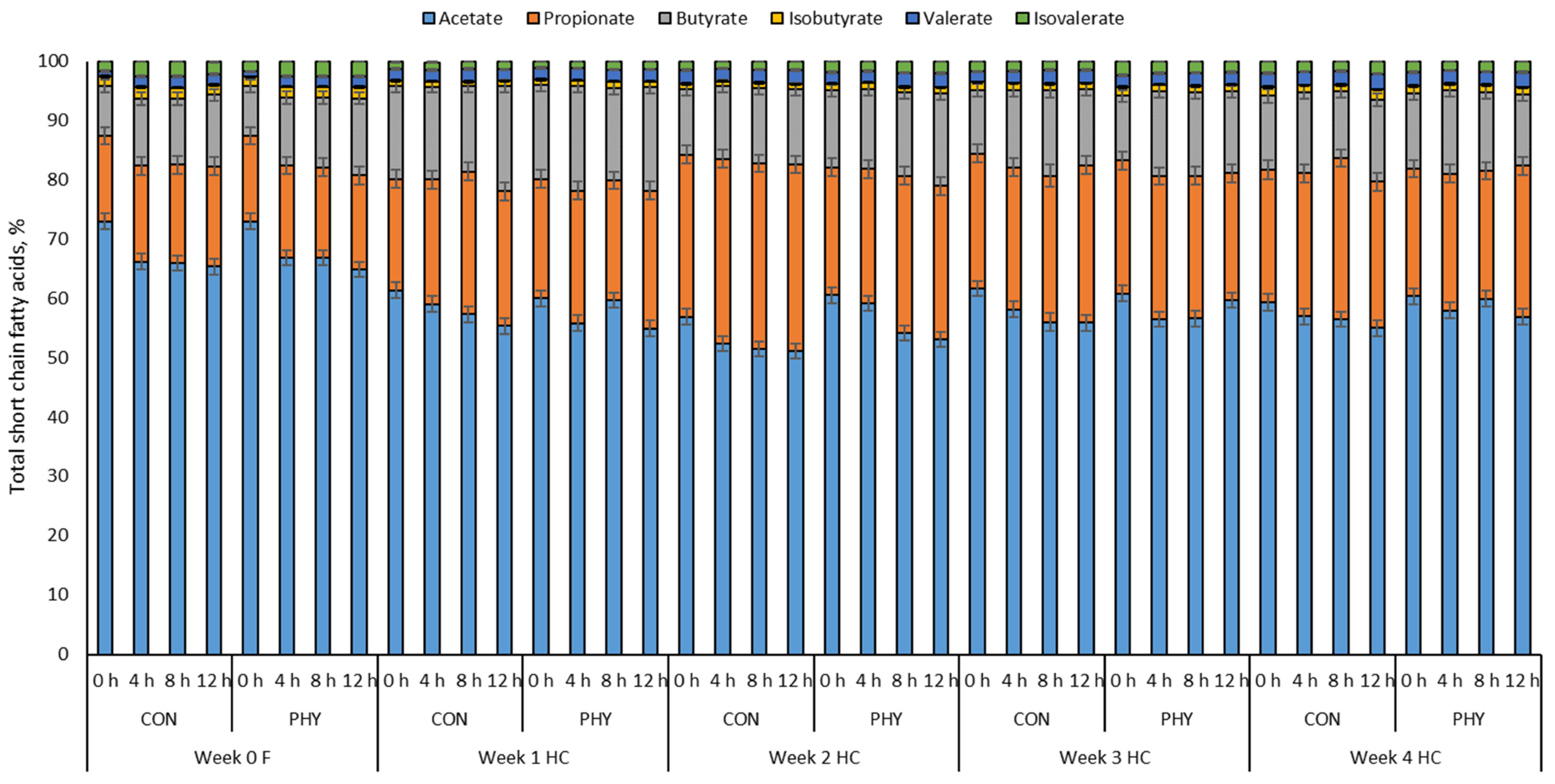
3.2. Ruminal and Reticular Short Chain Fatty Acids Profile
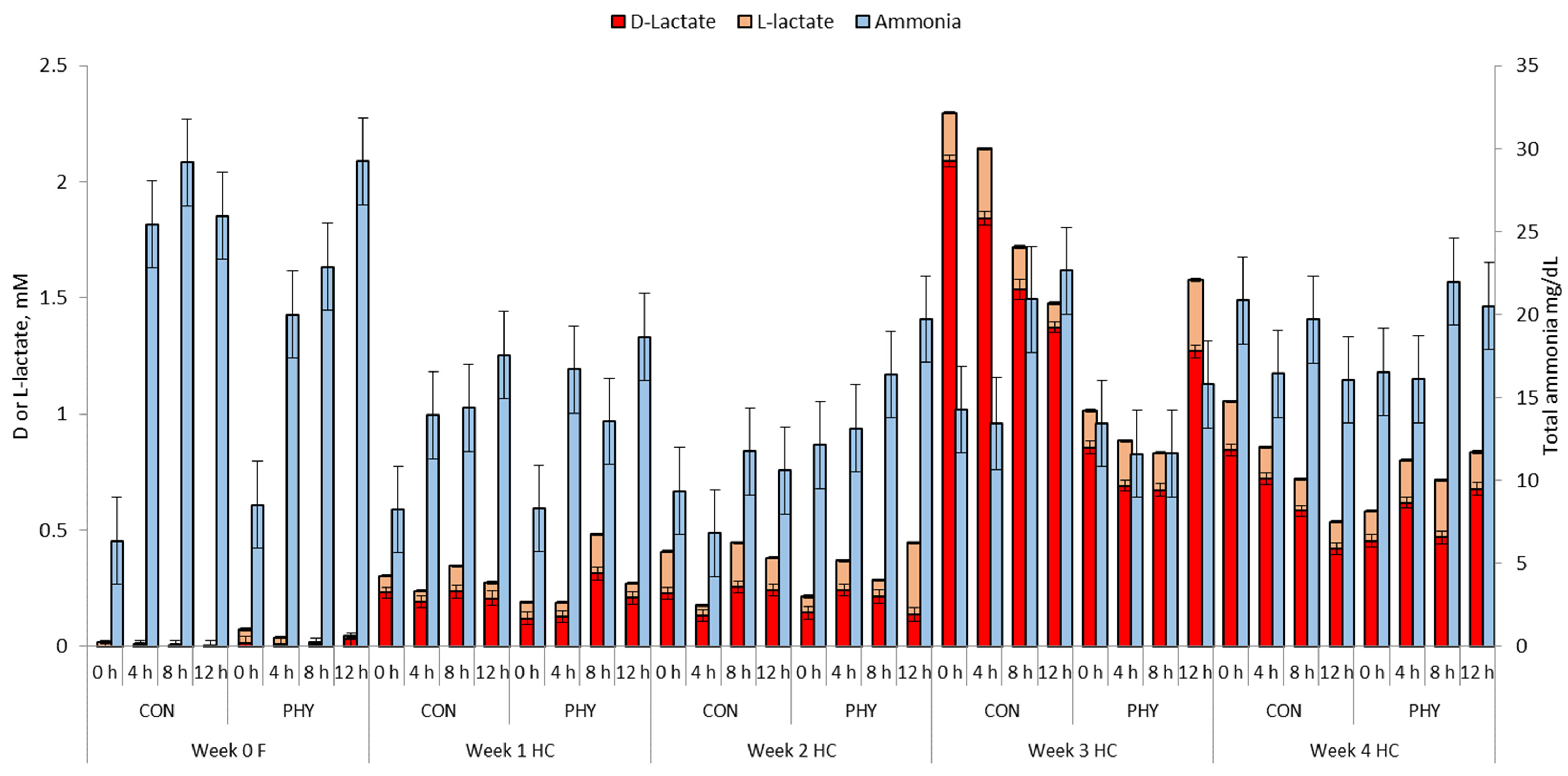
3.3. Ruminal and Reticular Lactate and Ammonia
3.4. Systemic Inflammation and Liver Health Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khorrami, B.; Khiaosa-ard, R.; Zebeli, Q. Models to predict the risk of subacute ruminal acidosis in dairy cows based on dietary and cow factors: A meta-analysis. J. Dairy Sci. 2021, 104, 7761–7780. [Google Scholar] [CrossRef] [PubMed]
- Sutton, J.D.; Dhanoa, M.S.; Morant, S.V.; France, J.; Napper, D.J.; Schuller, E. Rates of Production of Acetate, Propionate, and Butyrate in the Rumen of Lactating Dairy Cows Given Normal and Low-Roughage Diets. J. Dairy Sci. 2003, 86, 3620–3633. [Google Scholar] [CrossRef] [Green Version]
- Bannink, A.; France, J.; Lopez, S.; Gerrits, W.J.J.; Kebreab, E.; Tamminga, S.; Dijkstra, J. Modelling the implications of feeding strategy on rumen fermentation and functioning of the rumen wall. Anim. Feed Sci. Technol. 2008, 43, 3–26. [Google Scholar] [CrossRef]
- Allen, M.S. Relationship between fermentation acid production in the rumen and the requirement for physically effective fibre. J. Dairy Sci. 1997, 80, 1447–1462. [Google Scholar] [CrossRef]
- Zebeli, Q.; Dijkstra, J.; Tafaj, M.; Steingass, H.; Ametaj, B.; Drochner, W. Modeling the adequacy of dietary fiber in dairy cows based on the responses of ruminal pH and milk fat production to composition of the diet. J. Dairy Sci. 2008, 91, 2046–2066. [Google Scholar] [CrossRef] [Green Version]
- Plaizier, J.C.; Khafipour, E.; Li, S.; Gozho, G.N.; Krause, D.O. Subacute ruminal acidosis (SARA), endotoxins and health consequences. Anim. Feed Sci. Technol. 2012, 172, 9–21. [Google Scholar] [CrossRef]
- Zebeli, Q.; Metzler-Zebeli, B.U. Interplay between rumen digestive disorders and diet-induced inflammation in dairy cattle. Res. Vet. Sci. 2012, 93, 1099–1108. [Google Scholar] [CrossRef]
- Stone, W.C. Nutritional approaches to minimize subacute ruminal acidosis and laminitis in dairy cattle. J. Dairy Sci. 2004, 87, E13–E26. [Google Scholar] [CrossRef]
- White, R.R.; Roman-Garcia, Y.; Firkins, J.L. Meta-analysis of postruminal microbial nitrogen flows in dairy cattle. II. Approaches to and implications of more mechanistic prediction. J. Dairy Sci. 2016, 99, 7932–7944. [Google Scholar] [CrossRef] [Green Version]
- Lillehoj, H.; Liu, Y.; Calsamiglia, S.; Fernandez Miyakawa, M.E.; Chi, F.; Cravens, R.L.; Oh, S.; Gay, C.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018, 49, 76. [Google Scholar] [CrossRef] [Green Version]
- Neubauer, V.; Petri, R.; Humer, E.; Kröger, I.; Mann, E.; Reisinger, N.; Wagner, M.; Zebeli, Q. High-grain diets supplemented with phytogenic compounds or autolyzed yeast modulate ruminal bacterial community and fermentation in dry cows. J. Dairy Sci. 2018, 101, 2335–2349. [Google Scholar] [CrossRef] [PubMed]
- Kröger, I.; Humer, E.; Neubauer, V.; Reisinger, N.; Aditya, S.; Zebeli, Q. Modulation of chewing behavior and reticular pH in nonlactating cows challenged with concentrate-rich diets supplemented with phytogenic compounds and autolyzed yeast. J. Dairy Sci. 2017, 100, 9702–9714. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Lopez, E.; Rivera-Chacon, R.; Ricci, S.; Petri, R.M.; Reisinger, N.; Zebeli, Q. Short-term screening of multiple phytogenic compounds for their potential to modulate chewing behavior, ruminal fermentation profile, and pH in cattle fed grain-rich diets. J. Dairy Sci. 2021, 104, 4271–4289. [Google Scholar] [CrossRef] [PubMed]
- Ricci, S.; Rivera-Chacon, R.; Castillo-Lopez, E.; Petri, R.M.; Sener-Aydemir, A.; Sharma, S.; Reisinger, N.; Zebeli, Q. Supplementation of phytogenic compounds modulates feed ensalivation and salivary composition in cattle fed an acidogenic diet. Front. Anim. Physiol. 2021, 12, 645529. [Google Scholar] [CrossRef]
- Kononoff, P.J.; Heinrichs, A.J.; Buckmaster, D.R. Modification of the penn state forage and total mixed ration particle separator and the effects of moisture content on its measurements. J. Dairy Sci. 2003, 86, 1858–1863. [Google Scholar] [CrossRef]
- Naumann, C.; Bassler, R. Handbuch der Landwirtschaftlichen Versuchs-und Untersuchungsmethodik (VDLUFA-Methodenbuch). In Band III Die Chemische Untersuchung Von Futtermitteln; VDLUFA-Verlag: Darmstadt, Germany, 2012. [Google Scholar]
- Beauchemin, K.A.; Yang, W.Z. Effects of Physically Effective Fiber on Intake, Chewing Activity, and Ruminal Acidosis for Dairy Cows Fed Diets Based on Corn Silage. J. Dairy Sci. 2005, 88, 2117–2129. [Google Scholar] [CrossRef] [Green Version]
- Penner, G.B.; Beauchemin, K.A.; Mutsvangwa, T. An evaluation of the accuracy and precision of a stand-alone submersible continuous ruminal pH measurement system. J. Dairy Sci. 2006, 89, 2132–2140. [Google Scholar] [CrossRef] [Green Version]
- Khiaosa-ard, R.; Pourazad, P.; Aditya, S.; Humer, E.; Zebeli, Q. Factors related to variation in the susceptibility to subacute ruminal acidosis in early lactating Simmental cows fed the same grain-rich diet. Anim. Feed Sci. Technol. 2018, 238, 111–122. [Google Scholar] [CrossRef]
- Qumar, M.; Khiaosa-ard, R.; Pourazad, P.; Wetzels, S.U.; Klevenhusen, F.; Kandler, W.; Aschenbach, J.R.; Zebeli, Q. Evidence of in vivo absorption of lactate and modulation of short chain fatty acid absorption from the reticulo rumen of non-lactating cattle fed high concentrate diets. PLoS ONE 2016, 11, e0164192. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Simpson, P.M.; Hamer, R.M.; Lansing, S. Cross crossover studies off your list. In Proceedings of the Twenty Fourth Annual SAS Users Group International Conference, Miami Beach, FL, USA, 11–14 April 1999; pp. 1303–1309. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org/ (accessed on 8 August 2021).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: Houston, TX, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 8 August 2021).
- Kholif, A.E.; Hassan, A.A.; Matloup, O.H.; El Ashry, G.M. Top-dressing of chelated phytogenic feed additives in the diet of lactating Friesian cows to enhance feed utilization and lactational performance. Ann. Anim. Sci. 2021, 21, 657–673. [Google Scholar] [CrossRef]
- Kholif, A.E.; Hassan, A.A.; El Ashry, G.M.; Bakr, M.H.; El-Zaiat, H.M.; Olafadehan, O.A.; Matloup, O.H.; Sallam, S.M.A. Phytogenic feed additives mixture enhances the lactational performance, feed utilization and ruminal fermentation of Friesian cows. Anim. Biotechnol. 2020, 32, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.; Bonifacio, M.A.; De Giglio, E.; Cometa, S.; Logrieco, A.F.; Baruzzi, F. Unravelling the antifungal effect of red Thyme oil (Thymus vulgaris L.) compounds in vapor phase. Molecules 2020, 25, 4761. [Google Scholar] [CrossRef]
- Beigi, M.; Torki-Harchegani, M.; Pirbalouti, A.G. Quantity and chemical composition of essential oil of peppermint (Mentha × piperita L.) leaves under different drying methods. Int. J. Food Prop. 2018, 21, 267–276. [Google Scholar] [CrossRef] [Green Version]
- Benchaar, C.; Petit, H.V.; Berthiaume, R.; Ouellet, D.R.; Chiquette, J.; Chouinard, P.Y. Effects of Essential Oils on Digestion, Ruminal Fermentation, Rumen Microbial Populations, Milk Production, and Milk Composition in Dairy Cows Fed Alfalfa Silage or Corn Silage. J. Dairy Sci. 2007, 90, 886–897. [Google Scholar] [CrossRef] [Green Version]
- Benchaar, C.; Petit, H.V.; Berthiaume, R.; Whyte, T.D.; Chouinard, P.Y. Effects of dietary addition of essential oils and monensin premix on digestion, ruminal fermentation characteristics, milk production, and milk composition in dairy cows. J. Dairy Sci. 2006, 89, 4352–4364. [Google Scholar] [CrossRef]
- Calsamiglia, S.; Busquet, M.; Cardozo, P.W.; Castillejos, L.; Ferret, A. Invited review: Essential oils as modifiers of rumen microbial fermentation. J. Dairy Sci. 2007, 90, 2580–2595. [Google Scholar] [CrossRef] [Green Version]
- Patra, A.K. Effects of essential oils on rumen fermentation, microbial ecology and ruminant production. Asian J. Anim. Vet. Adv. 2011, 6, 416–428. [Google Scholar] [CrossRef]
- Duval, S.M.; McEwan, N.R.; Graham, R.C.; Wallace, R.J.; Newbold, C.J. Effect of a blend of essential oil compounds on the colonization of starch-rich substrates by bacteria in the rumen. J. Appl. Microbiol. 2007, 103, 2132–2141. [Google Scholar] [CrossRef]
- Khafipour, E.; Krause, D.O.; Plaizier, J.C. A grain-based subacute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation. J. Dairy Sci. 2009, 92, 1060–1070. [Google Scholar] [CrossRef] [Green Version]
- Pourazad, P.; Khiaosa-ard, R.; Qumar, M.; Wetzels, S.U.; Klevenhusen, F.; Metzler-Zebeli, B.; Zebeli, Q. Transient feeding of a concentrate-rich diet increases the severity of subacute ruminal acidosis in dairy cattle. J. Anim. Sci. 2016, 94, 726–738. [Google Scholar] [CrossRef]
- Benchaar, C.; Hassanat, F.; Petit, H.V. Dose–response to eugenol supplementation to dairy cow diets: Methane production, N excretion, ruminal fermentation, nutrient digestibility, milk production, and milk fatty acid profile. Anim. Feed Sci. Technol. 2015, 209, 51–59. [Google Scholar] [CrossRef]
- Sato, S. Pathophysiological evaluation of subacute ruminal acidosis (SARA) by continuous ruminal pH monitoring. J. Anim. Sci. 2015, 87, 168–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lana, R.P.; Russell, J.B.; Van Amburgh, M.E. The Role of pH in Regulating Ruminal Methane and Ammonia Production. J. Anim. Sci. 1998, 76, 2190–2196. [Google Scholar] [CrossRef] [PubMed]
- Hristov, A.N.; Ropp, J.K. Effect of dietary carbohydrate composition and availability on utilization of ruminal ammonia nitrogen for milk protein synthesis in dairy cows. J. Dairy Sci. 2003, 86, 2416–2427. [Google Scholar] [CrossRef]
- Flythe, M.D.; Harrison, B.; Kagan, I.A.; Klotz, J.L.; Gellin, G.L.; Goff, B.M.; Aike, G.E. Antimicrobial Activity of Red Clover (Trifolium pratense L.) Extract on Caprine Hyper Ammonia-Producing Bacteria. Agric. Food Anal. Bacteriol. 2013, 3, 176–185. [Google Scholar]
- Eckel, E.F.; Ametaj, B.N. Invited review: Role of bacterial endotoxins in the etiopathogenesis of periparturient diseases of transition dairy cows. J. Dairy Sci. 2016, 99, 5967–5990. [Google Scholar] [CrossRef]
- Yang, W.Z.; Ametaj, B.N.; Benchaar, C.; He, M.L.; Beauchemin, K.A. Cinnamaldehyde in feedlot cattle diets: Intake, growth performance, carcass characteristics, and blood metabolites. J. Anim. Sci. 2010, 88, 1082–1092. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.; Wall, E.H.; Bravo, D.M.; Hristov, A.N. Host-mediated effects of phytonutrients in ruminants: A review. J. Dairy Sci. 2017, 100, 5974–5983. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, R.O.; Cooke, R.F.; Firmino, F.C.; Moura, M.K.R.; Angeli, B.F.; Ferreira, H.A.O.; Brandão, A.P.; Gex-Fabry, M.; Ostrensky, A.; Vasconcelos, J.L.M. Productive and physiological responses of lactating dairy cows supplemented with phytogenic feed ingredients. Transl. Anim. Sci. 2019, 3, 1133–1142. [Google Scholar] [CrossRef]
- Wille, S.; Simon, A.; Platen, M.; Oertel, C. Factors influencing the activity of liver enzymes of clinically healthy dairy cows under field conditions. Züchtungskunde 2010, 82, 155–164. [Google Scholar]
- Kröger, I.; Humer, E.; Neubauer, V.; Reisinger, N.; Zebeli, Q. Feeding diets moderate in physically effective fibre alters eating and feed sorting patterns without improving ruminal pH, but impaired liver health in dairy cows. Animals 2019, 9, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stengärde, L.; Holtenius, K.; Travén, M.; Hultgren, J.; Niskanen, R.; Emanuelson, U. Blood profiles in dairy cows with displaced abomasum. J. Dairy Sci. 2010, 93, 4691–4699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakhani, N.; Kamra, D.N.; Lakhani, P.; Alhussien, M.N. Immune status and haemato-biochemical profile of buffalo calves supplemented with phytogenic feed additives rich in tannins, saponins and essential oils. Trop. Anim. Health Prod. 2018, 51, 565–573. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Kim, I.H. Efficacy of phytogenic feed additive on performance, production and health status of monogastric animals—A review. Ann. Anim. Sci. 2017, 17, 929–948. [Google Scholar] [CrossRef] [Green Version]
 ). (A) Week 0 forage, SEM = 0.05. (B) Day 4 of adaptation week, 40% concentrate, SEM = 0.08. (C) Week 1 of high concentrate, SEM = 0.09. (D) Week 2 of high concentrate, SEM = 0.12. (E) Week 3 of high concentrate, SEM = 0.12. (F) Week 4 of high concentrate, SEM = 0.12. p-Values: Time < 0.01; Week < 0.01; Time × Week < 0.01. SEM: The largest standard error of the mean.
). (A) Week 0 forage, SEM = 0.05. (B) Day 4 of adaptation week, 40% concentrate, SEM = 0.08. (C) Week 1 of high concentrate, SEM = 0.09. (D) Week 2 of high concentrate, SEM = 0.12. (E) Week 3 of high concentrate, SEM = 0.12. (F) Week 4 of high concentrate, SEM = 0.12. p-Values: Time < 0.01; Week < 0.01; Time × Week < 0.01. SEM: The largest standard error of the mean.
 ). (A) Week 0 forage, SEM = 0.05. (B) Day 4 of adaptation week, 40% concentrate, SEM = 0.08. (C) Week 1 of high concentrate, SEM = 0.09. (D) Week 2 of high concentrate, SEM = 0.12. (E) Week 3 of high concentrate, SEM = 0.12. (F) Week 4 of high concentrate, SEM = 0.12. p-Values: Time < 0.01; Week < 0.01; Time × Week < 0.01. SEM: The largest standard error of the mean.
). (A) Week 0 forage, SEM = 0.05. (B) Day 4 of adaptation week, 40% concentrate, SEM = 0.08. (C) Week 1 of high concentrate, SEM = 0.09. (D) Week 2 of high concentrate, SEM = 0.12. (E) Week 3 of high concentrate, SEM = 0.12. (F) Week 4 of high concentrate, SEM = 0.12. p-Values: Time < 0.01; Week < 0.01; Time × Week < 0.01. SEM: The largest standard error of the mean.
| Item | Diet and Treatment 1 | ||
|---|---|---|---|
| Forage Diet | High Concentrate Diet | ||
| CON | PHY | ||
| Ingredients (% of DM) | |||
| Grass silage | 75 | 26.25 | 26.25 |
| Corn silage | 15 | 8.75 | 8.75 |
| Grass hay | 10 | 0 | 0 |
| CONTROL concentrate 1 | 0 * | 65 | 0 |
| TREATMENT concentrate 2 | 0 * | 0 | 65 |
| Chemical composition (% of DM unless stated) | |||
| DM, % as fresh | 32.4 ± 5.16 | 45.1 ± 0.83 | 44.0 ± 2.09 |
| Crude protein (CP) | 17.2 ± 1.08 | 19.6 ± 0.80 | 19.3 ± 1.15 |
| Neutral detergent fiber (NDF) | 50.4 ± 1.58 | 30.2 ± 2.09 | 31.6 ± 2.44 |
| Acid detergent fiber (ADF) | 36.6 ± 6.39 | 19.9 ± 2.12 | 20.0 ± 1.89 |
| Starch | 4.2 ± 1.3 | 28.9 ± 1.85 | 28.0 ± 1.59 |
| Ether extract (EE) | 2.9 ± 1.32 | 3.2 ± 0.16 | 3.2 ± 0.21 |
| Non-fiber carbohydrates | 18.4 ± 0.47 | 39.5 ± 1.85 | 39.0 ± 1.83 |
| Ash | 11.0 ± 1.87 | 6.8 ± 0.26 | 6.7 ± 0.25 |
| Particle fraction (% retained) 3 | |||
| Long | 86.7 | 27.8 ± 4.95 | 29.2 ± 6.57 |
| Medium | 5.54 | 29.3 ± 1.74 | 29.7 ± 2.55 |
| Short | 7.30 | 20.3 ± 2.20 | 18.8 ± 3.21 |
| Fine | 0.50 | 1.4 ± 0.93 | 1.1 ± 0.80 |
| pef 4 > 8 mm | 0.92 | 0.6 ± 0.02 | 0.6 ± 0.04 |
| peNDF 5 > 8 mm, % of DM | 47.5 | 17.3 ± 0.71 | 18.6 ± 0.25 |
| Item | Forage Diet Week 0 | High Concentrate Week 1 | High Concentrate Week 2 | High Concentrate Week 3 | High Concentrate Week 4 | p-Values 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | PHY | CON | PHY | CON | PHY | CON | PHY | CON | PHY | SEM 2 | D | T | I | |
| DMI, kg | 8.43 | 7.57 | 13.31 | 12.85 | 13.93 | 13.09 | 14.09 | 13.32 | 12.91 | 11.77 | 0.602 | <0.01 | 0.08 | 0.23 |
| Maximum pH | 6.89 | 6.82 | 6.64 | 6.60 | 6.71 | 6.66 | 6.60 | 6.64 | 6.59 | 6.66 | 6.7 × 10−11 | <0.01 | 0.76 | 0.10 |
| Minimum pH | 6.30 | 6.31 | 5.61 | 5.60 | 5.50 | 5.44 | 5.52 b | 5.66 a | 5.51 b | 5.67 a | 9.2 × 10−10 | <0.01 | 0.12 | <0.01 |
| Mean pH | 6.58 | 6.56 | 6.04 | 6.05 | 6.03 | 6.00 | 6.02 b | 6.15 a | 6.02 b | 6.16 a | 2.8 × 10−10 | <0.01 | 0.17 | <0.05 |
| Difference * | 0.55 a | 0.48 b | 1.01 | 0.99 | 1.22 | 1.22 | 1.05 | 0.96 | 1.07 | 0.99 | 0.042 | <0.01 | <0.05 | <0.01 |
| Dur 4 6.0, min * | 2.45 | 0.31 | 581.2 | 651.9 | 620.5 | 662.4 | 538.3 a | 364.2 b | 653.1 a | 410.9 b | 2.12 | <0.01 | <0.05 | <0.01 |
| Dur 4 5.8, min * | 1.27 | 0.62 | 239.6 | 244.2 | 304.7 | 349.1 | 286.8 a | 148.4 b | 330.1 x | 195.5 y | 2.64 | <0.01 | 0.07 | <0.05 |
| Area 6.0, min × pH * | 1.13 | 0.01 | 143.6 | 115.6 | 169.0 | 184.3 | 108.3 a | 49.9 b | 146.9 a | 69.67 b | 1.48 | <0.01 | <0.05 | <0.01 |
| Area 5.8, min × pH * | 0.20 | 0.00 | 68.06 | 38.89 | 92.14 | 89.48 | 51.87 x | 23.80 y | 70.85 | 42.72 | 1.29 | <0.01 | <0.05 | <0.05 |
| Acidosis index, area pH < 5.8/kg DMI * | 0.01 | 0.00 | 5.16 | 3.18 | 7.21 | 7.44 | 4.75 a | 1.75 b | 6.99 | 3.08 | 0.16 | <0.01 | <0.05 | <0.05 |
| Acidosis index, time pH < 5.8/kg DMI * | 0.09 | 0.02 | 17.88 | 18.60 | 22.82 | 27.00 | 20.92 a | 10.83 b | 30.93 a | 13.20 b | 0.25 | <0.01 | <0.05 | <0.01 |
| Item | Forage Diet Week 0 | High Concentrate Week 1 | High Concentrate Week 2 | High Concentrate Week 3 | High Concentrate Week 4 | p-Values 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | PHY | CON | PHY | CON | PHY | CON | PHY | CON | PHY | SEM 2 | D | T | I | |
| Total SCFA concentration, mM | 86.0 | 84.0 | 105 | 108 | 116 | 118 | 106 | 98.0 | 112 | 114 | 4.98 | <0.01 | 0.82 | 0.71 |
| % of total SCFA | ||||||||||||||
| Acetate | 67.4 | 67.8 | 58.1 | 56.9 | 52.6 b | 55.4 a | 57.8 | 57.1 | 56.9 | 57.9 | 0.76 | <0.01 | 0.57 | <0.01 |
| Propionate | 15.6 | 15.1 | 20.5 | 21.6 | 29.5 a | 24.1 b | 23.9 | 23.3 | 23.7 | 23.2 | 0.70 | <0.01 | 0.08 | <0.01 |
| Butyrate | 10.5 | 10.8 | 16.0 | 16.7 | 12.0 b | 14.0 a | 12.6 | 12.9 | 12.8 | 12.8 | 0.60 | <0.01 | 0.16 | 0.17 |
| Isobutyrate | 1.70 | 1.80 | 0.84 | 0.90 | 0.80 b | 1.00 a | 1.15 | 1.18 | 1.23 | 1.11 | 0.07 | <0.01 | 0.49 | 0.01 |
| Isovalerate | 2.17 | 2.29 | 1.27 | 1.30 | 1.43 b | 1.90 a | 1.65 b | 2.08 a | 1.92 | 1.78 | 0.08 | <0.01 | <0.05 | <0.01 |
| Valerate | 1.48 | 1.45 | 1.90 | 1.98 | 2.22 | 2.19 | 2.01 | 2.06 | 2.29 | 2.37 | 0.08 | <0.01 | 0.70 | 0.87 |
| Ratio of acetate to propionate | 4.39 | 4.54 | 2.86 | 2.77 | 1.90 b | 2.60 a | 2.46 | 2.67 | 2.37 | 2.68 | 0.15 | <0.01 | 0.20 | <0.01 |
| Ammonia, mg/dL | 21.72 | 20.16 | 13.53 | 14.30 | 9.64 b | 15.35 a | 17.66 a | 13.12 b | 18.26 | 18.77 | 1.61 | <0.01 | 0.88 | <0.01 |
| Lactate 4 | ||||||||||||||
| D-lactate, mM | 0.037 b | 0.062 a | 0.433 | 0.347 | 0.386 | 0.317 | 1.053 a | 0.765 b | 0.718 | 0.669 | 0.0027 | <0.01 | 0.35 | <0.01 |
| L-lactate, mM | 0.062 | 0.131 | 0.259 | 0.260 | 0.293 | 0.231 | 0.372 | 0.408 | 0.363 | 0.386 | 0.0010 | <0.01 | 0.29 | <0.01 |
| Total lactate, mM | 0.118 | 0.218 | 0.711 | 0.618 | 0.714 | 0.558 | 1.539 x | 1.183 y | 1.107 | 1.089 | 0.0033 | <0.01 | 0.46 | <0.01 |
| Item 4 | Forage Diet Week 0 | High Concentrate Week 1 | High Concentrate Week 2 | High Concentrate Week 3 | High Concentrate Week 4 | p-Values 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | PHY | CON | PHY | CON | PHY | CON | PHY | CON | PHY | SEM 2 | D | T | I | |
| Hp, μg/mL | 103.0 | 141.9 | 142.3 | 308.6 | 257.9 | 452.0 | 621.2 a | 90.6 b | 429.4 x | 96.4 y | 1.82 | 0.12 | 0.46 | 0.19 |
| SAA, μg/mL | 2.83 | 2.86 | 10.25 | 15.04 | 19.61 | 29.85 | 28.67 a | 8.87 b | 12.7 | 9.14 | 1.53 | <0.01 | 0.60 | 0.23 |
| ALP, U/L | 7.70 | 7.10 | 7.43 | 8.10 | 7.59 | 8.33 | 8.49 | 8.20 | 6.78 | 7.33 | 1.10 | 0.50 | 0.73 | 0.27 |
| AST, U/L | 67.85 | 72.80 | 63.21 | 66.40 | 76.28 | 86.98 | 98.06 | 94.92 | 85.31 | 98.12 | 1.08 | <0.01 | 0.32 | <0.01 |
| GLDH, U/L | 4.80 | 6.01 | 5.56 | 5.22 | 9.94 | 10.87 | 13.26 | 12.17 | 10.86 | 11.67 | 1.20 | <0.01 | 0.78 | <0.01 |
| GGT, U/L | 21.21 | 20.99 | 21.56 | 20.89 | 25.64 | 25.11 | 30.78 | 25.64 | 29.26 | 29.59 | 1.09 | <0.01 | 0.57 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Chacon, R.; Castillo-Lopez, E.; Ricci, S.; Petri, R.M.; Reisinger, N.; Zebeli, Q. Supplementing a Phytogenic Feed Additive Modulates the Risk of Subacute Rumen Acidosis, Rumen Fermentation and Systemic Inflammation in Cattle Fed Acidogenic Diets. Animals 2022, 12, 1201. https://doi.org/10.3390/ani12091201
Rivera-Chacon R, Castillo-Lopez E, Ricci S, Petri RM, Reisinger N, Zebeli Q. Supplementing a Phytogenic Feed Additive Modulates the Risk of Subacute Rumen Acidosis, Rumen Fermentation and Systemic Inflammation in Cattle Fed Acidogenic Diets. Animals. 2022; 12(9):1201. https://doi.org/10.3390/ani12091201
Chicago/Turabian StyleRivera-Chacon, Raul, Ezequias Castillo-Lopez, Sara Ricci, Renee M. Petri, Nicole Reisinger, and Qendrim Zebeli. 2022. "Supplementing a Phytogenic Feed Additive Modulates the Risk of Subacute Rumen Acidosis, Rumen Fermentation and Systemic Inflammation in Cattle Fed Acidogenic Diets" Animals 12, no. 9: 1201. https://doi.org/10.3390/ani12091201






