Effect of Acute Melatonin Injection on Metabolomic and Testicular Artery Hemodynamic Changes and Circulating Hormones in Shiba Goats under Sub-Tropical Environmental Conditions
Abstract
:Simple Summary
Abstract
1. Introduction
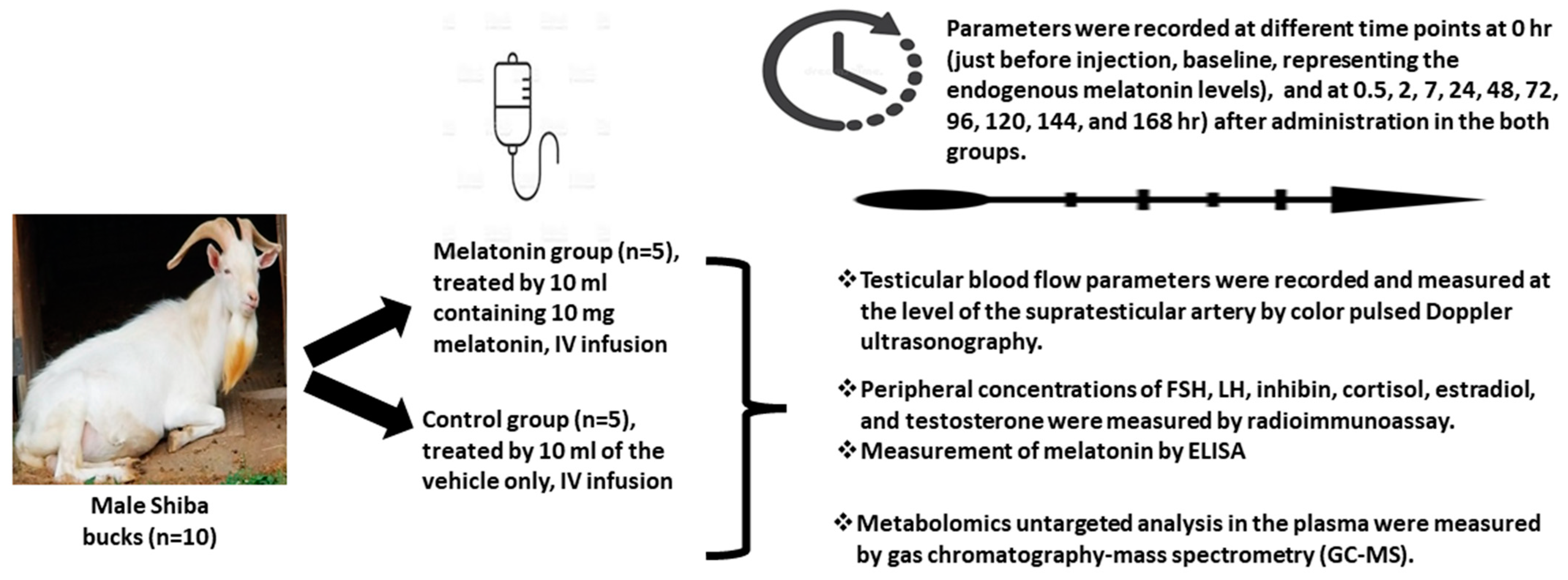
2. Materials and Methods
2.1. Animals and Management
2.2. Experimental Design
How to Prepare and Inject Melatonin Intravenously?
2.3. Triplex Ultrasonographic Examinations
2.4. Blood Sampling and Hormonal Analysis
2.5. Metabolomic Analysis
2.6. Statistical Analysis
3. Results
3.1. Testicular Artery Hemodynamics
3.2. Circulating Hormones
3.3. Metabolomic Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samir, H.; Radwan, F.; El-Khawagah, A.R.; Kandiel, M.; El Sayed, M.A.; Elfadadny, A.; Karen, A.; El-Sherbiny, H.R. Ultrasonography and computer-assisted assessment of postpartum uterine echotexture and its relationship with peripheral oxidative stress biomarkers in goats. Small Rumin. Res. 2023, 221, 106947. [Google Scholar] [CrossRef]
- Samir, H.; El-Sherbiny, H.; El-Shalofy, A. Seasonal alterations in testicular hemodynamics and echotexture in relation to semen quality in buffalo bulls. Andrologia 2023, 2023, el5003366. [Google Scholar] [CrossRef]
- Ahmad Para, I.; Ahmad Dar, P.; Ahmad Malla, B.; Punetha, M.; Rautela, A.; Maqbool, I.; Mohd, A.; Ahmad Shah, M.; Ahmad War, Z.; Ishaaq, R. Impact of heat stress on the reproduction of farm animals and strategies to ameliorate it. Biol. Rhyth. Res. 2020, 51, 616–632. [Google Scholar] [CrossRef]
- Samir, H.; ElSayed, M.I.; Radwan, F.; Hedia, M.; Hendawy, H.; Hendawy, A.O.; Elbadawy, M.; Watanabe, G. An updated insight on testicular hemodynamics: Environmental, physiological, and technical perspectives in farm and companion animals. Vet. Res. Commun. 2022, 47, 323–345. [Google Scholar] [CrossRef] [PubMed]
- Samir, H.; Nyametease, P.; Nagaoka, K.; Watanabe, G. Effect of seasonality on testicular blood flow as determined by color Doppler ultrasonography and hormonal profiles in Shiba goats. Anim. Reprod. Sci. 2018, 197, 185–192. [Google Scholar] [CrossRef]
- El-Sherbiny, H.R.; Abdelnaby, E.A.; El-Shahat, K.; Salem, N.Y.; Ramadan, E.S.; Yehia, S.G.; Fathi, M. Coenzyme Q10 Supplementation enhances testicular volume and hemodynamics, reproductive hormones, sperm quality, and seminal antioxidant capacity in goat bucks under summer hot humid conditions. Vet. Res. Commun. 2022, 46, 1245–1257. [Google Scholar] [CrossRef]
- El-Sherbiny, H.R.; El-Shalofy, A.S.; Samir, H. Exogenous L-carnitine administration ameliorates the adverse effects of heat stress on testicular hemodynamics, echotexture, and total antioxidant capacity in rams. Front. Vet. Sci. 2022, 9, 860771. [Google Scholar] [CrossRef]
- Setchell, B.; Bergh, A.; Widmark, A.; Damber, J.E. Effect of testicular temperature on vasomotion and blood flow. Int. J. Androl. 1995, 18, 120–126. [Google Scholar] [CrossRef]
- Setchell, B.P. The parkes lecture heat and the testis. J. Reprod. Fertil. 1998, 114, 179–194. [Google Scholar] [CrossRef]
- Kastelic, J.; Wilde, R.; Rizzoto, G.; Thundathil, J. Hyperthermia and not hypoxia may reduce sperm motility and morphology following testicular hyperthermia. Vet. Med. 2017, 62, 437–4420. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef]
- Delgadillo, J.; Velez, L.; Flores, J. Continuous light after a long-day treatment is equivalent to melatonin implants to stimulate testosterone secretion in Alpine male goats. Animal 2016, 10, 649–654. [Google Scholar] [CrossRef]
- Gallego-Calvo, L.; Gatica, M.; Santiago-Moreno, J.; Guzmán, J.; Zarazaga, L. Exogenous melatonin does not improve the freezability of Blanca Andaluza goat semen over exposure to two months of short days. Anim. Reprod. Sci. 2015, 157, 24–32. [Google Scholar] [CrossRef]
- Dönmez, N.; Karaca, F.; Belge, F.; Ateş, C.T. The effects of melatonin application on some haematological parameters and thyroid hormones and testosterone in male goats’ non-breeding season. Vet. Arhiv 2004, 74, 281–287. [Google Scholar]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Lee, M.Y.; Kuan, Y.H.; Chen, H.Y.; Chen, T.Y.; Chen, S.T.; Huang, C.C.; Yang, I.P.; Hsu, Y.S.; Wu, T.S.; Lee, E.J. Intravenous administration of melatonin reduces the intracerebral cellular inflammatory response following transient focal cerebral ischemia in rats. J. Pineal Res. 2007, 42, 297–309. [Google Scholar] [CrossRef]
- Vargas, Á.; Bustos-Obregón, E.; Hartley, R. Effects of hypoxia on epididymal sperm parameters and protective role of ibuprofen and melatonin. Biol. Res. 2011, 44, 161–167. [Google Scholar] [CrossRef]
- Yuan, X.C.; Wang, P.; Li, H.W.; Wu, Q.B.; Zhang, X.Y.; Li, B.W.; Xiu, R.J. Effects of melatonin on spinal cord injury-induced oxidative damage in mice testis. Andrologia 2017, 49, e12692. [Google Scholar] [CrossRef]
- Ali, T.; Rehman, S.U.; Shah, F.A.; Kim, M.O. Acute dose of melatonin via Nrf2 dependently prevents acute ethanol-induced neurotoxicity in the developing rodent brain. J. Neuroinflamm. 2018, 15, 119. [Google Scholar] [CrossRef] [PubMed]
- Goldansaz, S.A.; Guo, A.C.; Sajed, T.; Steele, M.A.; Plastow, G.S.; Wishart, D.S. Livestock metabolomics and the livestock metabolome: A systematic review. PLoS ONE 2017, 12, e0177675. [Google Scholar] [CrossRef] [PubMed]
- El-Tarabany, M.S.; El-Tarabany, A.A.; Atta, M.A. Physiological and lactation responses of Egyptian dairy Baladi goats to natural thermal stress under subtropical environmental conditions. Int. J. Biometeorol. 2017, 61, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Kendall, P.; Webster, J. Season and physiological status affects the circadian body temperature rhythm of dairy cows. Livestock Sci. 2009, 125, 155–160. [Google Scholar] [CrossRef]
- Andersen, L.P.; Werner, M.U.; Rosenkilde, M.M.; Fenger, A.Q.; Petersen, M.C.; Rosenberg, J.; Gögenur, I. Pharmacokinetics of high-dose intravenous melatonin in humans. J. Clin. Pharmacol. 2016, 56, 324–329. [Google Scholar] [CrossRef]
- Andersen, L.P.; Werner, M.U.; Rosenkilde, M.M.; Harpsøe, N.G.; Fuglsang, H.; Rosenberg, J.; Gögenur, I. Pharmacokinetics of oral and intravenous melatonin in healthy volunteers. BMC Pharmacol. Toxicol. 2016, 17, 8. [Google Scholar] [CrossRef]
- Zetner, D.; Andersen, L.P.K.; Alder, R.; Jessen, M.L.; Tolstrup, A.; Rosenberg, J. Pharmacokinetics and safety of intravenous, intravesical, rectal, transdermal, and vaginal melatonin in healthy female volunteers: A cross-over study. Pharmacology 2021, 106, 169–176. [Google Scholar] [CrossRef]
- Yellon, S.M.; Hilliker, S. Influence of acute melatonin treatment and light on the circadian melatonin rhythm in the Djungarian hamster. J. Biol. Rhythms 1994, 9, 71–81. [Google Scholar] [CrossRef]
- Samir, H.; Sasaki, K.; Ahmed, E.; Karen, A.; Nagaoka, K.; El Sayed, M.; Taya, K.; Watanabe, G. Effect of a single injection of gonadotropin-releasing hormone (GnRH) and human chorionic gonadotropin (hCG) on testicular blood flow measured by color doppler ultrasonography in male Shiba goats. J. Vet. Med. Sci. 2015, 77, 549–556. [Google Scholar] [CrossRef]
- Samir, H.; Nyametease, P.; Elbadawy, M.; Fathi, M.; Mandour, A.S.; Radwan, F.; Nagaoka, K.; Sasaki, K.; Watanabe, G. Assessment of correlations and concentrations of salivary and plasma steroids, testicular morphometry, and semen quality in different climatic conditions in goats. Theriogenology 2020, 157, 238–244. [Google Scholar] [CrossRef]
- Blanco, P. Volumetric blood flow measurement using Doppler ultrasound: Concerns about the technique. J. Ultrasound 2015, 18, 201–204. [Google Scholar] [CrossRef]
- Araki, K.; Arai, K.Y.; Watanabe, G.; Taya, K. Involvement of inhibin in the regulation of follicle-stimulating hormone secretion in the young adult male Shiba Goat. J. Androl. 2000, 21, 558–565. [Google Scholar]
- Mori, Y.; Kano, Y. Changes in plasma concentrations of LH, progesterone and oestradiol in relation to the occurrence of luteolysis, oestrus and time of ovulation in the Shiba goat (Capra hircus). J. Reprod. Fertil. 1984, 72, 223–230. [Google Scholar] [CrossRef]
- Hamada, T.; Watanabe, G.; Kokuho, T.; Taya, K.; Sasamoto, S.; Hasegawa, Y.; Miyamoto, K.; Igarashi, M. Radioimmunoassay of inhibin in various mammals. J. Endocrinol. 1989, 122, 697–704. [Google Scholar] [CrossRef]
- Taya, K. Radioimmunoassay for progesterone, testosterone, and estradiol-17β using 125 I-iodohistamine radioligands. Jpn. J. Anim. Reprod. 1985, 31, 186–197. [Google Scholar] [CrossRef]
- Arai, K.; Watanabe, G.; Fujimoto, M.; Nagata, S.; Takemura, Y.; Taya, K.; Sasamoto, S. A sensitive radioimmunoassay for cortisol using 125I-labeled radioligand. J. Reprod. Dev. 1995, 41, 15–20. [Google Scholar] [CrossRef]
- Pu, S.; Usuda, K.; Nagaoka, K.; Watanabe, G. Heat challenge influences serum metabolites concentrations and liver lipid metabolism in Japanese quail (Coturnix japonica). J. Vet. Med. Sci. 2019, 81, 77–83. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef]
- Kusama, K.; Bai, R.; Matsuno, Y.; Ideta, A.; Sakurai, T.; Nagaoka, K.; Hori, M.; Imakawa, K. Characterization of Serum Metabolome and Proteome Profiles Identifies SNX5 Specific for Pregnancy Failure in Holstein Heifers. Life 2022, 12, 309. [Google Scholar] [CrossRef]
- Samir, H.; Radwan, F.; Watanabe, G. Advances in applications of color Doppler ultrasonography in the andrological assessment of domestic animals: A review. Theriogenology 2021, 161, 252–261. [Google Scholar] [CrossRef]
- Samir, H.; Nyametease, P.; Elbadawy, M.; Nagaoka, K.; Sasaki, K.; Watanabe, G. Administration of melatonin improves testicular blood flow, circulating hormones, and semen quality in Shiba goats. Theriogenology 2020, 146, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Salama, A.; El-Shahat, K.; EL-Sherbiny, H.R.; Abdelnaby, E.A. Effect of melatonin supplementation during IVM of dromedary camel oocytes (Camelus dromedarius) on their maturation, fertilization, and developmental rates in vitro. Theriogenology 2021, 172, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Sewerynek, E. Melatonin and the cardiovascular system. Neuro. Endocrinol. Lett. 2002, 23, 79–83. [Google Scholar] [PubMed]
- Reiter, R.J.; Tan, D.-X.; Paredes, S.D.; Fuentes-Broto, L. Beneficial effects of melatonin in cardiovascular disease. Ann. Med. 2010, 42, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Ozkalayci, F.; Kocabas, U.; Altun, B.U.; Pandi-Perumal, S.; Altun, A. Relationship between melatonin and cardiovascular disease. Cureus 2021, 13, e12935. [Google Scholar] [CrossRef]
- Rosenfeld, C.R.; Roy, T.; Cox, B.E. Mechanisms modulating estrogen-induced uterine vasodilation. Vascul. Pharmacol. 2002, 38, 115–125. [Google Scholar] [CrossRef]
- Bollwein, H.; Schulze, J.J.; Miyamoto, A.; Sieme, H. Testicular blood flow and plasma concentrations of testosterone and total estrogen in the stallion after the administration of human chorionic gonadotropin. J. Reprod. Dev. 2008, 54, 335–339. [Google Scholar] [CrossRef]
- Odawara, H.; Iwasaki, T.; Horiguchi, J.; Rokutanda, N.; Hirooka, K.; Miyazaki, W.; Koibuchi, Y.; Shimokawa, N.; Iino, Y.; Takeyoshi, I. Activation of aromatase expression by retinoic acid receptor-related orphan receptor (ROR) α in breast cancer cells: Identification of a novel ROR response element. J. Biol. Chem. 2009, 284, 17711–17719. [Google Scholar] [CrossRef]
- Cardinali, D. Molecular biology of melatonin: Assessment of the “microtubule hypothesis of melatonin action”. In Melatonin: Current Status and Perspectives; Elsevier: Amsterdam, The Netherlands, 1981; pp. 247–256. [Google Scholar] [CrossRef]
- Benítez-King, G.; Ríos, A.; Martínez, A.; Antón-Tay, F. In vitro inhibition of Ca2+/calmodulin-dependent kinase II activity by melatonin. Biochim. Biophys Acta 1996, 1290, 191–196. [Google Scholar] [CrossRef]
- Pévet, P. Melatonin in animal models. Dialogues Clin. Neurosci. 2003, 5, 343–352. [Google Scholar] [CrossRef]
- Bothorel, B.; Barassin, S.; Saboureau, M.; Perreau, S.; Vivien-Roels, B.; Malan, A.; Pévet, P. In the rat, exogenous melatonin increases the amplitude of pineal melatonin secretion by a direct action on the circadian clock. Euro. J. Neurosci. 2002, 16, 1090–1098. [Google Scholar] [CrossRef]
- Deng, S.-L.; Zhang, Y.; Yu, K.; Wang, X.-X.; Chen, S.-R.; Han, D.-P.; Cheng, C.Y.; Lian, Z.-X.; Liu, Y.-X. Melatonin up-regulates the expression of the GATA-4 transcription factor and increases testosterone secretion from Leydig cells through RORα signaling in an in vitro goat spermatogonial stem cell differentiation culture system. Oncotarget 2017, 8, 110592. [Google Scholar] [CrossRef]
- Casao, A.; Pérez-Pé, R.; Abecia, J.A.; Forcada, F.; Muiño-Blanco, T.; Cebrián-Pérez, J.Á. The effect of exogenous melatonin during the non-reproductive season on the seminal plasma hormonal profile and the antioxidant defence system of Rasa Aragonesa rams. Anim. Reprod. Sci. 2013, 138, 168–174. [Google Scholar] [CrossRef]
- Rekik, M.; Taboubi, R.; Ben Salem, I.; Fehri, Y.; Sakly, C.; Lassoued, N.; Hilali, M.E. Melatonin administration enhances the reproductive capacity of young rams under a southern Mediterranean environment. Anim. Sci. J. 2015, 86, 666–672. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Krishna, A. Melatonin affects steroidogenesis and delayed ovulation during winter in vespertilionid bat, Scotophilus heathi. J. Steroid Biochem. Mol. Biol. 2010, 118, 107–116. [Google Scholar] [CrossRef]
- Rosa, H.; Juniper, D.; Bryant, M. Effects of recent sexual experience and melatonin treatment of rams on plasma testosterone concentration, sexual behaviour and ability to induce ovulation in seasonally anoestrous ewes. J. Reprod. Fertil. 2000, 120, 169–176. [Google Scholar] [CrossRef]
- Juszczak, M.; Michalska, M. The effect of melatonin on prolactin, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) synthesis and secretion. Postepy Hig. Med. Dosw. 2006, 60, 431–438. [Google Scholar]
- Portaluppi, F.; Vergnani, L.; Manfredini, R.; Fersini, C. Endocrine mechanisms of blood pressure rhythms. Ann. N. Y. Acad. Sci. 1996, 783, 113–131. [Google Scholar] [CrossRef]
- Portaluppi, F.; Boari, B.; Manfredini, R. Oxidative stress in essential hypertension. Curr. Pharm. Des. 2004, 10, 1695–1698. [Google Scholar] [CrossRef]
- Okatani, Y.; Watanabe, K.; Hayashi, K.; Wakatsuki, A.; Sagara, Y. Melatonin suppresses vasospastic effect of hydrogen peroxide in human umbilical artery: Relation to calcium influx. J. Pineal Res. 1997, 22, 232–237. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tamura, H.; Kashida, S.; Takayama, H.; Yamagata, Y.; Karube, A.; Sugino, N.; Kato, H. Changes of serum melatonin level and its relationship to feto-placental unit during pregnancy. J. Pineal Res. 2001, 30, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Galdames, H.A.; Torres-Farfan, C.; Spichiger, C.; Mendez, N.; Abarzua-Catalan, L.; Alonso-Vazquez, P.; Richter, H.G. Impact of gestational chronodisruption on fetal cardiac genomics. J. Mol. Cell. Cardiol. 2014, 66, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chaves, I.; van der Eerden, B.; Boers, R.; Boers, J.; Streng, A.A.; Ridwan, Y.; Schreuders-Koedam, M.; Vermeulen, M.; van der Pluijm, I.; Essers, J. Gestational jet lag predisposes to later-life skeletal and cardiac disease. Chronobiol. Int. 2019, 36, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, G.; De, K.; Maity, S.; Bandyopadhyay, D.; Bhattacharya, S.; Reiter, R.J.; Bandyopadhyay, A. Melatonin protects against oxidative damage and restores expression of GLUT4 gene in the hyperthyroid rat heart. J. Pineal Res. 2007, 42, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Durante, W. The emerging role of l-glutamine in cardiovascular health and disease. Nutrients 2019, 11, 2092. [Google Scholar] [CrossRef]
- Hussein, H.A.; Hassaneen, A.S.; Ali, M.E.; Sindi, R.A.; Ashour, A.M.; Fahmy, S.M.; Swelum, A.A.; Ahmed, A.E. The Impact of Rumen-Protected L-Arginine Oral Supplementation on Libido, Semen Quality, Reproductive Organ Biometry, and Serum Biochemical Parameters of Rams. Front. Vet. Sci. 2022, 9, 899434. [Google Scholar] [CrossRef]
- Burggraaf, J.; Schoemaker, R.; Lentjes, E.; Cohen, A. Sorbitol as a marker for drug-induced decreases of variable duration in liver blood flow in healthy volunteers. Euro. J. Pharm. Sci. 2000, 12, 133–139. [Google Scholar] [CrossRef]
- Samanta, P.K.; Manna, I.; Jana, K. Effect of L-ascorbic acid supplementation on testicular oxidative stress and endocrine disorders in mature male rats exposed to intensive swimming exercise. Reprod. Med. Biol. 2006, 5, 145–153. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samir, H.; Mandour, A.S.; Radwan, F.; Ahmed, A.E.; Momenah, M.A.; Aldawood, N.A.; Yoshida, T.; Watanabe, G.; El-Sherbiny, H.R. Effect of Acute Melatonin Injection on Metabolomic and Testicular Artery Hemodynamic Changes and Circulating Hormones in Shiba Goats under Sub-Tropical Environmental Conditions. Animals 2023, 13, 1794. https://doi.org/10.3390/ani13111794
Samir H, Mandour AS, Radwan F, Ahmed AE, Momenah MA, Aldawood NA, Yoshida T, Watanabe G, El-Sherbiny HR. Effect of Acute Melatonin Injection on Metabolomic and Testicular Artery Hemodynamic Changes and Circulating Hormones in Shiba Goats under Sub-Tropical Environmental Conditions. Animals. 2023; 13(11):1794. https://doi.org/10.3390/ani13111794
Chicago/Turabian StyleSamir, Haney, Ahmed S. Mandour, Faten Radwan, Ahmed Ezzat Ahmed, Maha Abdullah Momenah, Nouf Arkan Aldawood, Tomihiko Yoshida, Gen Watanabe, and Hossam R. El-Sherbiny. 2023. "Effect of Acute Melatonin Injection on Metabolomic and Testicular Artery Hemodynamic Changes and Circulating Hormones in Shiba Goats under Sub-Tropical Environmental Conditions" Animals 13, no. 11: 1794. https://doi.org/10.3390/ani13111794
APA StyleSamir, H., Mandour, A. S., Radwan, F., Ahmed, A. E., Momenah, M. A., Aldawood, N. A., Yoshida, T., Watanabe, G., & El-Sherbiny, H. R. (2023). Effect of Acute Melatonin Injection on Metabolomic and Testicular Artery Hemodynamic Changes and Circulating Hormones in Shiba Goats under Sub-Tropical Environmental Conditions. Animals, 13(11), 1794. https://doi.org/10.3390/ani13111794






