The Effect of Microbial Inoculum and Urea Supplements on Nutritive Value, Amino Acids Profile, Aerobic Stability and Digestibility of Wheat and Corn Silages
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Chemical Analyses of Silages
2.3. Aerobic Stability Assay
2.4. Statistical Analysis
3. Results
3.1. pH of Wheat and Corn Silage
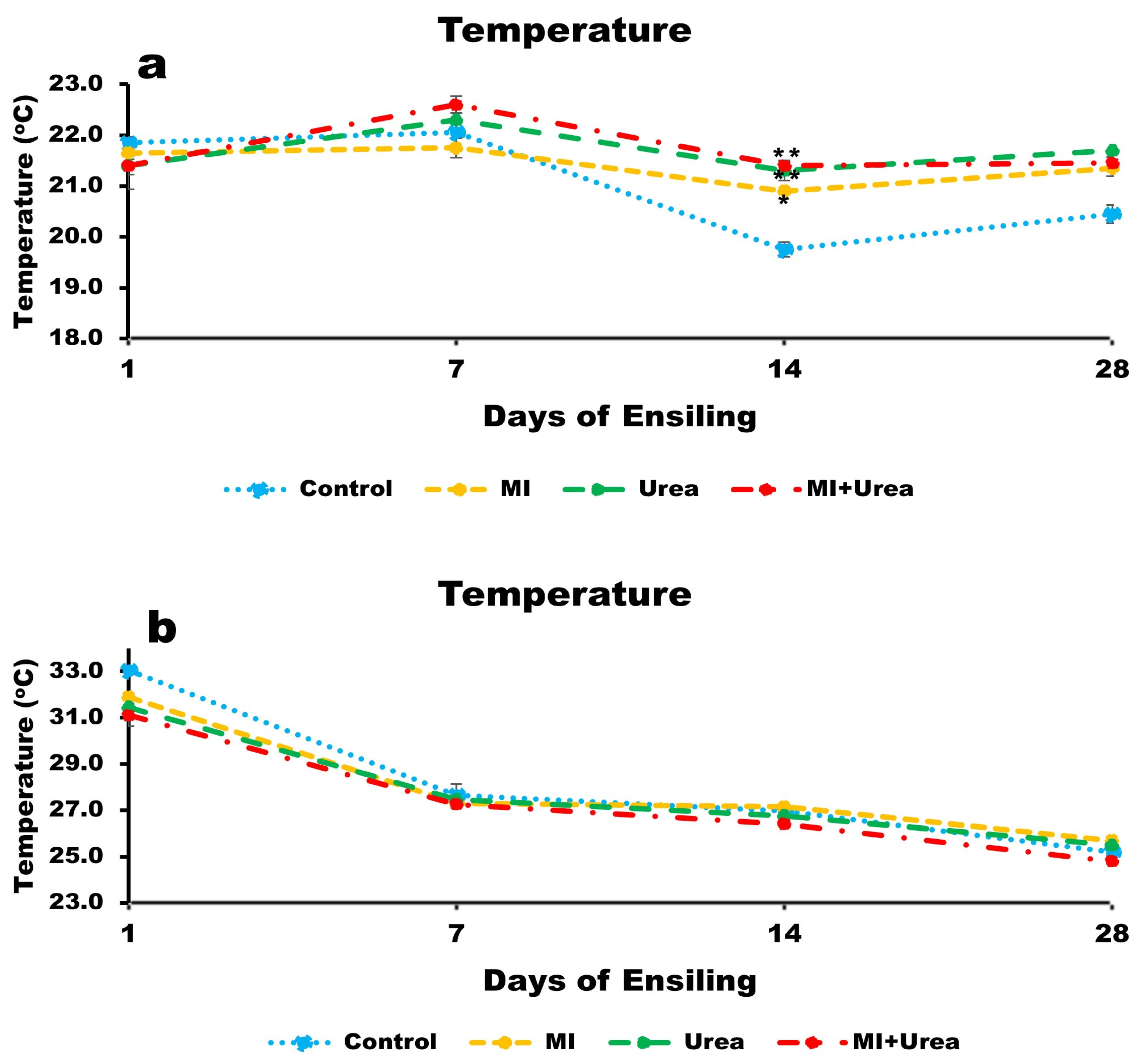
3.2. Temperature of Wheat and Corn Silages
3.3. In Vitro Digestibility of Wheat and Corn Silage
3.3.1. In Vitro DMD of Wheat and Corn Silage
3.3.2. In Vitro NDFD of Wheat and Corn Silage
3.4. Volatile Fatty Acids’ Concentration of Wheat and Corn Silage
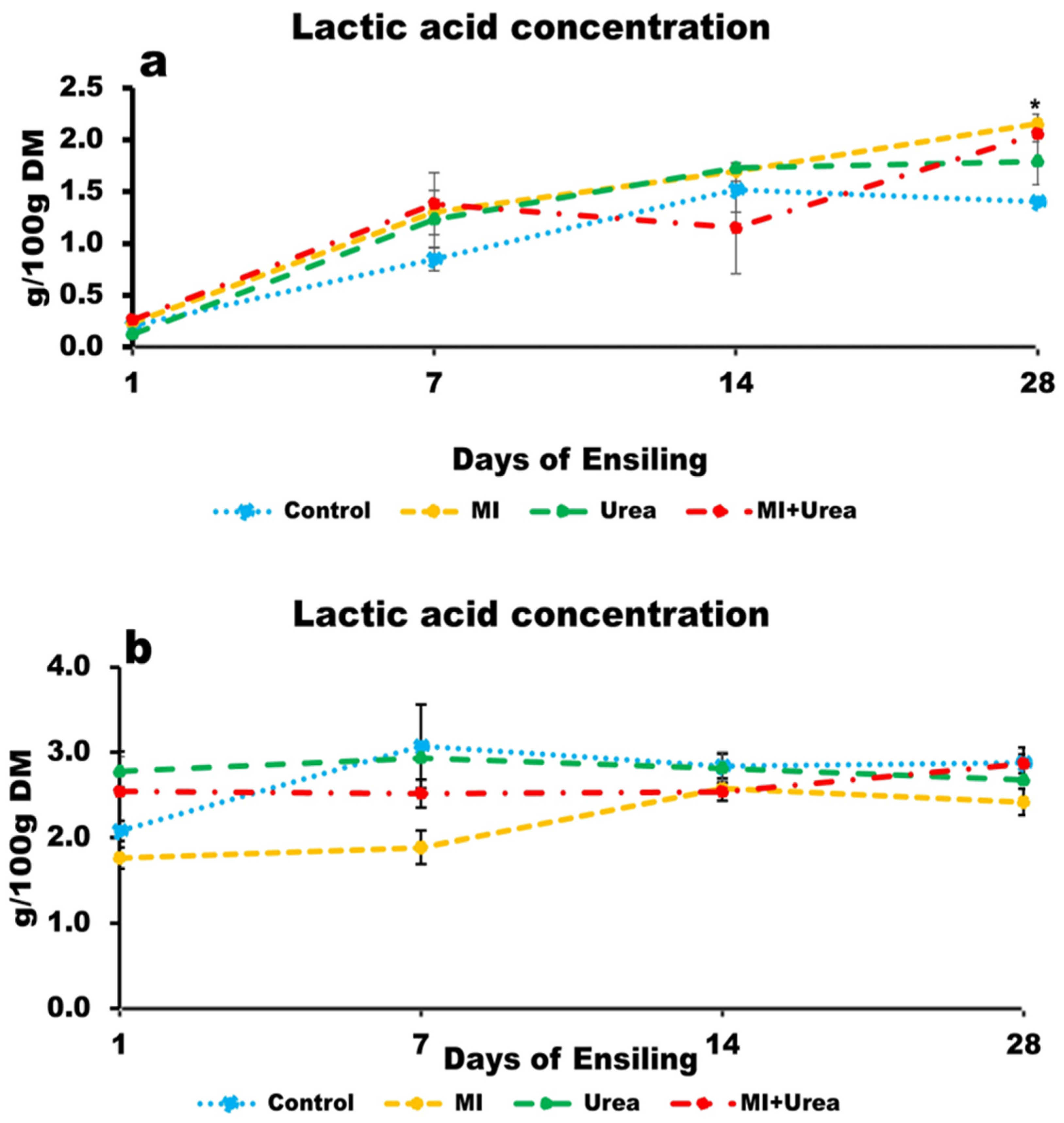
3.5. Lactic Acid Concentration of Wheat and Corn Silage
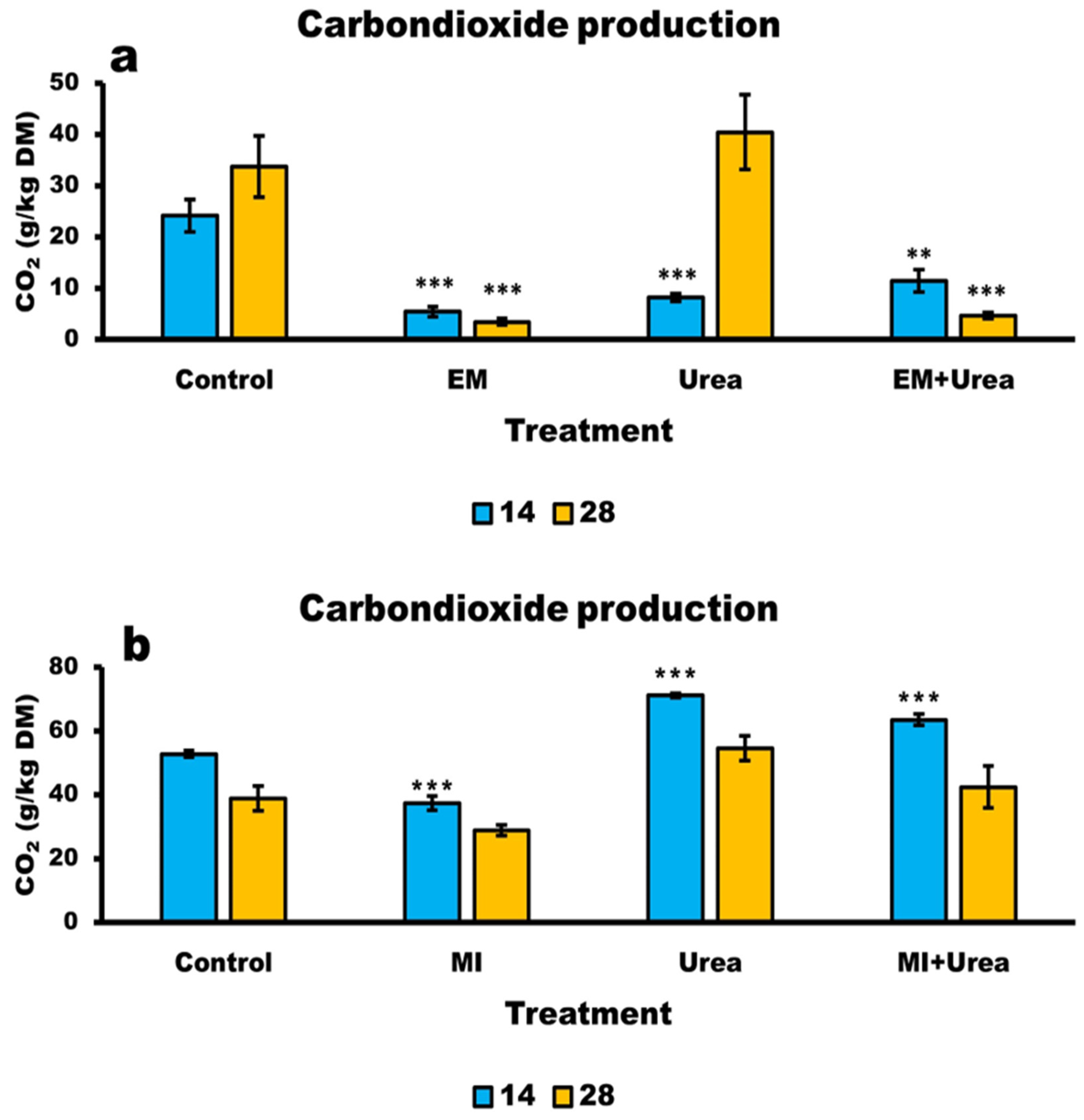
3.6. Aerobic Stability of Wheat and Corn Silage
3.7. Chemical Composition of Wheat and Corn Silages
3.7.1. Crude Protein Content of Wheat and Corn Silages
3.7.2. Cell Wall Carbohydrates and Other Parameters of Wheat and Corn Silages
3.8. Amino Acids Profile of Wheat and Corn Silages
4. Discussion
4.1. Dry Matter of Forages and pH Value of Silages
4.2. Temperature Dynamics in Silages
4.3. In Vitro Digestibility of Silages
4.4. Cell Wall Carbohydrates’ Contents of Silages
4.5. Lactic Acid and Volatile Fatty Acids in Silages
4.6. Aerobic Stability of Silages
4.7. Crude Protein and Amino Acids’ Content in Silages
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolsen, K.K.; Ashbell, G.; Weinberg, Z.G. Silage fermentation and silage additives—Review. Asian Australas. J. Anim. Sci. 1996, 9, 483–494. [Google Scholar] [CrossRef]
- Schroeder, J.W. Silage Fermentation and Preservation. 2004. Available online: https://library.ndsu.edu/ir/bitstream/handle/10365/5102/as1254.pdf?sequence=1 (accessed on 1 June 2004).
- Seglar, B. Fermentation analysis and silage quality testing. In Proceedings of the Minnesota Dairy Health Conference; University of Minnesota: St. Paul, MN, USA, 2003; pp. 119–136. [Google Scholar]
- Pitt, R.E. Silage and Hay Preservation; Natural Resource, Agriculture, and Engineering Service: College Park, MD, USA, 1990; pp. 5–53. [Google Scholar]
- Charmley, E. Towards improved silage quality—A review. Can. J. Anim. Sci. 2001, 81, 157–168. [Google Scholar] [CrossRef]
- Weinberg, Z.G.; Muck, R.E. New trends and opportunities in the development and use of inoculants for silage. FEMS Microbiol. Rev. 1996, 19, 53–68. [Google Scholar] [CrossRef]
- Higa, T.; Parr, J.F. Beneficial and Effective Microorganisms for a Sustainable Agriculture and Environment; International Nature Farming Research Center: Atami, Japan, 1994; Volume 1. [Google Scholar]
- Higa, T.; Wididana, G. The concept and theories of effective microorganisms. In Proceedings of the First International Conference on Kyusei Nature Farming; US Department of Agriculture: Washington, DC, USA, 1991; pp. 118–124. [Google Scholar]
- Hardin, G. The Competitive Exclusion Principle: An idea that took a century to be born has implications in ecology, economics, and genetics. Science 1960, 131, 1292–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higa, T. The technology of effective microorganisms–Concept and philosophy. In Proceedings of the Seminar on the Application of Effective Microorganisms (EM) Techniques in Organic Farming, Organised by the International Society of the Royal Agricultural College, Okinawa, Japan, 25–27 October 2001. [Google Scholar]
- Higa, T. Kyusei Nature Farming and Environmental Management through Effective Microorganisms—The Past, Present and Future. In Proceedings of the Seventh International Conference on Kyusei Nature Farming, Christchurch, New Zealand, 15–18 January 2003. [Google Scholar]
- Higa, T. Effective microorganisms–A holistic technology for humankind. In Proceedings of the Fix International Conference on Kyusei Nature Farming, Atami, Japan, 1–4 September 1999; pp. 19–28. [Google Scholar]
- Cherdthong, A.; Wanapat, M. Development of urea products as rumen slow-release feed for ruminant production: A review. Aust. J. Basic Appl. Sci. 2010, 4, 2232–2241. [Google Scholar]
- Bentley, O.G.; Klosterman, E.W.; Engle, P. The Use of Urea to Increase the Crude Protein Content of Corn Silage for Fattening Steers. 1955. Available online: https://kb.osu.edu/bitstream/handle/1811/63063/1/OARDC_research_bulletin_n0766.pdf (accessed on 22 May 2023).
- Elferink, S.; Driehuis, F.; Gottschal, J.C.; Spoelstra, S.F. Silage Fermentation Processes and Their Manipulation; FAO Plant Production and Protection Paper; Food and Agriculture Organization (FAO): Rome, Italy, 2000; pp. 17–30. [Google Scholar]
- Rogosa, M.; Mitchell, J.A.; Wiseman, R.F. A selective medium for the isolation and enumeration of oral and fecal lactobacilli. J. Bacteriol. 1951, 62, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashbell, G.; Pahlow, G.; Dinter, B.; Weinberg, Z. Dynamics of orange peel fermentation during ensilage. J. Appl. Bacteriol. 1987, 63, 275–279. [Google Scholar] [CrossRef]
- Erwin, E.; Marco, G.J.; Emery, E. Volatile fatty acid analyses of blood and rumen fluid by gas chromatography. J. Dairy Sci. 1961, 44, 1768–1771. [Google Scholar] [CrossRef]
- Barker, S.; Summerson, W.H. The colorimetric determination of lactic acid in biological material. J. Biol. Chem. 1941, 138, 535–554. [Google Scholar] [CrossRef]
- Van Soest, P.v.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Tilley, J.; Terry, D.R. A two-stage technique for the in vitro digestion of forage crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Tagari, H.; Bergman, E. Intestinal disappearance and portal blood appearance of amino acids in sheep. J. Nutr. 1978, 108, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Ashbell, G.; Weinberg, Z.; Azrieli, A.; Hen, Y.; Horev, B. A simple system to study the aerobic determination of silages. Can. Agric. Eng. 1991, 34, 171–175. [Google Scholar]
- Yitbarek, M.B.; Tamir, B. Silage Additives: Review. Open J. Appl. Sci. 2014, 04, 258–274. [Google Scholar] [CrossRef] [Green Version]
- Weinberg, Z.; Chen, Y. Effects of storage period on the composition of whole crop wheat and corn silages. Anim. Feed Sci. Technol. 2013, 185, 196–200. [Google Scholar] [CrossRef]
- Henderson, N. Silage additives. Anim. Feed Sci. Technol. 1993, 45, 35–56. [Google Scholar] [CrossRef]
- Demirel, M.; Yilmaz, I.; Deniz, S.; Kaplan, O.; Akdeniz, H. Effect of addition of urea or urea plus molasses to different corn silages harvested at dough stage on silage quality and digestible dry matter yield. J. Appl. Anim. Res. 2003, 24, 7–16. [Google Scholar] [CrossRef]
- Weinberg, Z.; Chen, Y.; Solomon, R. The quality of commercial wheat silages in Israel. J. Dairy Sci. 2009, 92, 638–644. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.S.; Weinberg, Z.G.; Ogunade, I.M.; Cervantes, A.A.P.; Arriola, K.G.; Jiang, Y.; Kim, D.; Li, X.; Gonçalves, M.C.M.; Vyas, D.; et al. Meta-analysis of effects of inoculation with homofermentative and facultative heterofermentative lactic acid bacteria on silage fermentation, aerobic stability, and the performance of dairy cows. J. Dairy Sci. 2017, 100, 4587–4603. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Tan, Z.; Wu, G.; Wang, L.; Qin, G.; Wang, Y.; Pang, H. Microbial community and fermentation characteristic of whole-crop wheat silage treated by lactic acid bacteria and Artemisia argyi during ensiling and aerobic exposure. Front. Microbiol. 2022, 13, 1004495. [Google Scholar] [CrossRef] [PubMed]
- Kleinschmit, D.H.; Schmidt, R.J.; Kung, L. The effects of various antifungal additives on the fermentation and aerobic stability of corn silage. J. Dairy Sci. 2005, 88, 2130–2139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanderson, M.A. Aerobic stability and in vitro fiber digestibility of microbially inoculated corn and sorghum silages. J. Anim. Sci. 1993, 71, 505–514. [Google Scholar] [CrossRef] [Green Version]
- Pahlow, G.; Muck, R.E.; Driehuis, F.; Elferink, S.J.O.; Spoelstra, S.F. Microbiology of ensiling. Silage Sci. Technol. 2003, 42, 31–93. [Google Scholar]
- Kaiser, A.G.; Piltz, J.W.; Burns, H.M.; Griffiths, N.W. Top Fodder Successful Silage, 2nd ed.; Dairy Australia and New South Wales Department of Primary Industries: Victoria, BC, Canada, 2004; pp. 1–33. [Google Scholar]
- Zhang, M.; Lv, H.; Tan, Z.; Li, Y.; Wang, Y.; Pang, H.; Li, Z.; Jiao, Z.; Jin, Q. Improving the fermentation quality of wheat straw silage stored at low temperature by psychrotrophic lactic acid bacteria. Anim. Sci. J. 2017, 88, 277–285. [Google Scholar] [CrossRef]
- Weinberg, Z.G.; Shatz, O.; Chen, Y.; Yosef, E.; Nikbahat, M.; Ben-Ghedalia, D.; Miron, J. Effect of lactic acid bacteria inoculants on in vitro digestibility of wheat and corn silages. J. Dairy Sci. 2007, 90, 4754–4762. [Google Scholar] [CrossRef] [Green Version]
- Griswold, K.E.; Hoover, W.H.; Miller, T.K.; Thayne, W.V. Effect of form of nitrogen on growth of ruminal microbes in continuous culture. J. Anim. Sci. 1996, 74, 483. [Google Scholar] [CrossRef] [PubMed]
- Niimi, M.; Kawamura, O. Fermentation and cell wall degradation in guineagrass and Italian ryegrass silages. In Proceedings of the International Grassland Congress Proceedings, Virtual, 25–29 October 2021; pp. 1–7. [Google Scholar]
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef] [PubMed]
- Filya, I.; Ashbell, G.; Hen, Y.; Weinberg, Z.G. The effect of bacterial inoculants on the fermentation and aerobic stability of whole crop wheat silage. Anim. Feed Sci. Technol. 2000, 88, 39–46. [Google Scholar] [CrossRef]
- Weinberg, Z.G.; Ashbell, G.; Hen, Y.; Azrieli, A. The effect of applying lactic acid bacteria at ensiling on the aerobic stability of silages. J. Appl. Bacteriol. 1993, 75, 512–518. [Google Scholar] [CrossRef]
- Kristensen, N.B.; Sloth, K.H.; Højberg, O.; Spliid, N.H.; Jensen, C.; Thøgersen, R. Effects of microbial inoculants on corn silage fermentation, microbial contents, aerobic stability, and milk production under field conditions. J. Dairy Sci. 2010, 93, 3764–3774. [Google Scholar] [CrossRef] [Green Version]
- Schmutz, W.G.; Brown, L.D.; Thomas, J.W. Nutritive Value of Corn Silages Treated with Chemical Additives for Lactation. J. Dairy Sci. 1969, 52, 1408–1412. [Google Scholar] [CrossRef]
- Huber, J.; Bucholtz, H.; Boman, R. Ammonia versus urea-treated silages with varying urea in concentrate. J. Dairy Sci. 1980, 63, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, Z.G.; Szakacs, G.; Ashbell, G.; Hen, Y. The effect of Lactobacillus buchneri and L. plantarum, applied at ensiling, on the ensiling fermentation and aerobic stability of wheat and sorghum silages. J. Ind. Microbiol. Biotechnol. 1999, 23, 218–222. [Google Scholar] [CrossRef]
- Kung, L.; Schmidt, R.J.; Ebling, T.E.; Hu, W. The effect of Lactobacillus buchneri 40788 on the fermentation and aerobic stability of ground and whole high-moisture corn. J. Dairy Sci. 2007, 90, 2309–2314. [Google Scholar] [CrossRef] [PubMed]
- Ashbell, G.; Theune, H.; Sklan, D. Changes in amino acid compounds of wheat plants during ensiling and aerobic exposure: The influence of propionic acid and urea phosphate-calcium propionate. J. Agric. Sci. 1984, 102, 667–672. [Google Scholar] [CrossRef]
- Winters, A.L.; Fychan, R.; Jones, R. Effect of formic acid and a bacterial inoculant on the amino acid composition of grass silage and on animal performance. Grass Forage Sci. 2001, 56, 181–192. [Google Scholar] [CrossRef]
- Luo, C.; Wang, D.; Lu, N.; Li, H.; Liu, G.; Cao, Z.; Yang, H.; Li, S.; Yu, X.; Shao, W. Analysis of chemical composition, amino acid content, and rumen degradation characteristics of six organic feeds. Animals 2022, 12, 682. [Google Scholar] [CrossRef]
| pH | DM | OM | CP | NDF | ADF | Hemicellulose | |
|---|---|---|---|---|---|---|---|
| Corn forage | 5.50 ± 0.08 | 30.6 ± 0.48 | 95.7 ± 0.24 | 8.7 ± 0.65 | 52.2 ± 2.46 | 28.4 ± 1.62 | 23.9 ± 0.85 |
| Wheat forage | 6.49 ± 0.01 | 43.8 ± 0.25 | 93.3 ± 0.18 | 9.7 ± 0.45 | 57.2 ± 1.01 | 29.5 ± 0.54 | 27.7 ± 0.53 |
| Wheat Silage | ||||||||
| Days | Treatment | Main Effect (p-Value) | ||||||
| Control | MI | Urea | MI + Urea | SEM | Trt | Day | Trt × Day | |
| 1 | 6.64 b | 6.52 b | 6.97 a** | 6.99 a** | 0.078 | <0.0001 | <0.0001 | <0.0001 |
| 7 | 4.33 a | 4.16 b* | 4.36 a | 4.41 a | 0.037 | |||
| 14 | 3.96 ab | 3.81 b | 4.00 a | 4.02 a | 0.033 | |||
| 28 | 3.84 c | 3.73 d*** | 3.93 a*** | 3.90 b** | 0.029 | |||
| Corn Silage | ||||||||
| Days | Treatment | Main Effect (p-Value) | ||||||
| Control | MI | Urea | MI + Urea | SEM | Trt | Day | Trt × Day | |
| 1 | 3.75 | 3.86 | 3.90 | 3.86 | 0.031 | 0.0047 | 0.0008 | 0.0399 |
| 7 | 3.62 | 3.68 | 3.65 | 3.73 | 0.021 | |||
| 14 | 3.89 | 3.98 | 3.80 | 3.72 | 0.045 | |||
| 28 | 3.62 b | 3.97 a** | 3.74 b | 3.68 b | 0.053 | |||
| Wheat Silage | ||||||||
| Treatment | Main Effect (p-Value) | |||||||
| Days | Control | MI | Urea | MI + Urea | SEM | Trt | Day | Trt × Day |
| 1 | 57.2 | 60.2 | 54.0 | 55.7 | 1.001 | 0.0058 | 0.7763 | 0.0099 |
| 7 | 55.6 | 57.8 | 55.9 | 54.1 | 0.710 | |||
| 14 | 59.0 | 54.7 | 57.0 | 53.7 * | 0.840 | |||
| 28 | 61.7 a | 54.2 ab | 52.6 b* | 56.8 ab | 1.436 | |||
| Corn Silage | ||||||||
| Treatment | Main Effect (p-Value) | |||||||
| Days | Control | MI | Urea | MI + Urea | SEM | Trt | Day | Trt × Day |
| 1 | 60.8 a | 57.2 ab | 52.2 bc** | 48.6 c** | 1.804 | <0.0001 | 0.1549 | 0.1328 |
| 7 | 60.4 | 54.1 | 49.9 * | 50.2 * | 1.838 | |||
| 14 | 60.4 a | 61.0 a | 50.0 b | 47.1 b | 2.393 | |||
| 28 | 55.8 ab | 56.7 a | 45.9 b* | 49.9 ab | 1.793 | |||
| Wheat Silage | ||||||||
| Treatment | Main Effect (p-Value) | |||||||
| Days | Control | MI | Urea | MI + Urea | SEM | Trt | Day | Trt × Day |
| 1 | 39.5 c | 48.1 a*** | 43.2 b* | 45.1 ab*** | 0.771 | <0.0001 | 0.0014 | <0.0001 |
| 7 | 39.9 b | 45.1 a** | 41.9 ab | 43.7 b* | 0.575 | |||
| 14 | 40.6 b | 43.0 ab | 44.0 a* | 41.9 ab | 0.461 | |||
| 28 | 40.3 b | 41.6 b | 39.9 b | 44.8 a*** | 0.528 | |||
| Corn Silage | ||||||||
| Treatment | Main Effect (p-Value) | |||||||
| Days | Control | MI | Urea | MI + Urea | SEM | Trt | Day | Trt × Day |
| 1 | 42.6 bc | 38.9 c | 45.2 ab | 47.5 a* | 0.807 | <0.0001 | 0.0368 | 0.0008 |
| 7 | 43.4 ab | 43.2 b | 45.3 ab | 47.5 a* | 0.607 | |||
| 14 | 40.0 b | 36.5 b | 48.3 a*** | 45.2 a* | 0.990 | |||
| 28 | 38.2 b | 40.3 b | 47.6 a*** | 47.3 a*** | 0.903 | |||
| Wheat Silage | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | ||||||||
| Control | MI | Urea | MI + Urea | Main Effect (p-Value) | ||||
| Days | Acetic Acid (g/100 g DM) | SEM | Trt | Day | Trt × Day | |||
| 1 | 1.89 | 1.75 | 2.01 | 2.08 | 0.130 | 0.2098 | 0.6174 | 0.9963 |
| 7 | 1.98 | 1.84 | 2.18 | 2.27 | 0.084 | |||
| 14 | 1.89 | 1.93 | 1.91 | 2.15 | 0.051 | |||
| 28 | 1.89 | 2.07 | 2.24 | 2.31 | 0.118 | Main Effect (p-Value) | ||
| Days | Propionic Acid (g/100 g DM) | SEM | Trt | Day | Trt × Day | |||
| 1 | 0.10 | 0.05 | 0.05 | 0.06 | 0.012 | 0.2001 | 0.0256 | 0.4722 |
| 7 | 0.04 | 0.04 | 0.04 | 0.01 | 0.008 | |||
| 14 | 0.05 | 0.01 | 0.03 | 0.03 | 0.008 | |||
| 28 | 0.02 | 0.02 | 0.04 * | 0.04 * | 0.004 | Main Effect (p-Value) | ||
| Days | Butyric Acid (g/100 g DM) | SEM | Trt | Day | Trt × Day | |||
| 1 | 0.02 | 0.01 | N.D | N.D | 0.004 | 0.0513 | 0.0771 | 0.3841 |
| 7 | N.D | N.D | N.D | N.D | 0.000 | |||
| 14 | N.D | N.D | N.D | N.D | 0.000 | |||
| 28 | N.D | N.D | N.D | N.D | 0.000 | Main Effect (p-Value) | ||
| Days | Total Acid (g/100 g DM) | SEM | Trt | Day | Trt × Day | |||
| 1 | 2.08 | 1.91 | 2.07 | 2.15 | 0.130 | 0.4212 | 0.8159 | 0.9945 |
| 7 | 2.11 | 1.95 | 2.22 | 2.28 | 0.081 | |||
| 14 | 2.03 | 1.94 | 1.95 | 2.18 | 0.050 | |||
| 28 | 1.91 | 2.09 | 2.28 | 2.35 | 0.120 | |||
| Corn Silage | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | ||||||||
| Control | MI | Urea | MI + Urea | Main Effect (p-Value) | ||||
| Days | Acetic Acid (g/100 g DM) | SEM | Trt | Day | Trt × Day | |||
| 1 | 1.59 b | 0.87 b | 2.50 a* | 2.86 a* | 0.300 | <0.0001 | <0.0001 | 0.1581 |
| 7 | 1.68 bc | 1.34 c | 2.96 ab | 3.45 a* | 0.346 | |||
| 14 | 2.31 bc | 1.73 c | 3.00 ab | 3.60 a* | 0.274 | |||
| 28 | 3.42 | 2.27 | 3.57 | 3.60 | 0.227 | Main Effect (p-Value) | ||
| Days | Propionic Acid (g/100 g DM) | SEM | Trt | Day | Trt × Day | |||
| 1 | 0.02 | 0.16 | 0.02 | 0.02 | 0.027 | <0.0001 | 0.0274 | 0.0328 |
| 7 | 0.01 b | 0.08 a* | 0.02 ab | 0.02 ab | 0.012 | |||
| 14 | 0.02 b | 0.22 a*** | 0.02 b | 0.01 b | 0.033 | |||
| 28 | 0.03 b | 0.29 a** | 0.03 b | 0.02 b | 0.044 | Main Effect (p-Value) | ||
| Days | Butyric Acid (g/100 g DM) | SEM | Trt | Day | Trt × Day | |||
| 1 | 0.08 ab | 0.05 b | 0.12 a | 0.11 a | 0.012 | <0.0001 | 0.0122 | 0.3739 |
| 7 | 0.07 | 0.05 | 0.12 | 0.13 | 0.013 | |||
| 14 | 0.10 | 0.05 | 0.23 | 0.14 | 0.028 | |||
| 28 | 0.15 ab | 0.07 b | 0.18 a | 0.16 ab | 0.017 | Main Effect (p-Value) | ||
| Days | Total Acid (g/100 g DM) | SEM | Trt | Day | Trt × Day | |||
| 1 | 1.68 bc | 1.09 c | 2.65 ab* | 3.12 a* | 0.308 | <0.0001 | <0.0001 | 0.2612 |
| 7 | 1.77 b | 1.47 b | 3.11 ab | 3.60 a* | 0.354 | |||
| 14 | 2.44 bc | 2.01 c | 3.25 ab | 4.05 a** | 0.303 | |||
| 28 | 3.60 ab | 2.63 b | 3.79 ab | 4.09 a | 0.226 | |||
| Wheat Silage | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | ||||||||
| Control | MI | Urea | MI + Urea | Main Effect (p-Value) | ||||
| Days | 1 CO2 (g/kg DM) | SEM | Trt | Day | Trt × Day | |||
| 14 | 24.1 a | 5.4 b*** | 8.2 b*** | 11.4 b** | 2.166 | <0.0001 | 0.0217 | 0.0011 |
| 28 | 33.7 a | 3.4 b*** | 40.4 a | 4.7 b*** | 4.122 | |||
| 2 Sugar loss (g) | ||||||||
| 14 | 16.4 a | 3.7 b*** | 5.6 b*** | 7.8 b** | 1.473 | <0.0001 | 0.0217 | 0.0011 |
| 28 | 22.9 a | 2.3 b*** | 27.5 a | 3.2 b*** | 2.803 | |||
| 3 Molds (Log CFU g/DM) | SEM | |||||||
| 28 | 9.1 | 7.9 *** | 8.2 *** | 8.1 *** | 0.112 | <0.0001 | ||
| pH | SEM | |||||||
| 28 | 7.1 | 3.9 *** | 5.1 *** | 4.1 *** | 0.272 | <0.0001 | ||
| Corn silage | ||||||||
| CO2 (g/kg DM) | SEM | Trt | Day | Trt × Day | ||||
| 14 | 52.7 c | 37.2 d*** | 71.2 a*** | 63.5 b** | 3.273 | <0.0001 | <0.0001 | 0.3411 |
| 28 | 38.8 ab | 28.7 b | 54.5 a | 42.4 ab | 3.007 | |||
| Sugar loss (g) | SEM | |||||||
| 14 | 35.5 c | 27.3 d*** | 47.8 a*** | 43.2 b*** | 1.868 | <0.0001 | <0.0001 | 0.3208 |
| 28 | 26.4 ab | 20.4 b | 34.7 a | 35.5 a | 1.927 | |||
| Molds (Log CFU g/DM) | SEM | |||||||
| 28 | 9.2 | 9.1 | 9.3 | 9.1 | 0.059 | 0.3948 | ||
| pH | SEM | |||||||
| 28 | 4.3 | 4.1 | 3.9 | 3.7 | 0.097 | 0.1882 | ||
| Wheat Silage | ||||||||
| Days | Treatment | Main Effect (p-Value) | ||||||
| Control | MI | Urea | MI + Urea | SEM | Trt | Day | Trt × Day | |
| 1 | 9.02 bc | 8.81 c | 9.83 ab* | 9.89 a* | 0.152 | 0.0005 | 0.3004 | 0.2427 |
| 7 | 8.53 b | 9.25 ab | 9.66 a** | 9.60 a** | 0.144 | |||
| 14 | 9.30 | 8.90 | 9.45 | 9.47 | 0.163 | |||
| 28 | 9.17 | 9.73 | 9.60 | 9.80 | 0.133 | |||
| Corn Silage | ||||||||
| Days | Treatment | Main Effect (p-Value) | ||||||
| Control | MI | Urea | MI + Urea | SEM | Trt | Day | Trt × Day | |
| 1 | 8.33 b | 8.35 b | 10.8 a*** | 10.5 a*** | 0.318 | <0.0001 | <0.0001 | <0.0001 |
| 7 | 8.28 c | 9.81 b*** | 10.9 a*** | 10.5 ab*** | 0.281 | |||
| 14 | 9.18 b | 9.28 b | 11.0 a*** | 11.0 ac*** | 0.255 | |||
| 28 | 7.99 c | 10.5 b*** | 11.1 b*** | 12.5 a*** | 0.420 | |||
| Additive | ||||||
|---|---|---|---|---|---|---|
| Amino Acid | Control | MI | Urea | MI + Urea | SEM | Main Effect (p-Value) |
| Essential | ||||||
| Lysine | 1.78 | 1.94 | 2.01 | 1.91 | 0.107 | 0.9385 |
| Histidine | 1.15 | 1.17 | 1.26 | 1.03 | 0.066 | 0.7777 |
| Valine | 2.31 | 3.29 | 4.04 | 3.31 | 0.286 | 0.1883 |
| Phenylalanine | 3.13 | 3.06 | 3.51 | 2.84 | 0.171 | 0.6873 |
| Arginine | 1.84 | 1.50 | 2.04 | 1.49 | 0.112 | 0.2126 |
| Threonine | 2.83 | 2.85 | 3.09 | 2.64 | 0.107 | 0.6365 |
| Methionine | 0.64 | 0.67 | 0.76 | 0.37 | 0.064 | 0.1307 |
| Isoleucine | 10.10 | 11.80 | 12.14 | 11.51 | 0.753 | 0.8641 |
| Leucine | 3.42 | 3.39 | 4.18 | 3.34 | 0.221 | 0.5864 |
| Non-essential | ||||||
| Serine | 2.71 | 2.78 | 2.87 | 2.41 | 0.130 | 0.7342 |
| Glutamic acid | 9.95 | 8.84 | 10.71 | 9.34 | 0.405 | 0.4836 |
| Glycine | 3.66 | 3.95 | 4.09 | 4.04 | 0.106 | 0.6061 |
| Tyrosine | 1.77 | 1.93 | 2.20 | 2.00 | 0.121 | 0.7670 |
| γ-Aminobutyric acid | 1.38 | 1.48 | 1.25 | 1.61 | 0.073 | 0.4426 |
| Proline | 4.40 | 4.32 | 4.70 | 4.37 | 0.156 | 0.8209 |
| Alanine | 4.23 | 4.43 | 5.62 | 4.84 | 0.316 | 0.5081 |
| Hydroxyproline | 0.50 | 0.49 | 0.41 | 0.51 | 0.017 | 0.1235 |
| Lysine% EAA 1 | 6.49 a | 6.56 a | 6.06 b | 6.71 a | 0.099 | 0.0480 |
| Methionine% EAA 2 | 2.37 a | 2.27 a | 2.29 a | 1.31 b* | 0.172 | 0.0132 |
| TEAA% CP 3 | 27.19 | 29.67 | 33.03 | 28.44 | 1.692 | 0.7537 |
| TAA% CP | 56.37 | 58.72 | 58.30 | 65.54 | 9.343 | 0.7815 |
| Additive | ||||||
|---|---|---|---|---|---|---|
| Amino Acid | Control | MI | Urea | MI + Urea | SEM | Main Effect (p-Value) |
| Essential | ||||||
| Lysine | 2.56 a | 2.08 b* | 2.04 b* | 1.81 b** | 0.107 | 0.0118 |
| Histidine | 1.46 a | 1.38 a | 1.21 b* | 1.02 c** | 0.065 | 0.0028 |
| Valine | 4.42 a | 3.84 a | 3.97 a | 2.98 b* | 0.213 | 0.0326 |
| Phenylalanine | 3.96 a | 3.59 ab | 3.22 bc* | 2.72 c** | 0.181 | 0.0125 |
| Arginine | 2.27 a | 2.15 a | 1.85 ab | 1.64 b | 0.104 | 0.0509 |
| Threonine | 3.58 | 3.16 | 2.76 | 2.64 | 0.154 | 0.0617 |
| Methionine | 0.90 | 0.82 | 0.54 | 0.65 | 0.061 | 0.0792 |
| Isoleucine | 9.54 | 10.92 | 11.35 | 8.59 | 0.698 | 0.5852 |
| Leucine | 6.44 | 5.42 | 4.76 | 4.23 | 0.350 | 0.0764 |
| Non-essential | ||||||
| Serine | 3.43 a | 2.78 b* | 2.90 b* | 2.62 b** | 0.020 | 0.0096 |
| Glutamic acid | 8.84 a | 8.29 a | 9.33 a | 6.68 b* | 0.407 | 0.0346 |
| Glycine | 4.66 a | 3.91 b | 4.05 ab | 3.36 b* | 0.188 | 0.0311 |
| Tyrosine | 2.17 a | 1.94 a | 1.90 a | 1.58 b* | 0.083 | 0.0217 |
| γ-Aminobutyric acid | 2.15 a | 1.57 b | 1.36b * | 1.51 b* | 0.122 | 0.0309 |
| Proline | 4.82 | 4.39 | 4.25 | 3.75 | 0.162 | 0.0707 |
| Alanine | 7.65 | 6.67 | 5.90 | 5.70 | 0.319 | 0.0550 |
| Hydroxyproline | 0.63 | 0.48 | 0.48 | 0.43 | 0.033 | 0.0941 |
| Lysine% EAA 1 | 7.32 | 6.27 | 6.45 | 6.88 | 0.205 | 0.2976 |
| Methionine% EAA 2 | 2.58 | 2.47 | 1.69 | 2.49 | 0.160 | 0.1296 |
| TEAA% CP 3 | 35.14 | 33.37 | 31.70 | 26.28 | 1.473 | 0.1288 |
| TAA% CP | 69.86 | 63.58 | 62.33 | 52.22 * | 4.106 | 0.0533 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagali, P.; Pelech, I.; Sabastian, C.; Ben Ari, J.; Tagari, H.; Mabjeesh, S.J. The Effect of Microbial Inoculum and Urea Supplements on Nutritive Value, Amino Acids Profile, Aerobic Stability and Digestibility of Wheat and Corn Silages. Animals 2023, 13, 2197. https://doi.org/10.3390/ani13132197
Wagali P, Pelech I, Sabastian C, Ben Ari J, Tagari H, Mabjeesh SJ. The Effect of Microbial Inoculum and Urea Supplements on Nutritive Value, Amino Acids Profile, Aerobic Stability and Digestibility of Wheat and Corn Silages. Animals. 2023; 13(13):2197. https://doi.org/10.3390/ani13132197
Chicago/Turabian StyleWagali, Philip, Ira Pelech, Chris Sabastian, Julius Ben Ari, Haim Tagari, and Sameer J. Mabjeesh. 2023. "The Effect of Microbial Inoculum and Urea Supplements on Nutritive Value, Amino Acids Profile, Aerobic Stability and Digestibility of Wheat and Corn Silages" Animals 13, no. 13: 2197. https://doi.org/10.3390/ani13132197






