A Comparison of Dobutamine, Norepinephrine, Vasopressin, and Hetastarch for the Treatment of Isoflurane-Induced Hypotension in Healthy, Normovolemic Dogs
Abstract
:Simple Summary
Abstract
1. Introduction
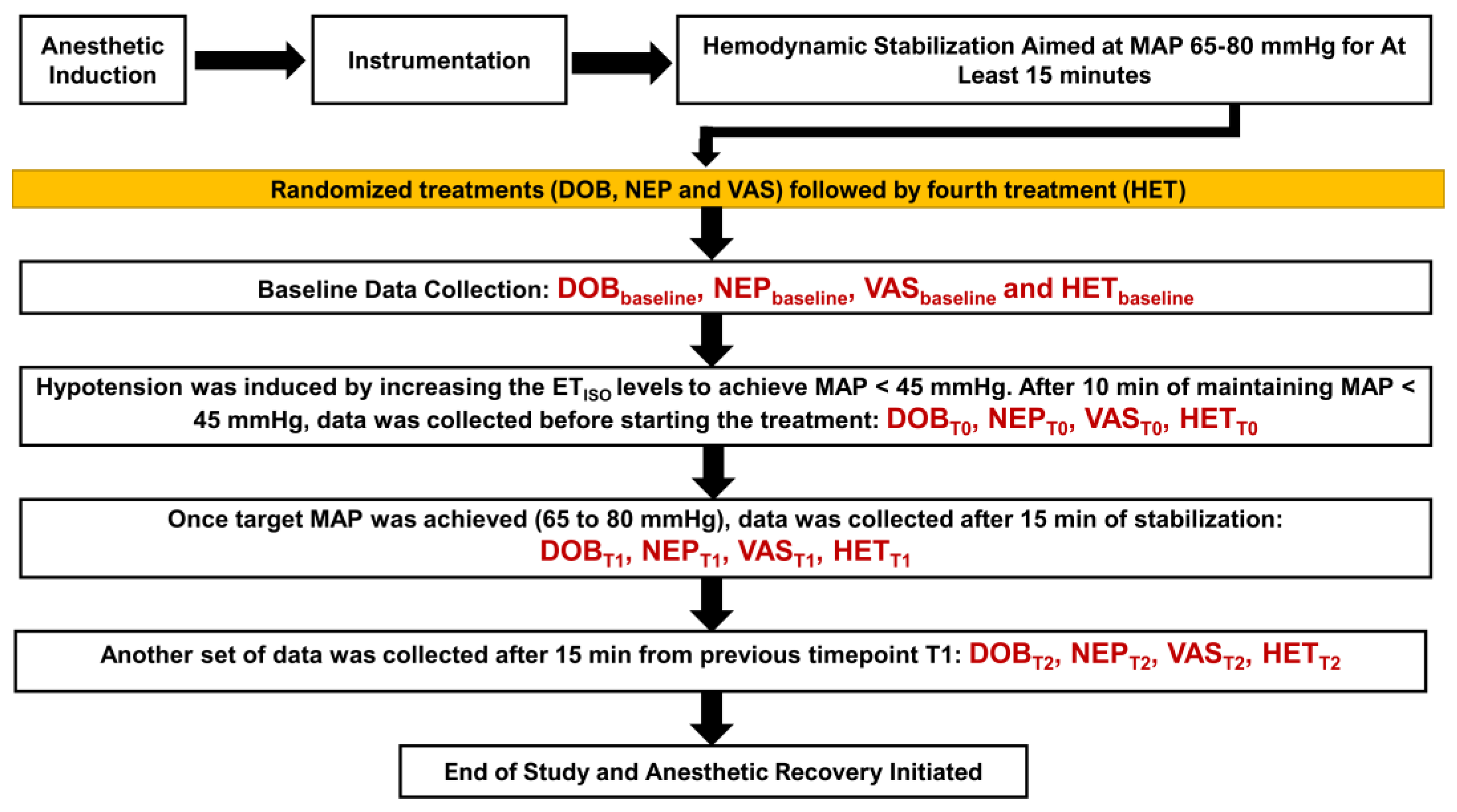
2. Materials and Methods
2.1. Animals
2.2. General Anesthesia and Anesthetic Monitoring
2.3. Instrumentation for Intermittent Pulmonary Artery Thermodilution to Measure CO and Other Hemodynamic Variables
2.4. Calculations for Other Hemodynamic Variables Derived from the Thermodilution Catheter
2.5. Administration for Treatments and Data Collection
2.6. Anesthetic Recovery
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitter, N.; Grogan, K.; Berkowitz, D.E.; Nyhan, D. Pharmacology of Anesthetic Drugs. In Kaplan’s Cardiac Anesthesia: For Cardiac and Noncardiac Surgery, 7th ed.; Kaplan, J.A., Ed.; Elsevier: Philadelphia, PA, USA, 2017; pp. 247–291. [Google Scholar]
- Meng, T.; Bu, W.; Ren, X.; Chen, X.; Yu, J.; Eckenhoff, R.G.; Gao, W.D. Molecular mechanism of anesthetic-induced depression of myocardial contraction. FASEB J. 2016, 30, 2915–2925. [Google Scholar] [CrossRef]
- Hüneke, R.; Fassl, J.; Rossaint, R.; Lückhoff, A. Effects of volatile anesthetics on cardiac ion channels. Acta Anaesthesiol. Scand. 2004, 48, 547–561. [Google Scholar] [CrossRef]
- Yamada, T.; Takeda, J.; Koyama, K.; Sekiguchi, H.; Fukushima, K.; Kawazoe, T. Effects of sevoflurane, isoflurane, enflurane, and halothane on left ventricular diastolic performance in dogs. J. Cardiothorac. Vasc. Anesth. 1994, 8, 618–624. [Google Scholar] [CrossRef]
- Conzen, P.F.; Hobbhahn, J.; Goetz, A.E.; Habazettl, H.; Granetzny, T.; Peter, K.; Brendel, W. Myocardial contractility, blood flow, and oxygen consumption in healthy dogs during anesthesia with isoflurane or enflurane. J. Cardiothorac. Anesth. 1989, 3, 70–77. [Google Scholar] [CrossRef]
- Torri, G. Inhalation anesthetics: A review. Minerva Anestesiol. 2010, 76, 215–228. [Google Scholar] [PubMed]
- Tanaka, S.; Tsuchida, H.; Nakabayashi, K.; Seki, S.; Namiki, A. The effects of sevoflurane, isoflurane, halothane, and enflurane on hemodynamic responses during an inhaled induction of anesthesia via a mask in humans. Anesth. Analg. 1996, 82, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Brioni, J.D.; Varughese, S.; Ahmed, R.; Bein, B. A clinical review of inhalation anesthesia with sevoflurane: From early research to emerging topics. J. Anesth. 2017, 31, 764–778. [Google Scholar] [CrossRef] [PubMed]
- Wesselink, E.M.; Kappen, T.H.; Torn, H.M.; Slooter, A.J.C.; van Klei, W.A. Intraoperative hypotension and the risk of postoperative adverse outcomes: A systematic review. Br. J. Anaesth. 2018, 121, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Muzi, M.; Ebert, T.J. Randomized, prospective comparison of halothane, isoflurane, and enflurane on baroreflex control of heart rate in humans. Adv. Pharmacol. 1994, 31, 379–387. [Google Scholar] [CrossRef]
- Picker, O.; Scheeren, T.W.; Arndt, J.O. Inhalation anaesthetics increase heart rate by decreasing cardiac vagal activity in dogs. Br. J. Anaesth. 2001, 87, 748–754. [Google Scholar] [CrossRef]
- Raatikainen, M.J.; Trankina, M.F.; Morey, T.E.; Dennis, D.M. Effects of volatile anesthetics on atrial and AV nodal electrophysiological properties in guinea pig isolated perfused heart. Anesthesiology 1995, 89, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Sumikawa, K.; Tashiro, C.; Yamatodani, A.; Yoshiya, I. Arrhythmogenic threshold of epinephrine during sevoflurane, enflurane, and isoflurane anesthesia in dogs. Anesthesiology 1988, 69, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Imamura, S.; Ikeda, K. Comparison of the epinephrine-induced arrhythmogenic effect of sevoflurane with isoflurane and halothane. J. Anesth. 1987, 1, 62–68. [Google Scholar] [CrossRef]
- Wijnberge, M.; Schenk, J.; Bulle, E.; Vlaar, A.P.; Maheshwari, K.; Hollmann, M.W.; Binnekade, J.M.; Geerts, B.F.; Veelo, D.P. Association of intraoperative hypotension with postoperative morbidity and mortality: Systematic review and meta-analysis. Br. J. Surg. Open 2021, 5, zraa018. [Google Scholar] [CrossRef]
- Grubb, T.; Sager, J.; Gaynor, J.S.; Montgomery, E.; Parker, J.A.; Shafford, H.; Tearney, C. 2020 AAHA Anesthesia and Monitoring Guidelines for Dogs and Cats. J. Am. Anim. Hosp. Assoc. 2020, 56, 59–82. [Google Scholar] [CrossRef]
- Skelding, A.; Valverde, A. Review of non-invasive blood pressure measurement in animals: Part 2—Evaluation of the performance of non-invasive devices. Can. Vet. J. 2020, 61, 481–498. [Google Scholar]
- Costa, R.S.; Raisis, A.L.; Hosgood, G.; Musk, G.C. Preoperative factors associated with hypotension in young anaesthetised dogs undergoing elective desexing. Aust. Vet. J. 2015, 93, 99–104. [Google Scholar] [CrossRef]
- Kojima, K.; Ishizuka, T.; Sasaki, N.; Nakamura, K.; Takiguchi, M. Cardiovascular effects of dose escalating of norepinephrine in healthy dogs anesthetized with isoflurane. Vet. Anaesth. Analg. 2021, 48, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Skelding, A.M.; Valverde, A. Sympathomimetics in veterinary species under anesthesia. Vet. J. 2020, 258, 105455. [Google Scholar] [CrossRef]
- Valverde, A. Fluid Resuscitation for Refractory Hypotension. Front. Vet. Sci. 2021, 8, 621696. [Google Scholar] [CrossRef] [PubMed]
- Montealegre, F.; Lyons, B.M. Fluid Therapy in Dogs and Cats with Sepsis. Front. Vet. Sci. 2021, 8, 622127. [Google Scholar] [CrossRef] [PubMed]
- Adamik, K.N.; Yozova, I.D. Colloids Yes or No?—A “Gretchen Question” Answered. Front. Vet. Sci. 2021, 8, 624049. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Sinclair, M.D.; Dyson, D.H. Use of ephedrine and dopamine in dogs for the management of hypotension in routine clinical cases under isoflurane anesthesia. Vet. Anaesth. Analg. 2007, 34, 301–311. [Google Scholar] [CrossRef]
- Muir, W.W.; Wiese, A.J. Comparison of lactated Ringer’s solution and a physiologically balanced 6% hetastarch plasma expander for the treatment of hypotension induced via blood withdrawal in isoflurane-anesthetized dogs. Am. J. Vet. Res. 2004, 65, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Paranjape, V.V.; Henao-Guerrero, N.; Menciotti, G.; Saksena, S. Volumetric evaluation of fluid responsiveness using a modified passive leg raise maneuver during experimental induction and correction of hypovolemia in anesthetized dogs. Vet. Anaesth. Analg. 2023, 50, 211–219. [Google Scholar] [CrossRef]
- Paranjape, V.V.; Henao-Guerrero, N.; Menciotti, G.; Saksena, S. Esophageal Doppler-derived indices and arterial load variables provide useful hemodynamic information during assessment of fluid responsiveness in anesthetized dogs undergoing acute changes in blood volume. Am. J. Vet. Res. 2023, 84, 1–11. [Google Scholar] [CrossRef]
- Paranjape, V.V.; Henao-Guerrero, N.; Menciotti, G.; Saksena, S.; Agostinho, M. Agreement between Electrical Cardiometry and Pulmonary Artery Thermodilution for Measuring Cardiac Output in Isoflurane-Anesthetized Dogs. Animals 2023, 13, 1420. [Google Scholar] [CrossRef]
- Grasso, S.C.; Ko, J.C.; Weil, A.B.; Paranjape, V.; Constable, P.D. Hemodynamic influence of acepromazine or dexmedetomidine premedication in isoflurane-anesthetized dogs. J. Am. Vet. Med. Assoc. 2015, 246, 754–764. [Google Scholar] [CrossRef]
- Guyton, A.C. The relationship of cardiac output and arterial pressure control. Circulation 1981, 64, 1079–1088. [Google Scholar] [CrossRef]
- Aarnes, T.K.; Bednarski, R.M.; Lerche, P.; Hubbell, J.A.; Muir, W.W. Effect of intravenous administration of lactated Ringer’s solution or hetastarch for the treatment of isoflurane-induced hypotension in dogs. Am. J. Vet. Res. 2009, 70, 1345–1353. [Google Scholar] [CrossRef]
- Hauptman, J.G.; Richter, M.A.; Wood, S.L.; Nachreiner, R.F. Effects of anesthesia, surgery, and intravenous administration of fluids on plasma antidiuretic hormone concentrations in healthy dogs. Am. J. Vet. Res. 2000, 61, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, J.S.; Wertz, E.M.; Kesel, L.M.; Baker, G.E.; Cecchini, C.; Rice, K.; Mallinckrodt, C.M. Effect of intravenous administration of fluids on packed cell volume, blood pressure, and total protein and blood glucose concentrations in healthy halothane-anesthetized dogs. J. Am. Vet. Med. Assoc. 1996, 208, 2013–2015. [Google Scholar] [PubMed]
- Valverde, A.; Gianotti, G.; Rioja-Garcia, E.; Hathway, A. Effects of high-volume, rapid-fluid therapy on cardiovascular function and hematological values during isoflurane-induced hypotension in healthy dogs. Can. J. Vet. Res. 2012, 76, 99–108. [Google Scholar]
- McBride, D.; Raisis, A.L.; Hosgood, G.; Smart, L. Hydroxyethyl starch 130/0.4 compared with 0.9% NaCl administered to greyhounds with haemorrhagic shock. Vet. Anaesth. Analg. 2017, 44, 444–451. [Google Scholar] [CrossRef]
- Udelsmann, A.; Bonfim, M.R.; Silva, W.A.; Moraes, A.C. Hemodynamic effects of volume replacement with saline solution and hypertonic hydroxyethyl starch in dogs. Acta Cir. Bras. 2009, 24, 87–92. [Google Scholar] [CrossRef]
- Chohan, A.S.; Greene, S.A.; Grubb, T.L.; Keegan, R.D.; Wills, T.B.; Martinez, S.A. Effects of 6% hetastarch (600/0.75) or lactated Ringer’s solution on hemostatic variables and clinical bleeding in healthy dogs anesthetized for orthopedic surgery. Vet. Anaesth. Analg. 2011, 38, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Crystal, G.J.; Salem, M.R. The Bainbridge and the “reverse” Bainbridge reflexes: History, physiology, and clinical relevance. Anesth. Analg. 2012, 114, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Iannucci, C.; Dirkmann, D.; Howard, J.; Adamik, K.N. A prospective randomized open-label trial on the comparative effects of 6% hydroxyethyl starch 130/0.4 versus polyionic isotonic crystalloids on coagulation parameters in dogs with spontaneous hemoperitoneum. J. Vet. Emerg. Crit. Care 2021, 31, 32–42. [Google Scholar] [CrossRef]
- Smith, M.D.; Barletta, M.; Young, C.N.; Hofmeister, E.H. Retrospective study of intra-anesthetic predictors of prolonged hospitalization, increased cost of care and mortality for canine patients at a veterinary teaching hospital. Vet. Anaesth. Analg. 2017, 44, 1321–1331. [Google Scholar] [CrossRef]
- Ruffolo, R.R., Jr.; Messick, K. Effects of dopamine, (+/−)-dobutamine and the (+)- and (−)-enantiomers of dobutamine on cardiac function in pithed rats. J. Pharmacol. Exp. Ther. 1995, 235, 558–565. [Google Scholar]
- Robie, N.W.; Nutter, D.O.; Moody, C.; McNay, J.L. In vivo analysis of adrenergic receptor activity of dobutamine. Circ. Res. 1974, 34, 663–671. [Google Scholar] [CrossRef]
- Vainionpää, V.; Nuutinen, L.; Kairaluoma, M.; Mokka, R.; Tuononen, S. Haemodynamic comparison of dopamine and dobutamine in normovolaemic and hypovolaemic dogs. Acta Anaesthesiol. Scand. 1983, 27, 490–494. [Google Scholar] [CrossRef]
- Curtis, M.B.; Bednarski, R.M.; Majors, L. Cardiovascular effects of vasopressors in isoflurane-anesthetized dogs before and after hemorrhage. Am. J. Vet. Res. 1989, 50, 1866–1871. [Google Scholar]
- Dyson, D.H.; Sinclair, M.D. Impact of dopamine or dobutamine infusions on cardiovascular variables after rapid blood loss and volume replacement during isoflurane-induced anesthesia in dogs. Am. J. Vet. Res. 2006, 67, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Paranjape, V.V.; Garcia-Pereira, F.L.; Menciotti, G.; Saksena, S.; Henao-Guerrero, N.; Ricco-Pereira, C.H. Agreement of cardiac output measurements by esophageal doppler and transesophageal echocardiography with intermittent pulmonary artery thermodilution during pharmacologic manipulation of hemodynamics in anesthetized dogs. Am. J. Vet. Res. 2023. advance online publication. [Google Scholar] [CrossRef]
- Rosati, M.; Dyson, D.H.; Sinclair, M.D.; Sears, W.C. Response of hypotensive dogs to dopamine hydrochloride and dobutamine hydrochloride during deep isoflurane anesthesia. Am. J. Vet. Res. 2007, 68, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.; Vincent, J.L. Effects of norepinephrine and dobutamine on oxygen transport and consumption in a dog model of endotoxic shock. Crit. Care Med. 1993, 21, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Shankar, A.; Gurumurthy, G.; Sridharan, L.; Gupta, D.; Nicholson, W.J.; Jaber, W.A.; Vallabhajosyula, S. A Clinical Update on Vasoactive Medication in the Management of Cardiogenic Shock. Clin. Med. Insights Cardiol. 2022, 16, 11795468221075064. [Google Scholar] [CrossRef]
- Silverstein, D.C.; Beer, K.A. Controversies regarding choice of vasopressor therapy for management of septic shock in animals. J. Vet. Emerg. Crit. Care 2015, 25, 48–54. [Google Scholar] [CrossRef]
- Melchior, J.C.; Pinaud, M.; Blanloeil, Y.; Bourreli, B.; Potel, G.; Souron, R. Hemodynamic effects of continuous norepinephrine infusion in dogs with and without hyperkinetic endotoxic shock. Crit. Care Med. 1987, 15, 687–691. [Google Scholar] [CrossRef]
- Luo, X.; Huang, Y.; Hayes, J.K.; Wong, K.C.; Yee, J.B.; McJames, S. Effects of dobutamine, epinephrine and norepinephrine on the hemodynamics of dogs during hemorrhagic shock. Acta Anaesthesiol. Sin. 1997, 35, 61–71. [Google Scholar] [PubMed]
- Holt, N.F.; Haspel, K.L. Vasopressin: A review of therapeutic applications. J. Cardiothorac. Vasc. Anesth. 2010, 24, 330–347. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Kim, M.S.; Park, H.M. Hemodynamic characteristics of vasopressin in dogs with severe hemorrhagic shock. J. Vet. Med. Sci. 2006, 68, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Park, C.; Hahm, D.H.; Lee, H.J.; Park, H.M. Determination of optimal dose of arginine vasopressin in hemorrhagic shock in dogs. J. Vet. Med. Sci. 2007, 69, 755–758. [Google Scholar] [CrossRef]
- Yoo, J.H.; Kim, M.S.; Eom, K.D.; Park, J.I.; Park, C.; Park, H.M. Vasopressor therapy using vasopressin prior to crystalloid resuscitation in irreversible hemorrhagic shock under isoflurane anesthesia in dogs. J. Vet. Med. Sci. 2007, 69, 459–464. [Google Scholar] [CrossRef]
- Monge García, M.I.; Santos, A. Understanding ventriculo-arterial coupling. Ann. Transl. Med. 2020, 8, 795. [Google Scholar] [CrossRef]
| Treatment Timepoint | ETISO (%) | SpO2 (%) | HR (bpm) | SAP (mmHg) | MAP (mmHg) | DAP (mmHg) | CO (L/min) | CVP (mmHg) | MPAP (mmHg) | PAWP (mmHg) |
|---|---|---|---|---|---|---|---|---|---|---|
| DOBbaseline | 1.4 ± 0.1 | 99 ± 1 | 100 ± 15 | 118 ± 8 | 72 ± 9 | 49 ± 7 | 1.75 ± 0.32 | 4.0 ± 0.3 | 11 ± 3 | 7 ± 2 |
| NEPbaseline | 1.5 ± 0.1 | 99 ± 2 | 96 ± 11 | 115 ± 7 | 69 ± 10 | 46 ± 8 | 1.69 ± 0.44 | 3.6 ± 0.2 | 10 ± 2 | 6 ± 1 |
| VASbaseline | 1.5 ± 0.0 | 98 ± 2 | 102 ± 12 | 120 ± 8 | 68 ± 9 | 42 ± 8 | 1.72 ± 0.25 | 3.8 ± 0.1 | 11 ± 2 | 7 ± 2 |
| HETbaseline | 1.5 ± 0.1 | 98 ± 1 | 93 ± 10 | 119 ± 7 | 71 ± 7 | 47 ± 6 | 1.64 ± 0.29 | 3.4 ± 0.1 | 11 ± 3 | 7 ± 1 |
| DOBT0 | 3.0 ± 0.2 * | 99 ± 1 | 107 ± 12 | 89 ± 5 * | 42 ± 4 * | 19 ± 4 * | 0.88 ± 0.13 * | 4.6 ± 0.4 | 7 ± 1 * | 3 ± 1 * |
| NEPT0 | 2.9 ± 0.1 † | 99 ± 1 | 100 ± 11 | 86 ± 3 † | 41 ± 3 † | 18 ± 5 † | 0.95 ± 0.19 † | 4.1 ± 0.3 | 6 ± 2 † | 4 ± 1 † |
| VAST0 | 3.0 ± 0.1 ‡ | 98 ± 1 | 108 ± 10 | 90 ± 5 ‡ | 44 ± 2 ‡ | 21 ± 4 ‡ | 0.99 ± 0.11 ‡ | 4.4 ± 0.2 | 7 ± 2 ‡ | 3 ± 1 ‡ |
| HETT0 | 3.0 ± 0.1 § | 98 ± 1 | 99 ± 11 | 85 ± 5 § | 43 ± 2 § | 22 ± 4 § | 0.87 ± 0.15 § | 4.0 ± 0.2 | 6 ± 2 § | 4 ± 1 § |
| DOBT1 | 3.0 ± 0.1 * | 98 ± 2 | 115 ± 10 | 123 ± 6 | 63 ± 3 * | 33 ± 4 * | 2.10 ± 0.32 * | 4.9 ± 0.2 * | 11 ± 2 | 7 ± 1 |
| NEPT1 | 2.9 ± 0.2 † | 98 ± 2 | 76 ± 11 †,c | 130 ± 5 †,c | 72 ± 6 c | 43 ± 3 c | 1.26 ± 0.40 †,c | 4.6 ± 0.2 † | 15 ± 3 †,c | 10 ± 2 †,c |
| VAST1 | 3.0 ± 0.1 ‡ | 99 ± 2 | 80 ± 9 ‡,c | 102 ± 5 ‡,c | 58 ± 4 ‡ | 36 ± 5 ‡ | 0.76 ± 0.16 ‡,c | 4.8 ± 0.3 ‡ | 14 ± 3 ‡,c | 8 ± 2 |
| HETT1 | 3.0 ± 0.1 § | 98 ± 1 | 134 ± 12 §,c | 83 ± 5 §,c | 47 ± 5 §,c | 29 ± 4 § | 1.06 ± 0.24 §,c | 5.9 ± 0.3 §,c | 19 ± 2 §,c | 10 ± 2 §,c |
| DOBT2 | 2.9 ± 0.1 * | 98 ± 1 | 120 ± 11 * | 122 ± 7 * | 60 ± 6 * | 29 ± 5 * | 1.97 ± 0.22 * | 5.1 ± 0.1 * | 13 ± 2 | 7 ± 1 |
| NEPT2 | 2.9 ± 0.1 † | 98 ± 1 | 70 ± 10 †,d | 133 ± 8 †,d | 75 ± 8 d | 46 ± 6 d | 1.40 ± 0.31 †,d | 4.8 ± 0.2 † | 17 ± 3 †,d | 13 ± 2 †,d |
| VAST2 | 3.0 ± 0.1 ‡ | 98 ± 2 | 77 ± 8 ‡,d | 109 ± 5 ‡,d | 61 ± 7 ‡ | 37 ± 4 ‡ | 0.68 ± 0.23 ‡,d | 4.7 ± 0.2 ‡ | 16 ± 2 ‡,d | 10 ± 2 |
| HETT2 | 3.0 ± 0.1 § | 98 ± 1 | 138 ± 13 §,d | 84 ± 6 §,d | 48 ± 5 §,d | 30 ± 5 § | 1.14 ± 0.17 §,d | 7.4 ± 0.2 §,d | 21 ± 3 §,d | 14 ± 1 §,d |
| Treatment Timepoint | SVR (dyn · s/cm5) | PVR (dyn · s/cm5) | DO2 (mL/min) | VO2 (mL/min) | OER (%) |
|---|---|---|---|---|---|
| DOBbaseline | 3108 ± 387 | 182 ± 14 | 362 ± 19 | 77 ± 8 | 21 ± 2 |
| NEPbaseline | 3095 ± 293 | 189 ± 15 | 321 ± 16 | 71 ± 7 | 22 ± 2 |
| VASbaseline | 2986 ± 327 | 186 ± 12 | 326 ± 14 | 79 ± 9 | 24 ± 1 |
| HETbaseline | 3297 ± 304 | 195 ± 13 | 336 ± 17 | 68 ± 6 | 20 ± 2 |
| DOBT0 | 3400 ± 319 | 363 ± 21 * | 168 ± 12 * | 39 ± 9 * | 23 ± 2 |
| NEPT0 | 3107 ± 358 | 336 ± 20 † | 185 ± 15 † | 43 ± 7 † | 23 ± 3 |
| VAST0 | 3200 ± 304 | 323 ± 22 ‡ | 191 ± 13 ‡ | 41 ± 7 ‡ | 21 ± 1 |
| HETT0 | 3586 ± 371 | 367 ± 19 § | 170 ± 9 § | 36 ± 6 § | 21 ± 2 |
| DOBT1 | 2213 ± 385 * | 153 ± 16 * | 413 ± 11 | 90 ± 7 | 22 ± 1 |
| NEPT1 | 4279 ± 406 †,c | 318 ± 14 †,c | 244 ± 10 †,c | 56 ± 7 †,c | 23 ± 1 |
| VAST1 | 5600 ± 392 ‡,c | 631 ± 20 ‡,c | 146 ± 13 ‡,c | 31 ± 6 ‡,c | 21 ± 2 |
| HETT1 | 3101 ± 333 c | 679 ± 23 §,c | 206 ± 9 §,c | 47 ± 6 §,c | 23 ± 2 |
| DOBT2 | 2067 ± 402 * | 244 ± 21 * | 386 ± 12 | 88 ± 6 | 22 ± 1 |
| NEPT2 | 4011 ± 366 †,d | 228 ± 18 † | 266 ± 9 †,d | 60 ± 5 †,d | 23 ± 2 |
| VAST2 | 6343 ± 459 ‡,d | 705 ± 22 ‡,d | 129 ± 11 ‡,d | 28 ± 5 ‡,d | 22 ± 1 |
| HETT2 | 2849 ± 427 §,d | 491 ± 24 §,d | 222 ± 10 §,d | 50 ± 7 §,d | 23 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henao-Guerrero, N.; Ricco-Pereira, C.H.; Paranjape, V.V. A Comparison of Dobutamine, Norepinephrine, Vasopressin, and Hetastarch for the Treatment of Isoflurane-Induced Hypotension in Healthy, Normovolemic Dogs. Animals 2023, 13, 2674. https://doi.org/10.3390/ani13162674
Henao-Guerrero N, Ricco-Pereira CH, Paranjape VV. A Comparison of Dobutamine, Norepinephrine, Vasopressin, and Hetastarch for the Treatment of Isoflurane-Induced Hypotension in Healthy, Normovolemic Dogs. Animals. 2023; 13(16):2674. https://doi.org/10.3390/ani13162674
Chicago/Turabian StyleHenao-Guerrero, Natalia, Carolina H. Ricco-Pereira, and Vaidehi V. Paranjape. 2023. "A Comparison of Dobutamine, Norepinephrine, Vasopressin, and Hetastarch for the Treatment of Isoflurane-Induced Hypotension in Healthy, Normovolemic Dogs" Animals 13, no. 16: 2674. https://doi.org/10.3390/ani13162674
APA StyleHenao-Guerrero, N., Ricco-Pereira, C. H., & Paranjape, V. V. (2023). A Comparison of Dobutamine, Norepinephrine, Vasopressin, and Hetastarch for the Treatment of Isoflurane-Induced Hypotension in Healthy, Normovolemic Dogs. Animals, 13(16), 2674. https://doi.org/10.3390/ani13162674







