Unraveling the Sexual Dimorphism of First Instar Nymphs of the Giant Stick Insect, Cladomorphus phyllinus Gray, 1835, from the Atlantic Forest, Brazil
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
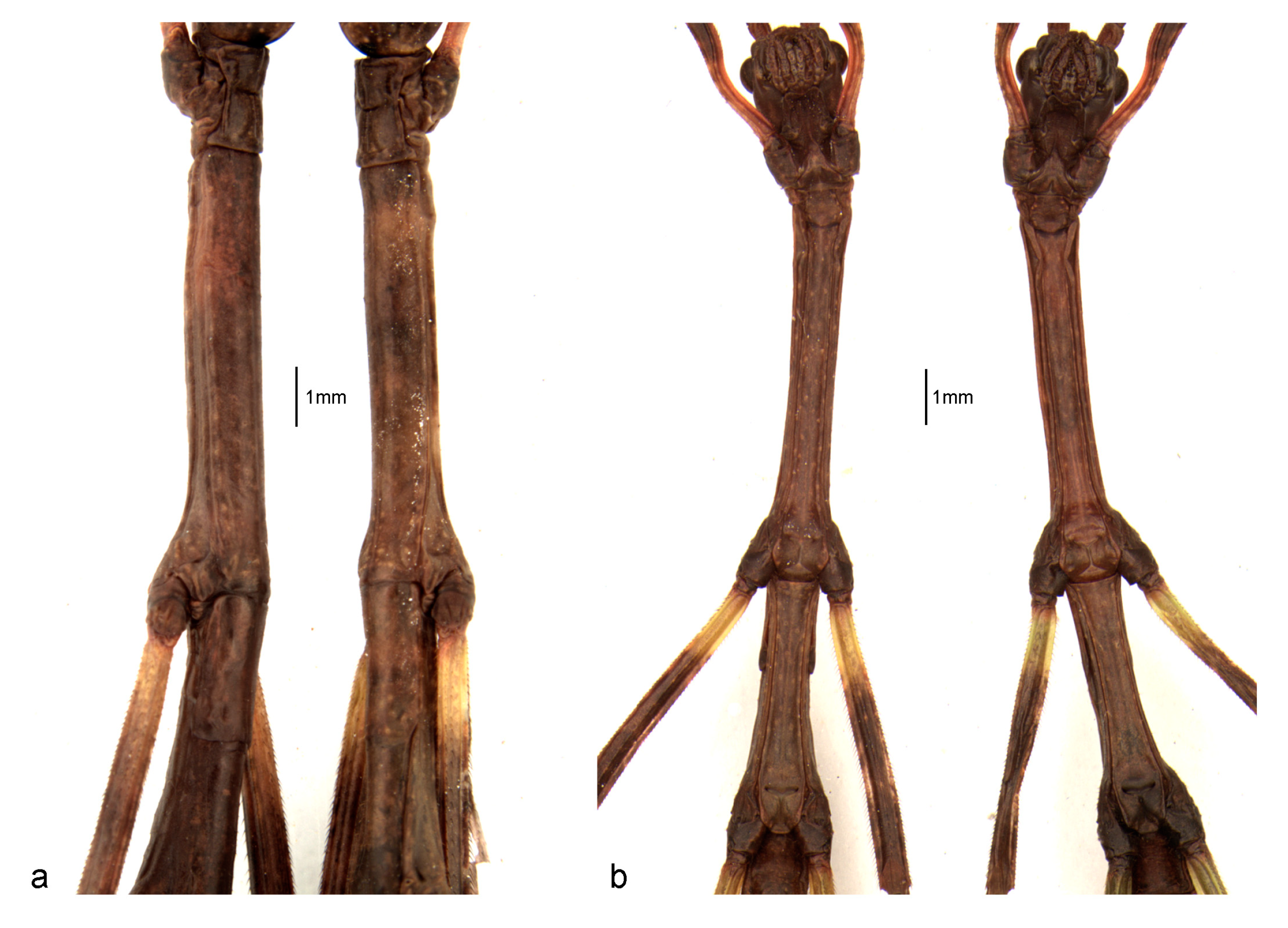
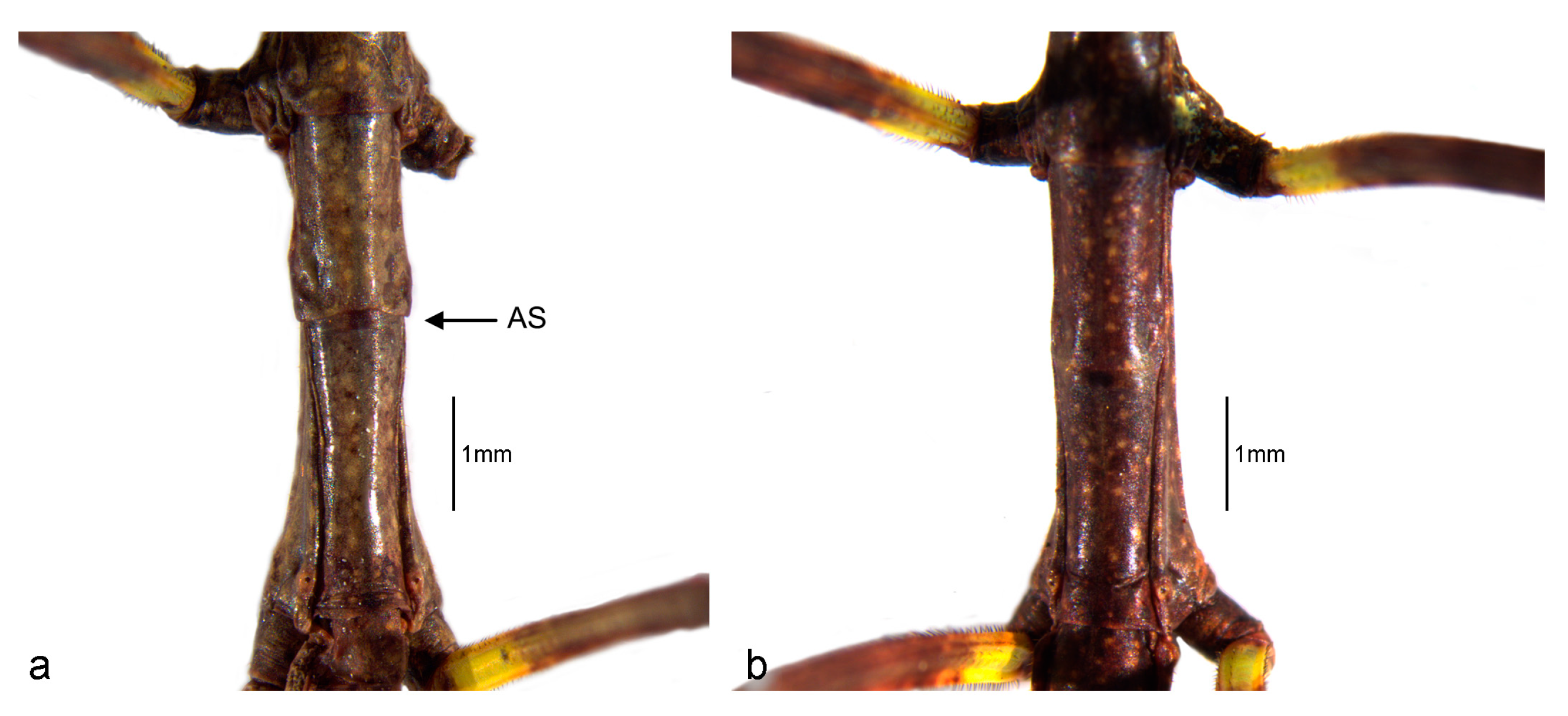
3.1. Phenotypic Description of the First Instar Nymph
3.1.1. General Aspect
| (±SD) | Median | IQR | p-Value | |||
|---|---|---|---|---|---|---|
| Overall length | Female | 22.2 (0.151) | 22.3 | 0.175 | 4.68 | <0.05 * |
| Male | 22.0 (0.241) | 22 | 0.100 | |||
| Head length | Female | 1.95 (0.053) | 1.95 | 0.100 | 0.192 | 0.661 |
| Male | 1.96 (0.0516) | 2 | 0.100 | |||
| Head width | Female | 2 (0.067) | 2 | 0 | 2.887 | 0.089 |
| Male | 1.95 (0.053) | 1.95 | 0.100 | |||
| Antenna | Female | 13.1 (0.225) | 13.0 | 0.175 | 0.0247 | 0.875 |
| Male | 13.1 (0.212) | 13.0 | 0.200 | |||
| Prothorax length | Female | 1.18 (0.103) | 1.2 | 0.175 | 0 | 1 |
| Male | 0.092 (1.2) | 1.2 | 0.075 | |||
| Mesothorax length | Female | 5.08 (0.123) | 5.05 | 0.175 | 4.263 | <0.05 * |
| Male | 4.91 (0.197) | 5 | 0.075 | |||
| Metathorax length | Female | 4.28 (0.162) | 4.3 | 0.175 | 3.41 | 0.065 |
| Male | 4.15 (0.127) | 4.1 | 0.100 | |||
| Abdomen length | Female | 9.76 (0.158) | 9.8 | 0.25 | 0.472 | 0.492 |
| Male | 9.82 (0.262) | 9.85 | 0.45 | |||
| Fore leg | Female | 15.9 (0.279) | 16 | 0.075 | 12.351 | <0.05 * |
| Male | 15.2 (0.179) | 15.2 | 0.275 | |||
| Middle leg | Female | 13.3 (0.246) | 13.4 | 0.40 | 0.997 | 0.318 |
| Male | 13.2 (0.211) | 13.2 | 0.425 | |||
| Hind leg | Female | 16.6 (0.392) | 16.8 | 0.550 | 4.229 | <0.05 * |
| Male | 16.2 (0.217) | 16.2 | 0.350 | |||
| Mesothoracic suture | Female | 0.99 (0.110) | 1 | 0.075 | 2.839 | 0.092 |
| Male | 1.07 (0.067) | 1 | 0.100 | |||
| Metathoracic suture | Female | 1.07 (0.082) | 1.1 | 0.075 | 3.344 | 0.067 |
| Male | 1.13 (0.048) | 1.1 | 0.075 |
3.1.2. Coloration
3.1.3. Structure and Vestiture
3.2. Sexual Dimorphism in First Instar Nymphs
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersson, M. Sexual Selection; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Benítez, H.A.; Vidal, M.; Briones, R.; Jerez, V. Sexual Dimorphism and Morphological Variation in Populations of Ceroglossus chilensis (Eschscholtz, 1829) (Coleoptera: Carabidae). J. Entomol. Res. Soc. 2010, 12, 87–95. [Google Scholar]
- Ralls, K.; Mesnick, S. Sexual dimorphism. In Encyclopedia of Marine Mammals, 2nd ed.; Perrin, W.F., Würsig, B., Thewissen, J.G.M., Eds.; Academic Press: London, UK, 2009. [Google Scholar]
- Esperk, T.; Tammaru, T.; Nylin, S.; Teder, T. Achieving high sexual size dimorphism in insects: Females add instars. Ecol. Entomol. 2007, 32, 243–256. [Google Scholar] [CrossRef]
- Blanckenhorn, W.U. Behavioral causes and consequences of sexual size dimorphism. Ethology 2005, 111, 977–1016. [Google Scholar] [CrossRef]
- Daly, H.V. Insect Morphometrics. Ann. Rev Entomol. 1985, 30, 415–438. [Google Scholar] [CrossRef]
- Benítez, H.A.; Sanzana, M.-J.; Jerez, V.; Parra, L.E.; Hernandez, C.E.; Canales-Aguirre, C.B. Sexual Shape and Size Dimorphism in Carabid Beetles of the Genus Ceroglossus: Is Geometric Body Size Similar between Sexes Due to Sex Ratio? Zool. Sci. 2013, 30, 289–295. [Google Scholar] [CrossRef]
- Bradler, S.; Buckley, T.R. Biodiversity of Phasmatodea. In Insect Biodiversity: Science and Society; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 281–313. [Google Scholar] [CrossRef]
- Simon, S.; Letsch, H.; Bank, S.; Buckley, T.R.; Donath, A.; Liu, S.; Machida, R.; Meusemann, K.; Misof, B.; Podsiadlowski, L.; et al. Old World and New World Phasmatodea: Phylogenomics resolve the evolutionary history of stick and leaf insects. Front. Ecol. Evol. 2019, 7, 345. [Google Scholar] [CrossRef]
- Brock, P.D.; Büsher, T. Stick and Leaf Insects of the World; NAP Editions: Verrières-le-Buisson, France, 2022. [Google Scholar]
- Brock, P.D.; Büscher, T.; Baker, E. Phasmida Species File Online (Version 5.0/5.0). Available online: http://phasmida.speciesfile.org/ (accessed on 5 May 2023).
- Madeira-Ott, T.; Thyssen, P.J.; Costa, J. Phasmatodea (Arthropoda, Insecta) in Brazil: Status, New Record and Proposal for using molecular tools to assist in species identification. Neotrop. Entomol. 2020, 49, 916–922. [Google Scholar] [CrossRef]
- Zompro, O. A key to the stick-insect genera of the ‘Anareolatae’ of the New World, with descriptions of several new taxa (Insecta: Phasmatodea). Stud. Neotrop. Fauna Environ. 2004, 39, 133–144. [Google Scholar] [CrossRef]
- Zompro, O. Phasmatodea. In Insetos do Brasil: Diversidade e Taxonomia; Holos Press: Ribeirão Preto, Brazil, 2012; pp. 289–306. [Google Scholar]
- Araujo, F.F.; Garraffoni, A.R.S. Sinopse dos Phasmatodea (Insecta) Descritos para o Brasil. Entomobrasilis 2012, 5, 232–237. [Google Scholar] [CrossRef]
- Hennemann, F.H.; Conle, O.V.; Perez-Gelabert, D.E. Studies on Neotropical Phasmatodea XVI: Revision of Haplopodini Günther, 1953 (rev. stat.), with notes on the subfamily Cladomorphinae Bradley and Galil, 1977 and the descriptions of a new tribe, four new genera and nine new species (Phasmatodea: “Anareolatae”: Phasmatidae: Cladomorphinae). Zootaxa 2016, 4128, 1–211. [Google Scholar] [CrossRef]
- Heleodoro, R.A.; Queiroz, L.; Rafael, J.A. Two new species of Periphloea Redtenbacher, 1906 (Insecta: Phasmatodea: Pseudophasmatidae) from the Brazilian Amazon Basin. Zootaxa 2021, 5047, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Chiquetto-Machado, P.I.; Morales, E.C.; Cancello, E.M. Taxonomic revision of Paraphasma Redtenbacher, 1906 (Phasmatodea, Pseudophasmatidae) based on phallic and external morphology. Zootaxa 2022, 5122, 1–80. [Google Scholar] [CrossRef] [PubMed]
- Heleodoro, R.A.; Rafael, J.A. Phasmatidae in: Catálogo Taxonômico da Fauna do Brasil. PNUD. Available online: http://fauna.jbrj.gov.br/fauna/faunadobrasil/33855 (accessed on 5 May 2023).
- Lima, A.R.; Kumagai, A.F.; Neto, F.C. Morphological and biological observations on the stick insect Tithonophasma tithonus (Gray, 1835) (Phasmida: Pseudophasmatidae: Pseudophasmatinae). Zootaxa 2013, 3700, 588–592. [Google Scholar] [CrossRef]
- Chiquetto-Machado, P.I. Redescription of the Brazilian stick insect Pseudophasma cambridgei Kirby (Phasmatodea: Pseudophasmatidae), with first description of the female and egg. Austral Entomol. 2018, 57, 392–402. [Google Scholar] [CrossRef]
- Kumagai, A.F.; Fonseca, N.G. Uma nova espécie de Cladomorphus (Phasmatidae, Cladomorphinae) de Minas Gerais, Brasil. Rev. Bras. Entomol. 2009, 53, 41–44. [Google Scholar] [CrossRef]
- Costa, J.; Mallet, J.R.S.; Takiya, D.M. Cladomorphus petropolisensis, a New Species of Stick Insect from the Atlantic Forest, Rio de Janeiro, Brazil. Animals 2022, 12, 2871. [Google Scholar] [CrossRef] [PubMed]
- Heleodoro, R.A. The first two cases of antisymmetry in the male genitalia of Phasmatodea reveal a new species of Isagoras Stål, 1875 (Phasmatodea: Pseudophasmatidae: Xerosomatinae) from the Brazilian Atlantic Forest. Zool. Anz. 2022, 296, 161–178. [Google Scholar] [CrossRef]
- Clark, J.T. The eggs of stick insects (Phasmida): A review with descriptions of the eggs of eleven species. Syst. Entomol. 1976, 1, 95–105. [Google Scholar] [CrossRef]
- Clark, J.T. A key to the eggs of stick and leaf insects (Phasmida). Syst. Entomol. 1979, 4, 325–331. [Google Scholar] [CrossRef]
- Sellick, J. The range of egg capsule morphology within the Phasmatodea and its relevance to the taxonomy of the order. Ital. J. Zool. 1997, 64, 97–104. [Google Scholar] [CrossRef]
- Goldberg, J.; Bresseel, C.J.; Kneubühler, B.; Leubner, F.; Michalik, P.; Bradler, S. Extreme convergence in egg-laying strategy across insect orders. Sci. Rep. 2015, 5, 7825. [Google Scholar] [CrossRef] [PubMed]
- Chiquetto-Machado, P.I.; Albertoni, F.F. Description of the female, egg and first instar nymph of the stick insect Paraphasma paulense (Phasmatodea: Pseudophasmatidae) from Southeast Brazil. J. Orthoptera Res. 2017, 26, 91–101. [Google Scholar] [CrossRef][Green Version]
- Bradler, S. Die Phylogenie der Stab- und Gespenstschrecken (Insecta: Phasmatodea). In Species, Phylogeny and Evolution; Georg-August-Universität: Göttingen, German, 2009; pp. 3–139. [Google Scholar]
- Bank, S.; Bradler, S.A. second view on the evolution of flight in stick and leaf insects (Phasmatodea). BMC Ecol. Evo. 2022, 22, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Dorval, A.; Peres-Filho, O.; Moraes, C.S.P.; Berti-Filho, E. Biologia e Estudo Comportamental de Bacteria tuberculata Piza Jr. 1939 (Phasmatodea; Phasmatidae) em Folhas de Anjico (Piptenia spp.). Sci. For. 2003, 63, 150–157. Available online: http://www.ipef.br/publicacoes/scientia/nr63/cap12.pdf (accessed on 4 May 2022).
- Alvarenga, C.D.; Souza, H.R.; Giustolin, T.A.; Matrangolo, C.A.R.; Silva, J.F. Biologia de Cladomorphus phyllinus Gray (Phasmatodea: Phasmatidae) em folhas de goiabeira (Psidium guajava). EntomoBrasilis 2018, 11, 65–69. [Google Scholar] [CrossRef]
- Costa, J.; Torres, L.; Provance, W.D.; Brugnera, R.; Grazia, J. First report of predation by a stink bug (Supputius cincticeps Stål) on a walking-stick insect (Cladomorphus phyllinus Gray), with reflections on evolutionary mechanisms for camouflage. Paraná Acta Biol. 2019, 48, 5–15. [Google Scholar]
- Costa, J.; Chiquetto-Machado, P.I.; Gil-Santana, H. Predation strategies of Harpactor angulosus (Lepeletier & Serville, 1825) (Hemiptera: Reduviidae) on Cladomorphus phyllinus Gray, 1835 (Phasmatodea: Phasmatidae) in captivity. Rev. Chilena Ent. 2022, 48, 531–543. [Google Scholar] [CrossRef]
- Torres, L.; Benitez, H.A.; Costa, J. Longevity, fertility and average eggs viability of parthenogenetic females of Cladomorphus phyllinus Gray (Phasmatodea, Phasmatide). Entomobrasilis 2022, 15, 998. [Google Scholar] [CrossRef]
- Torres, L.; Costa, J. Eu, Bicho-Pau; Fiocruz: Rio de Janeiro, Brasil, 2020; pp. 1–23. ISBN 978-65-00-21891-6. Available online: https://portal.fiocruz.br/noticia/fiocruz-lanca-livro-sobre-biodiversidade-para-criancas (accessed on 15 June 2023).
- Costa-Lima, A.M. Insetos do Brasil; Série didática; Escola Nacional de Agronomia: Rio de Janeiro, Brazil, 1938; pp. 2–453. [Google Scholar]
- Leuzinger, H.; Wiesmann, R.M.; Lehmann, F.E. Zur Kenntnis der Anatomie und Entwicklungsgeschichte der Stabheuschrecke Carausius Morosus; Gustav Fischer: Jena, Germany, 1926. [Google Scholar]
- Wilbert, H. Normales und experimentell beeinflusstes Auftreten von Männchen und Gynandromorphen der Stabheuschrecke. Zool. Jahrb. Physiol. 1953, 64, 470–495. [Google Scholar]
- Dürr, V.; Mesanovic, A. Behavioural function and development of body-to-limb proportions and active movement ranges in three stick insect species. J. Comp. Physiol. 2023, 209, 265–284. [Google Scholar] [CrossRef]
- Djordjevic, J.; Dumas, Z.; Robinson-Rechavi, M.; Schwander, T.; Parker, D.J. Dynamics of sex-biased gene expression during development in the stick insect Timema Californicum. Hered. 2022, 129, 113–122. [Google Scholar] [CrossRef]
- Badyaev, A.V.; Whittingham, L.A.; Hill, G.E. The evolution of sexual size dimorphism in house finch. III. Developmental basis. Evolution 2001, 55, 176–189. [Google Scholar]
- Badyaev, A.V. Growing apart: An ontogenetic perspective on the evolution of sexual size dimorphism. Trends Ecol. Evolut. 2002, 17, 369–378. [Google Scholar] [CrossRef]
- Langer, L.; Froschauer, C.; Reinhardt, K. Sex differences in bedbug nymphs, Cimex lectularius. J. Appl. Entomol. 2020, 144, 838–843. [Google Scholar] [CrossRef]
- Goncalves, T.C.M.; Jurberg, J.; Costa, J.M.; Souza, W. Estudo morfológico comparativo de ovos e ninfas de Triatoma maculata (Erichson, 1848) e Triatoma pseudomaculata Correa & Espínola, 1964 (Hemiptera, Reduviidae, Triatominae). Mem. Inst. Oswaldo Cruz. 1985, 80, 263–276. [Google Scholar]
- Jurberg, J.; Gonçalves, T.C.M.; Costa, J.M.; Souza, W. Contribuição ao estudo morfológico de ovos e ninfas de Triatoma brasiliensis Neiva, 1911 (Hemiptera, Reduviidae, Triatominae). Mem. Inst. Oswaldo Cruz. 1986, 81, 111–120. [Google Scholar] [CrossRef]
- Cook, L.G.; Gullan, P.J.; Stewart, A.C. First-instar morphology and sexual dimorphism in the gall-inducing scale insect Apiomorpha Rubsaamen (Hemiptera: Coccoidea: Eriococcidae). J. Nat. Hist. 2000, 34, 879–894. [Google Scholar] [CrossRef]
- Whiting, M.F.; Bradler, S.; Maxwell, T. Loss and recovery of wings in stick insects. Nature 2003, 421, 264–267. [Google Scholar] [CrossRef]
- Zeng, Y.; O’Malley, C.; Singhal, S.; Rahim, F.; Park, S.; Chen, X.; Dudley, R. A tale of winglets: Evolution of flight morphology in stick insects. Front. Ecol. Evol. 2020, 8, 1–121. [Google Scholar] [CrossRef]
- Brock, P.D.; Hasenpusch, J. Studies on the Australian stick insects (Phasmida), including a checklist of species and bibliography. In Zootaxa; Magnolia Press: Auckland, New Zealand, 2007; pp. 1–81. [Google Scholar]
- Brock, P.D. Studies on the Australasian stick-insect genus Extatosoma Gray (Phasmida: Phasmatidae: Tropoderinae: Extatosomatini). J. Orthoptera Res. 2001, 10, 303–313. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, J.; Torres, L.; Paschoaletto, L.; Pimenta, A.L.A.; Benítez, H.A.; Suazo, M.J.; Reigada, C.; Gil-Santana, H.R. Unraveling the Sexual Dimorphism of First Instar Nymphs of the Giant Stick Insect, Cladomorphus phyllinus Gray, 1835, from the Atlantic Forest, Brazil. Animals 2023, 13, 3474. https://doi.org/10.3390/ani13223474
Costa J, Torres L, Paschoaletto L, Pimenta ALA, Benítez HA, Suazo MJ, Reigada C, Gil-Santana HR. Unraveling the Sexual Dimorphism of First Instar Nymphs of the Giant Stick Insect, Cladomorphus phyllinus Gray, 1835, from the Atlantic Forest, Brazil. Animals. 2023; 13(22):3474. https://doi.org/10.3390/ani13223474
Chicago/Turabian StyleCosta, Jane, Lucas Torres, Leticia Paschoaletto, Ana Luiza Anes Pimenta, Hugo A. Benítez, Manuel J. Suazo, Carolina Reigada, and Hélcio R. Gil-Santana. 2023. "Unraveling the Sexual Dimorphism of First Instar Nymphs of the Giant Stick Insect, Cladomorphus phyllinus Gray, 1835, from the Atlantic Forest, Brazil" Animals 13, no. 22: 3474. https://doi.org/10.3390/ani13223474
APA StyleCosta, J., Torres, L., Paschoaletto, L., Pimenta, A. L. A., Benítez, H. A., Suazo, M. J., Reigada, C., & Gil-Santana, H. R. (2023). Unraveling the Sexual Dimorphism of First Instar Nymphs of the Giant Stick Insect, Cladomorphus phyllinus Gray, 1835, from the Atlantic Forest, Brazil. Animals, 13(22), 3474. https://doi.org/10.3390/ani13223474









