Evaluation of Renin–Angiotensin–Aldosterone System Components and Enzymes in Systemically Hypertensive Cats Receiving Amlodipine
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Equilibrium Analysis of RAAS Components
2.2. Enzyme Activity
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA2 | adrenal responsiveness marker (aldosterone/angiotensin II) |
| ACE | angiotensin-converting enzyme |
| ACE-S | ACE activity marker (angiotensin II/angiotensin I) |
| ALT-S | alternative RAAS activation marker ([angiotensin 1,7 + angiotensin 1,5]/[angiotensin I + angiotensin II + angiotensin 1,7 + angiotensin 1,5]) |
| BP | blood pressure |
| LC-MS/MS | liquid chromatography–tandem mass spectrometry |
| NT | normotensive |
| PRA-S | plasma renin activity marker (angiotensin I + angiotensin II) |
| RAAS | renin–angiotensin–aldosterone system |
| SHT | systemic hypertension |
| SHT/CKD-A | systemic hypertension with chronic kidney disease treated with amlodipine |
References
- Arendse, L.B.; Danser, A.H.J.; Poglitsch, M.; Touyz, R.M.; Burnett, J.C., Jr.; Llorens-Cortes, C.; Ehlers, M.R.; Sturrock, E.D. Novel therapeutic approaches targeting the renin-angiotensin system and associated peptides in hypertension and heart failure. Pharmacol. Rev. 2019, 71, 539–570. [Google Scholar] [CrossRef] [PubMed]
- Acierno, M.J.; Brown, S.; Coleman, A.E.; Jepson, R.E.; Papich, M.; Stepien, R.L.; Syme, H.M. ACVIM consensus statement: Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J. Vet. Intern. Med. 2018, 32, 1803–1822. [Google Scholar] [CrossRef] [PubMed]
- Ligtenberg, G.; Blankestijn, P.J.; Oey, P.L.; Klein, I.H.; Dijkhorst-Oei, L.-T.; Boomsma, F.; Wieneke, G.H.; van Huffelen, A.C.; Koomans, H.A. Reduction of sympathetic hyperactivity by enalapril in patients with chronic renal failure. N. Engl. J. Med. 1999, 340, 1321–1328. [Google Scholar] [CrossRef]
- Atkins, C.E.; Rausch, W.P.; Gardner, S.Y.; Defrancesco, T.C.; Keene, B.W.; Levine, J.F. The effect of amlodipine and the combination of amlodipine and enalapril on the renin-angiotensin-aldosterone system in the dog. J. Vet. Pharmacol. Ther. 2007, 30, 394–400. [Google Scholar] [CrossRef]
- Littman, M.P. Spontaneous Systemic Hypertension in 24 Cats. J. Vet. Intern. Med. 1994, 8, 79–86. [Google Scholar] [CrossRef]
- Snyder, P.S. Amlodipine: A randomized, blinded clinical trial in 9 cats with systemic hypertension. J. Vet. Intern. Med. Am. Coll. Vet. Intern. Med. 1998, 12, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Henik, R.; Snyder, P.; Volk, L. Treatment of systemic hypertension in cats with amlodipine besylate. J. Am. Anim. Hosp. Assoc. 1997, 33, 226–234. [Google Scholar] [CrossRef]
- Glaus, T.M.; Elliott, J.; Herberich, E.; Zimmering, T.; Albrecht, B. Efficacy of long-term oral telmisartan treatment in cats with hypertension: Results of a prospective European clinical trial. J. Vet. Intern. Med. 2018, 33, 413–422. [Google Scholar] [CrossRef]
- Coleman, A.E.; Brown, S.A.; Traas, A.M.; Bryson, L.; Zimmering, T.; Zimmerman, A. Safety and efficacy of orally administered telmisartan for the treatment of systemic hypertension in cats: Results of a double-blind, placebo-controlled, randomized clinical trial. J. Vet. Intern. Med. 2019, 33, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Inoue, T.; Suzuki, T.; Kawagoe, Y.; Ueda, Y.; Koide, H.; Node, K. Comparison of Renal and Vascular Protective Effects between Telmisartan and Amlodipine in Hypertensive Patients with Chronic Kidney Disease with Mild Renal Insufficiency. Hypertens. Res. 2008, 31, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Schiffrin, E.L.; Jeong, F.; Park, B.; Intengan, H.D.; Touyz, R.M. Correction of Arterial Structure and Endothelial Dysfunction in Human Essential Hypertension by the Angiotensin Receptor Antagonist Losartan. 2000, Volume 101. Available online: http://www.circulationaha.org (accessed on 10 July 2023).
- Jepson, R.; Syme, H.; Elliott, J. Plasma renin activity and aldosterone concentrations in hypertensive cats with and without azotemia and in response to treatment with amlodipine besylate. J. Vet. Intern. Med. 2013, 28, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.L.; Guillot, E.; Domenig, O.; Ware, W.A.; Yuan, L.; Mochel, J.P. Circulating renin-angiotensin-aldosterone system activity in cats with systemic hypertension or cardiomyopathy. J. Vet. Intern. Med. 2022, 36, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Potter, B.M.; Ames, M.K.; Hess, A.; Poglitsch, M. Comparison between the effects of torsemide and furosemide on the renin-angiotensin-aldosterone system of normal dogs. J. Vet. Cardiol. 2019, 26, 51–62. [Google Scholar] [CrossRef]
- Huh, T.; Larouche-Lebel, E.; Loughran, K.A.; Oyama, M.A. Effect of angiotensin receptor blockers and angiotensin-converting enzyme 2 on plasma equilibrium angiotensin peptide concentrations in cats with heart disease. J. Vet. Intern. Med. 2020, 35, 33–42. [Google Scholar] [CrossRef]
- Adin, D.; Atkins, C.; Domenig, O.; DeFrancesco, T.; Keene, B.; Tou, S.; Stern, J.A.; Meurs, K.M. Renin-angiotensin aldosterone profile before and after angiotensin-converting enzyme-inhibitor administration in dogs with angiotensin-converting enzyme gene polymorphism. J. Vet. Intern. Med. 2020, 34, 600–606. [Google Scholar] [CrossRef]
- Pavo, N.; Goliasch, G.; Wurm, R.; Novak, J.; Strunk, G.; Gyöngyösi, M.; Poglitsch, M.; Säemann, M.D.; Hülsmann, M. Low-and high-renin heart failure phenotypes with clinical implications. Clin Chem. 2018, 64, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Poglitsch, M.; McWhinney, B.C.; Ungerer, J.P.J.; Ahmed, A.H.; Gordon, R.D.; Wolley, M.; Stowasser, M. Measurement of Equilibrium Angiotensin II in the Diagnosis of Primary Aldosteronism. Clin. Chem. 2020, 66, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Hammond, H.H.; Ames, M.K.; Domenig, O.; Scansen, B.A.; Yang, N.T.; Wilson, M.D.; Sunshine, E.; Brunk, K.; Masters, A. The classical and alternative circulating renin-angiotensin system in normal dogs and dogs with stage B1 and B2 myxomatous mitral valve disease. J. Vet. Intern. Med. 2023, 37, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Ames, M.K.; Atkins, C.E.; Pitt, B. The renin-angiotensin-aldosterone system and its suppression. J. Vet. Intern. Med. 2019, 33, 363–382. [Google Scholar] [CrossRef]
- Ocaranza, M.P.; Riquelme, J.A.; García, L.; Jalil, J.E.; Chiong, M.; Santos, R.A.S.; Lavandero, S. Counter-regulatory renin–angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 116–129. [Google Scholar] [CrossRef]
- Lourenço, B.N.; Coleman, A.E.; Berghaus, R.D.; Tarigo, J.L.; Schmiedt, C.W.; Brown, S.A. Characterization of the intrarenal renin-angiotensin system in cats with naturally occurring chronic kidney disease. J. Vet. Intern. Med. 2022, 36, 647–655. [Google Scholar] [CrossRef]
- Lefebvre, H.; Duparc, C.; Naccache, A.; Lopez, A.G.; Castanet, M.; Louiset, E. Paracrine Regulation of Aldosterone Secretion in Physiological and Pathophysiological Conditions. Vitam. Horm. 2019, 109, 303–339. [Google Scholar]
- De Lean, A.; Racz Karoly McNicoll, N.; Luce Desrosiers, M. Direct beta-adrenergic stimulation of aldosterone secretion in cultured bovine adrenal subcapsular cells. Endocrinology 1984, 115, 485–492. [Google Scholar] [CrossRef]
- Hamming, I.; Van Goor, H.; Turner, A.J.; Rushworth, C.A.; Michaud, A.A.; Corvol, P.; Navis, G. Differential regulation of renal angiotensin-converting enzyme (ACE) and ACE2 during ACE inhibition and dietary sodium restriction in healthy rats. Exp. Physiol. 2008, 93, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Gowrisankar, Y.V.; Clark, M.A. Angiotensin II regulation of angiotensin-converting enzymes in spontaneously hypertensive rat primary astrocyte cultures. J. Neurochem. 2016, 138, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Te Riet, L.; Van Esch, J.H.M.; Roks, A.J.M.; Van Den Meiracker, A.H.; Danser, A.H.J. Hypertension: Renin-Angiotensin-Aldosterone System Alterations; Circulation Research; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2015; Volume 116, pp. 960–975. [Google Scholar]
- Stoll, D.; Yokota, R.; Sanches Aragão, D.; Casarini, D.E. Both aldosterone and spironolactone can modulate the intracellular ACE/ANG II/AT1 and ACE2/ANG (1-7)/MAS receptor axes in human mesangial cells. Physiol Rep. 2019, 7, e14105. [Google Scholar] [CrossRef]
- Zakrzewski-Jakubiak, M.; de Denus, S.; Dubé, M.-P.; Bélanger, F.; White, M.; Turgeon, J. Ten renin-angiotensin system-related gene polymorphisms in maximally treated Canadian Caucasian patients with heart failure. Br. J. Clin. Pharmacol. 2008, 65, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.C. Sex and the renin-angiotensin system: Inequality between the sexes in response to RAS stimulation and inhibition. Am. J. Physiol. Integr. Comp. Physiol. 2008, 294, R1220–R1226. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.; Elliott, J.; Syme, H. Renin-Angiotensin-Aldosterone System Activity in Hyperthyroid Cats with and without Concurrent Hypertension. J. Vet. Intern. Med. 2013, 27, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Bijsmans, E.; Doig, M.; Jepson, R.; Syme, H.; Elliott, J.; Pelligand, L. Factors Influencing the Relationship Between the Dose of Amlodipine Required for Blood Pressure Control and Change in Blood Pressure in Hypertensive Cats. J. Vet. Intern. Med. 2016, 30, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
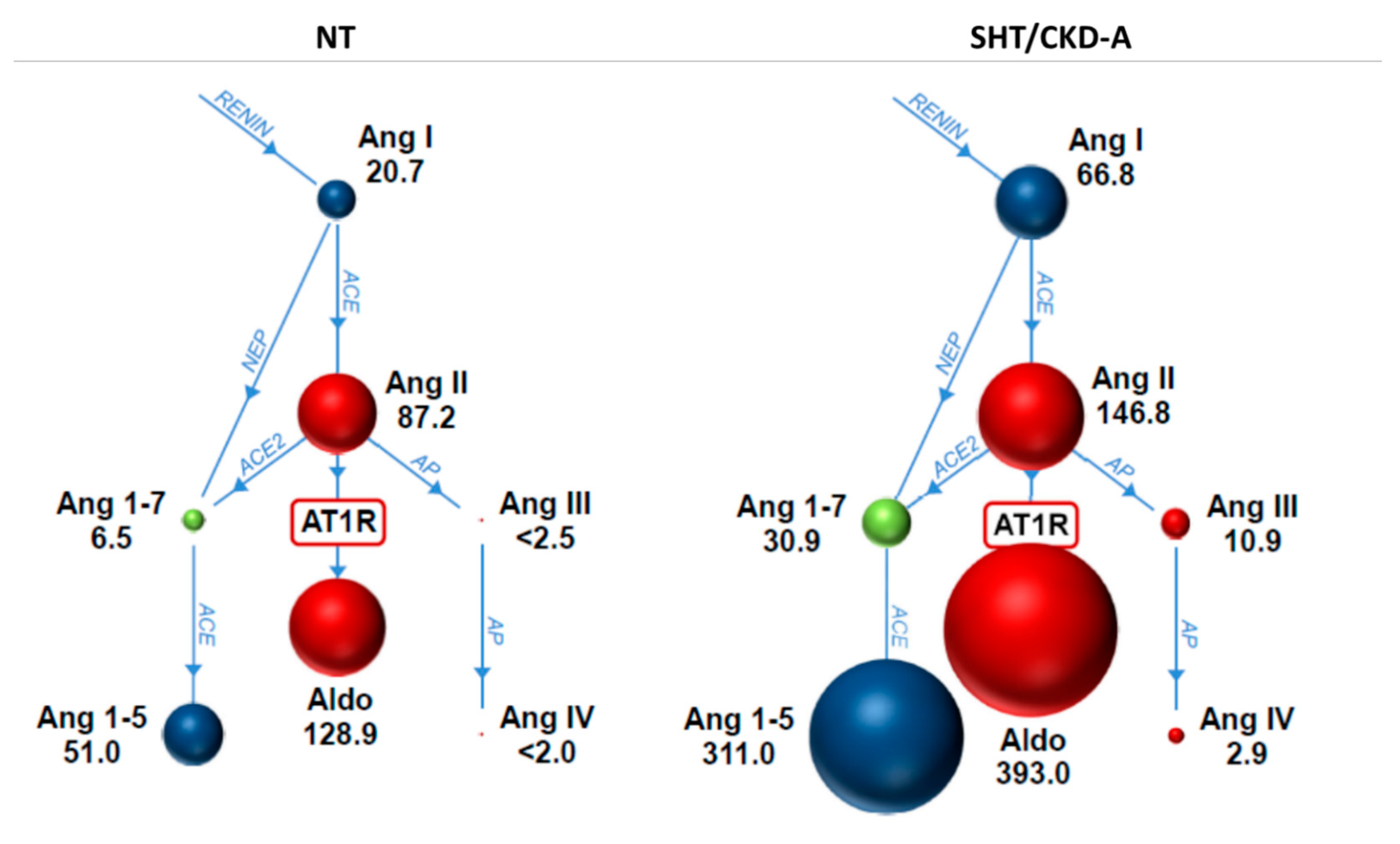
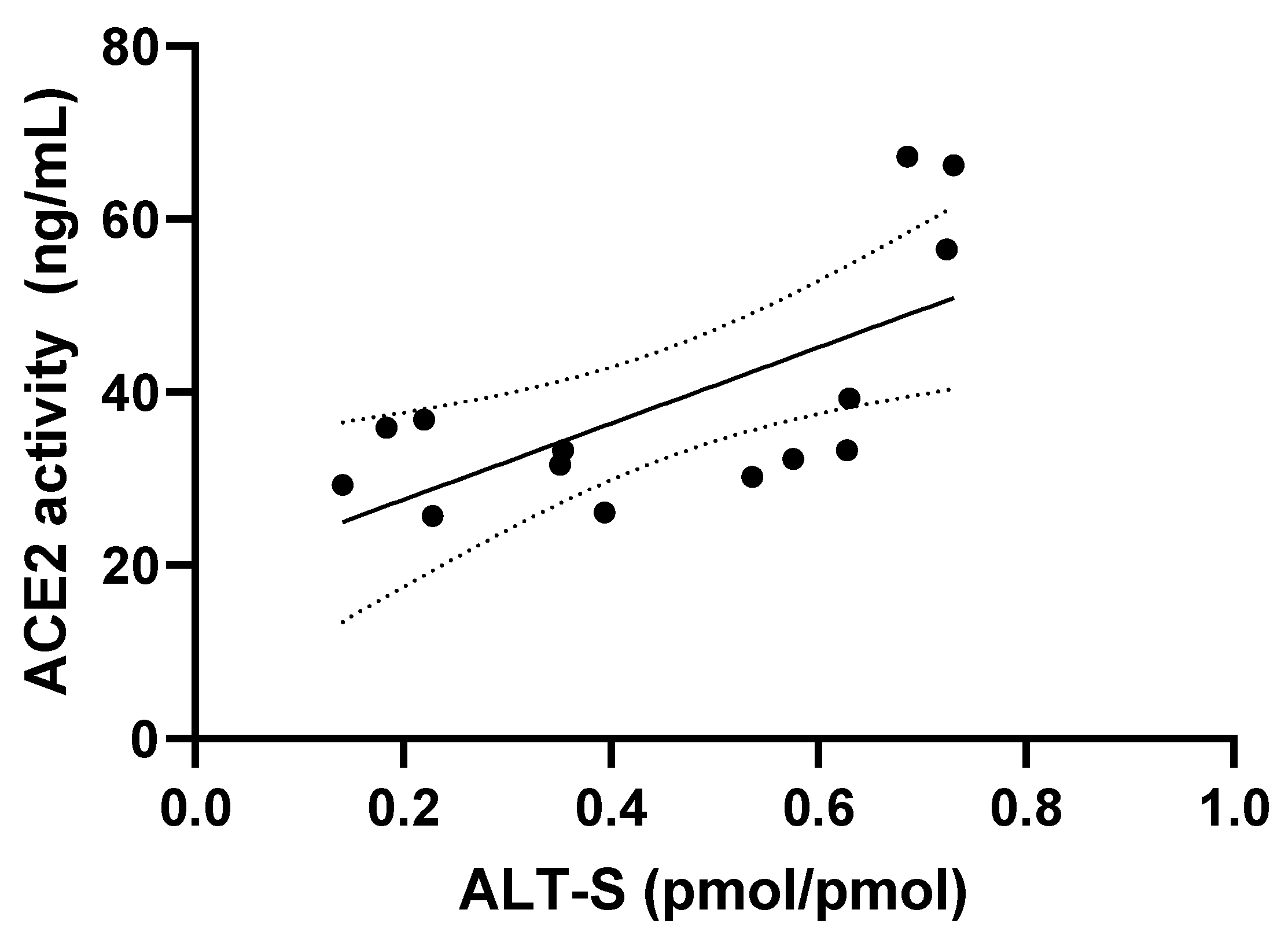
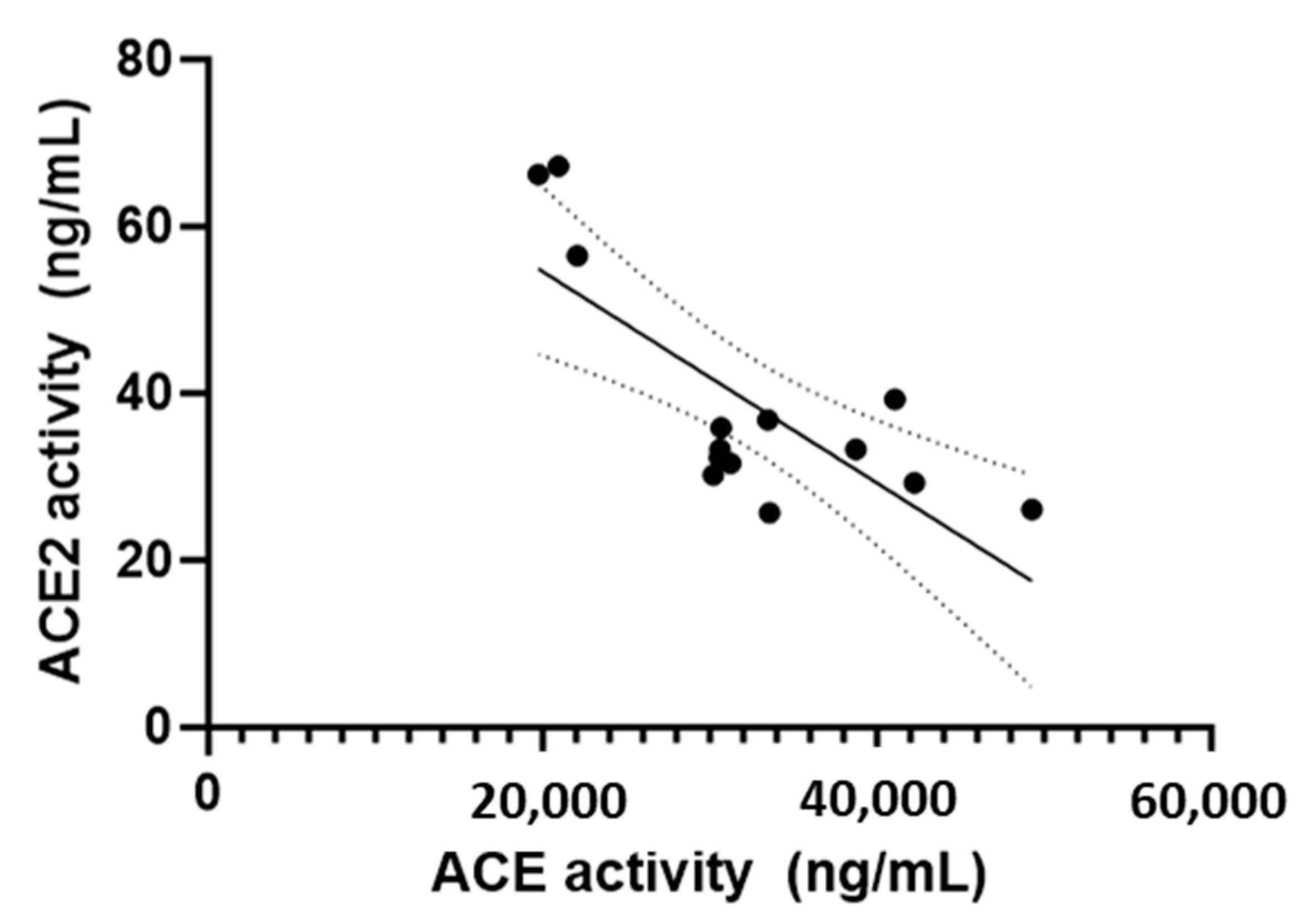
| Variable | NT Cats (n = 9) | SHT/CKD-A Cats (n = 5) | p Value |
|---|---|---|---|
| PRA-S (pmol/L) | 108 (34–206) | 199 (101–335) | 0.06 |
| angiotensin I (pmol/L) | 21 (2–36) | 67 (34–151) | 0.002 |
| angiotensin II (pmol/L) | 87 (30–177) | 147 (67–184) | 0.11 |
| angiotensin 1,7 (pmol/L) | 6.5 (1.3–11.7) | 30.9 (17.8–70.5) | 0.001 |
| angiotensin 1,5 (pmol/L) | 51 (16–135) | 311 (243–805) | 0.001 |
| angiotensin III (pmol/L) | 1.3 (1.3–10.0) | 10.9 (5.4–14.7) | 0.003 |
| angiotensin IV (pmol/L) | 1.3 (1.3–4.4) | 2.9 (2.2–3.1) | 0.21 |
| aldosterone (pmol/L) | 129 (28–206) | 393 (137–564) | 0.007 |
| AA2 (pmol/pmol) | 1.2 (0.2–3.3) | 2.7 (0.8–7.6) | 0.24 |
| ACE-S (pmol/pmol) | 5.4 (1.9–9.8) | 2.0 (1.1–3.0) | 0.007 |
| ACE activity (ng/mL) | 33.6 (30.2–49.3) | 22.1 (19.8–30.6) | 0.004 |
| ACE2 activity (ng/mL) | 32 (26–39) | 57 (32–67) | 0.045 |
| chymase activity (ng/mL) | 0.3 (0.1–2.0) | 0.8 (0.2–1.8) | 0.44 |
| ALT-S (pmol/pmol) | 35 (14–63) | 69 (58–73) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adin, D.; Atkins, C.; Domenig, O.; Glahn, C.; DeFrancesco, T.; Meurs, K. Evaluation of Renin–Angiotensin–Aldosterone System Components and Enzymes in Systemically Hypertensive Cats Receiving Amlodipine. Animals 2023, 13, 3479. https://doi.org/10.3390/ani13223479
Adin D, Atkins C, Domenig O, Glahn C, DeFrancesco T, Meurs K. Evaluation of Renin–Angiotensin–Aldosterone System Components and Enzymes in Systemically Hypertensive Cats Receiving Amlodipine. Animals. 2023; 13(22):3479. https://doi.org/10.3390/ani13223479
Chicago/Turabian StyleAdin, Darcy, Clarke Atkins, Oliver Domenig, Catherine Glahn, Teresa DeFrancesco, and Kathryn Meurs. 2023. "Evaluation of Renin–Angiotensin–Aldosterone System Components and Enzymes in Systemically Hypertensive Cats Receiving Amlodipine" Animals 13, no. 22: 3479. https://doi.org/10.3390/ani13223479





