Exploring the Effects of Acute Stress Exposure on Lumpfish Plasma and Liver Biomarkers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Acquisition and Aare
2.2. Study Design
2.3. Blood and Liver Sampling and Tissue Preparation
2.4. Plasma and Liver Parameters
2.5. Liver Oxidative Stress Assays
2.6. Data Analysis
3. Results
3.1. Cortisol
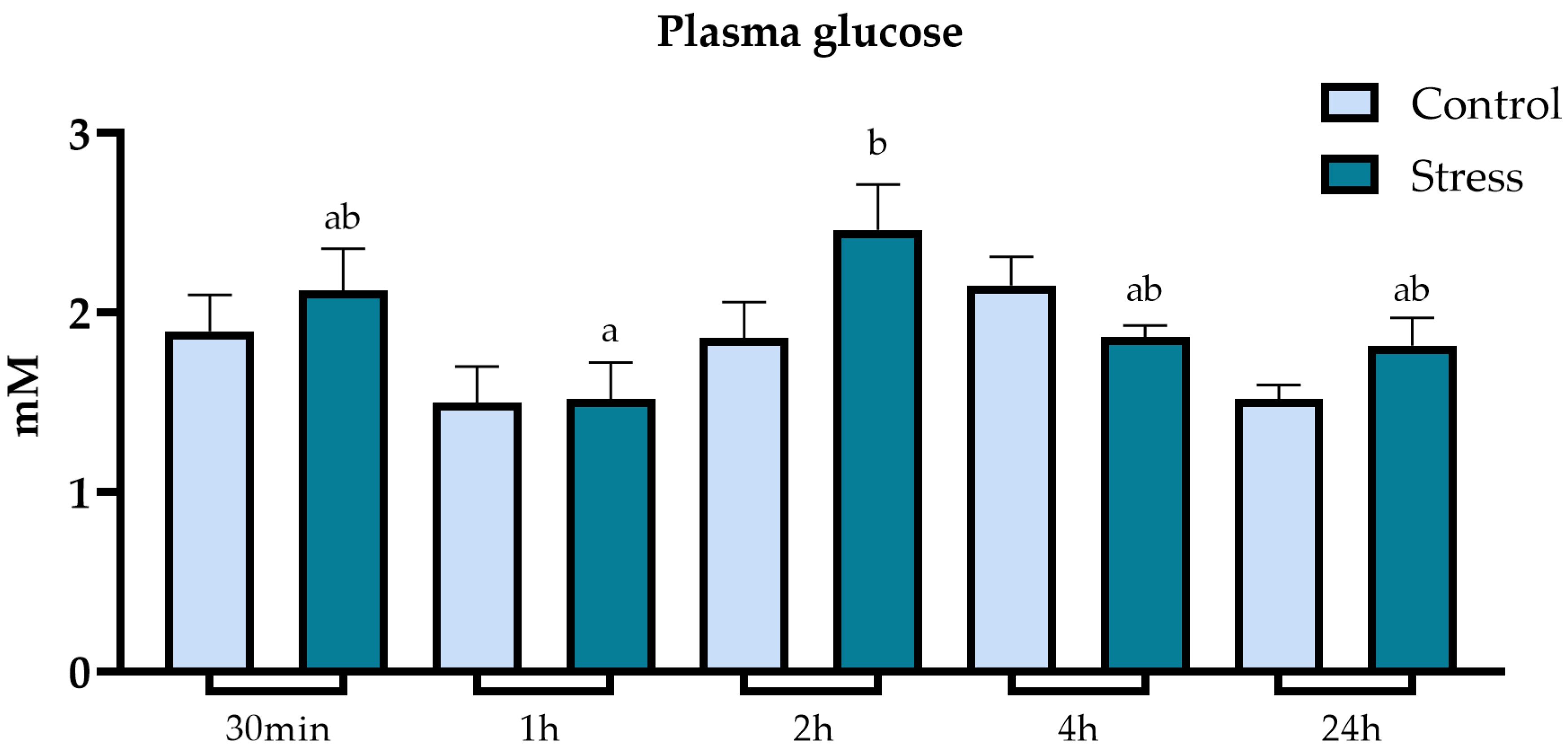
3.2. Metabolic Parameters
3.3. Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costello, M.J. How sea lice from salmon farms may cause wild salmonid declines in Europe and North America and be a threat to fishes elsewhere. Proc. R. Soc. B Biol. Sci. 2009, 276, 3385–3394. [Google Scholar] [CrossRef]
- Costello, M.J. The global economic cost of sea lice to the salmonid farming industry. J. Fish Dis. 2009, 32, 115–118. [Google Scholar] [CrossRef]
- Evans, J.; Sea Lice Report. Intrafish Industry Report. 2019. Available online: https://nhstglobalpublications.onfastspring.com/intrafish/sea-lice-2019-report (accessed on 8 August 2022).
- Aaen, S.M.; Helgesen, K.O.; Bakke, M.J.; Kaur, K.; Horsberg, T.E. Drug resistance in sea lice: A threat to salmonid aquaculture. Trends Parasitol. 2015, 31, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Strachan, F.; Kennedy, C.J. The environmental fate and effects of anti-sea lice chemotherapeutants used in salmon aquaculture. Aquaculture 2021, 544, 737079. [Google Scholar] [CrossRef]
- Barrett, L.T.; Oppedal, F.; Robinson, N.; Dempster, T. Prevention not cure: A review of methods to avoid sea lice infestations in salmon aquaculture. Rev. Aquac. 2020, 12, 2527–2543. [Google Scholar] [CrossRef]
- Powell, A.; Treasurer, J.W.; Pooley, C.L.; Keay, A.J.; Lloyd, R.; Imsland, A.K.; de Leaniz, C.G. Use of lumpfish for sea-lice control in salmon farming: Challenges and opportunities. Rev. Aquac. 2018, 10, 683–702. [Google Scholar] [CrossRef]
- Imsland, A.K.D.; Hanssen, A.; Nytrø, A.V.; Reynolds, P.; Jonassen, T.M.; Hangstad, T.A.; Elvegård, T.A.; Urskog, T.C.; Mikalsen, B. It works! Lumpfish can significantly lower sea lice infestation in large-scale salmon farming. Biol. Open 2018, 7, bio036301. [Google Scholar] [CrossRef] [PubMed]
- Imsland, A.K.; Micallef, G.; Korsnes, K.; Reynolds, P. Consumption of sea lice by lumpfish (Cyclopterus lumpus): qPCR quantification and use of a non-destructive sampling method. Aquaculture 2019, 500, 640–644. [Google Scholar] [CrossRef]
- Imsland, A.K.D.; Reynolds, P. In lumpfish We Trust? The Efficacy of Lumpfish Cyclopterus lumpus to Control Lepeophtheirus salmonis Infestations on Farmed Atlantic Salmon: A Review. Fishes 2022, 7, 220. [Google Scholar] [CrossRef]
- Fiskeridirektoratet. Aquaculture Statistics: Use of Cleanerfish 1998–2021. Norwegian Directorate of Fisheries. 2021. Available online: https://www.fiskeridir.no/English/Aquaculture/Statistics/Cleanerfish-Lumpfish-and-Wrasse (accessed on 8 August 2022).
- Stien, L.H.; Størkersen, K.V.; Gåsnes, S.K. Analyse Av Dødelighetsdata Fra Spørreundersøkelse Om Velferd Hos Rensefisk. 2020. Available online: https://samforsk.no/uploads/files/Publikasjoner/Analyse-av-dodelighetsdata-fra-sporreundersokelse-om-velferd-hos-rensefisk.pdf (accessed on 24 June 2020).
- Mattilsynet. Nasjonal Tilsynskampanje 2018/2019 Velferd Hos Rensefisk. The Norwegian Food Safety Authority’s National Inspection Projects. 2020. Available online: https://www.mattilsynet.no/fisk-og-akvakultur/rensefisk/nasjonal-tilsynskampanje-om-velferd-hos-rensefisk-2018-2019 (accessed on 9 August 2022).
- Jonassen, T.; Hamadi, M.; Remø, S.C.; Waagbø, R. An epidemiological study of cataracts in wild and farmed lumpfish (Cyclopterus lumpus L.) and the relation to nutrition. J. Fish Dis. 2017, 40, 1903–1914. [Google Scholar] [CrossRef]
- Imsland, A.K.; Reynolds, P.; Lorentzen, M.; Eilertsen, R.A.; Micallef, G.; Tvenning, R. Improving survival and health of lumpfish (Cyclopterus lumpus L.) by the use of feed blocks and operational welfare indicators (OWIs) in commercial Atlantic salmon cages. Aquaculture 2020, 527, 735476. [Google Scholar] [CrossRef]
- Staven, F.R.; Nordeide, J.T.; Imsland, A.K.; Andersen, P.; Iversen, N.S.; Kristensen, T. Is habituation measurable in lumpfish Cyclopterus lumpus when used as cleaner fish in atlantic salmon Salmo salar aquaculture? Front. Vet. Sci. 2019, 6, 227. [Google Scholar] [CrossRef] [PubMed]
- Sneddon, L.U.; Wolfenden, D.C.C.; Thomson, J.S. Stress Management and Welfare. Fish Physiol. 2016, 35, 463–539. [Google Scholar] [CrossRef]
- Urbinati, E.C.; Zanuzzo, F.S.; Biller, J.D. Stress and immune system in fish. In Biology and Physiology of Freshwater Neotropical Fish; Elsevier: Amsterdam, The Netherlands, 2020; pp. 93–114. [Google Scholar] [CrossRef]
- Reynolds, P.; Imsland, A.K.D.; Boissonnot, L. Causes of Mortality and Loss of Lumpfish Cyclopterus lumpus. Fishes 2022, 7, 328. [Google Scholar] [CrossRef]
- de Leaniz, C.G.; Rabadan, C.G.; Barrento, S.I.; Stringwell, R.; Howes, P.N.; Whittaker, B.A.; Minett, J.F.; Smith, R.G.; Pooley, C.L.; Overland, B.J.; et al. Addressing the welfare needs of farmed lumpfish: Knowledge gaps, challenges and solutions. Rev. Aquac. 2022, 14, 139–155. [Google Scholar] [CrossRef]
- Jørgensen, E.H.; Haatuft, A.; Puvanendran, V.; Mortensen, A. Effects of reduced water exchange rate and oxygen saturation on growth and stress indicators of juvenile lumpfish (Cyclopterus lumpus L.) in aquaculture. Aquaculture 2017, 474, 26–33. [Google Scholar] [CrossRef]
- Noble, C.; Iversen, M.H.; Lein, I.; Kolarevic, J.; Johansen, L.; Berge, G.M.; Burgerhout, E.; Mortensen, A.; Stene, A.; Espmark, Å.M. Rensvel OWI fact sheet series: An introduction to Operational and Laboratory-based Welfare Indicators for lumpfish (Cyclopterus lumpus L.). Nofima. 2019. Available online: https://www.fhf.no/prosjekter/prosjektbasen/901136/ (accessed on 19 May 2020).
- Imsland, A.K.; Reynolds, P.; Jonassen, T.M.; Hangstad, T.A.; Elvegård, T.A.; Urskog, T.C.; Hanssen, A.; Mikalsen, B. Effects of different feeding frequencies on growth, cataract development and histopathology of lumpfish (Cyclopterus lumpus L.). Aquaculture 2019, 501, 161–168. [Google Scholar] [CrossRef]
- Skår, M.W.; Haugland, G.T.; Powell, M.D.; Wergeland, H.I.; Samuelsen, O.B. Development of anaesthetic protocols for lumpfish (Cyclopterus lumpus L.): Effect of anaesthetic concentrations, sea water temperature and body weight. PLoS ONE 2017, 12, e0179344. [Google Scholar] [CrossRef]
- Mateus, A.P.; Power, D.M.; Canário, A.V.M. Stress and Disease in Fish. In Fish Diseases: Prevention and Control Strategies; Academic Press: Cambridge, MA, USA, 2017; pp. 187–220. [Google Scholar] [CrossRef]
- Segner, H.; Sundh, H.; Buchmann, K.; Douxfils, J.; Sundell, K.S.; Mathieu, C.; Ruane, N.; Jutfelt, F.; Toften, H.; Vaughan, L. Health of farmed fish: Its relation to fish welfare and its utility as welfare indicator. Fish Physiol. Biochem. 2012, 38, 85–105. [Google Scholar] [CrossRef]
- Sadoul, B.; Vijayan, M.M. Stress and Growth. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 35, pp. 167–205. [Google Scholar] [CrossRef]
- Yada, T.; Tort, L. Stress and Disease Resistance: Immune System and Immunoendocrine Interactions. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 35, pp. 365–403. [Google Scholar] [CrossRef]
- Perry, S.F.; McNeill, B.; Elia, E.; Nagpal, A.; Vulesevic, B.; Alsop, D.; Vijayan, M.M.; Bury, N.R.; Chung, M.J.; Sturm, A.; et al. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef]
- Balasch, J.C.; Tort, L. Netting the Stress Responses in Fish. Front. Endocrinol. 2019, 10, 62. [Google Scholar] [CrossRef]
- Faught, E.; Aluru, N.; Vijayan, M.M. The Molecular Stress Response. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 35, pp. 113–166. [Google Scholar] [CrossRef]
- Rodnick, K.J.; Planas, J.V. The Stress and Stress Mitigation Effects of Exercise: Cardiovascular, Metabolic, and Skeletal Muscle Adjustments. Fish Physiol. 2016, 35, 251–294. [Google Scholar] [CrossRef]
- Gorissen, M.; Flik, G. The Endocrinology of the Stress Response in Fish: An Adaptation-Physiological View. Fish Physiol. 2016, 35, 75–111. [Google Scholar] [CrossRef]
- Vijayan, M.M.; Aluru, N.; Leatherland, J.F. Stress response and the role of cortisol. Fish Dis. Disord. 2010, 2, 182–201. [Google Scholar] [CrossRef]
- Mommsen, T.P.; Vijayan, M.M.; Moon, T.W. Cortisol in teleosts: Dynamics, mechanisms of action, and metabolic regulation. Rev. Fish Biol. Fish. 1999, 9, 211–268. [Google Scholar] [CrossRef]
- Staven, F.R.; Nordeide, J.T.; Gesto, M.; Andersen, P.; Patel, D.M.; Kristensen, T. Behavioural and physiological responses of lumpfish (Cyclopterus lumpus) exposed to Atlantic salmon (Salmo salar) sensory cues. Aquaculture 2021, 544, 737066. [Google Scholar] [CrossRef]
- Aragão, C.; Corte-Real, J.; Costas, B.; Dinis, M.T.; Conceição, L.E.C. Stress response and changes in amino acid requirements in Senegalese sole (Solea senegalensis Kaup 1858). Amino Acids 2008, 34, 143–148. [Google Scholar] [CrossRef]
- Machado, M.; Malheiro, D.; Couto, A.; Wilson, J.M.; Guerreiro, M.; Azeredo, R.; Svendsen, J.C.; Afonso, A.; Serradeiro, R.; Costas, B. Acute hyperoxia induces systemic responses with no major changes in peripheral tissues in the Senegalese sole (Solea senegalensis Kaup, 1858). Fish Shellfish Immunol. 2018, 74, 260–267. [Google Scholar] [CrossRef]
- Costas, B.; Conceição, L.E.; Aragão, C.; Martos, J.A.; Ruiz-Jarabo, I.; Mancera, J.M.; Afonso, A. Physiological responses of Senegalese sole (Solea senegalensis Kaup, 1858) after stress challenge: Effects on non-specific immune parameters, plasma free amino acids and energy metabolism. Aquaculture 2011, 316, 68–76. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Oxidative stress and redox regulation: Adaptation, damage, repair, senescence, and death. Free Radic. Biol. Med. 2015, 199–283. [Google Scholar] [CrossRef]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I.; Bagnyukova, T.V. Hypoxia induces oxidative stress in tissues of a goby, the rotan Perccottus glenii. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 148, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Borowiec, B.G.; Scott, G.R. Hypoxia acclimation alters reactive oxygen species homeostasis and oxidative status in estuarine killifish (Fundulus heteroclitus). J. Exp. Biol. 2020, 223, jeb222877. [Google Scholar] [CrossRef] [PubMed]
- Gidron, Y.; Russ, K.; Tissarchondou, H.; Warner, J. The relation between psychological factors and DNA-damage: A critical review. Biol. Psychol. 2006, 72, 291–304. [Google Scholar] [CrossRef]
- Aschbacher, K.; O’donovan, A.; Wolkowitz, O.M.; Dhabhar, F.S.; Su, Y.; Epel, E. Good stress, bad stress and oxidative stress: Insights from anticipatory cortisol reactivity. Psychoneuroendocrinology 2013, 38, 1698–1708. [Google Scholar] [CrossRef]
- Hvas, M.; Folkedal, O.; Imsland, A.; Oppedal, F. Metabolic rates, swimming capabilities, thermal niche and stress response of the lumpfish, Cyclopterus lumpus. Biol. Open 2018, 7, bio036079. [Google Scholar] [CrossRef]
- Belanger, J.; Son, J.; Laugero, K.; Moberg, G.; Doroshov, S.; Lankford, S.; Cech, J. Effects of short-term management stress and ACTH injections on plasma cortisol levels in cultured white sturgeon, Acipenser transmontanus. Aquaculture 2001, 203, 165–176. [Google Scholar] [CrossRef]
- Arends, R.; Mancera, J.; Munoz, J.; Bonga, S.W.; Flik, G. The stress response of the gilthead sea bream (Sparus aurata L.) to air exposure and confinement. J. Endocrinol. 1999, 163, 149–157. [Google Scholar] [CrossRef]
- Torres, M.A.; Testa, C.P.; Gáspari, C.; Masutti, M.B.; Panitz, C.M.N.; Curi-Pedrosa, R.; de Almeida, E.A.; Di Mascio, P.; Filho, D.W. Oxidative stress in the mussel Mytella guyanensis from polluted mangroves on Santa Catarina Island, Brazil. Mar. Pollut. Bull. 2002, 44, 923–932. [Google Scholar] [CrossRef]
- Oliveira, C.C.V.; Aparício, R.; Blanco-Vives, B.; Chereguini, O.; Martín, I.; Sánchez-Vazquez, F.J. Endocrine (plasma cortisol and glucose) and behavioral (locomotor and self-feeding activity) circadian rhythms in Senegalese sole (Solea senegalensis Kaup 1858) exposed to light/dark cycles or constant light. Fish Physiol. Biochem. 2013, 39, 479–487. [Google Scholar] [CrossRef]
- Costas, B.; Simões, I.; Castro-Cunha, M.; Afonso, A. Non-specific immune responses of Senegalese sole, Solea senegalensis (Kaup), head-kidney leucocytes against Tenacibaculum maritimum. J. Fish Dis. 2014, 37, 765–769. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase Activity. In Handbook Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar] [CrossRef]
- Peixoto, D.; Pinto, W.; Gonçalves, A.T.; Machado, M.; Reis, B.; Silva, J.; Navalho, J.; Dias, J.; Conceição, L.; Costas, B. Microalgal biomasses have potential as ingredients in microdiets for Senegalese sole (Solea senegalensis) post-larvae. J. Appl. Phycol. 2021, 33, 2241–2250. [Google Scholar] [CrossRef]
- Lima, I.; Moreira, S.M.; Osten, J.R.-V.; Soares, A.M.; Guilhermino, L. Biochemical responses of the marine mussel Mytilus galloprovincialis to petrochemical environmental contamination along the North-western coast of Portugal. Chemosphere 2007, 66, 1230–1242. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.; Oliveira, C.; Gravato, C.; Guilhermino, L. Linking behavioural alterations with biomarkers responses in the European seabass Dicentrarchus labrax L. exposed to the organophosphate pesticide fenitrothion. Ecotoxicology 2010, 19, 1369–1381. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.P.; Draper, H.H. Comparative studies on different methods of malonaldehyde determination. Methods Enzymol 1984, 105, 299–305. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 2nd ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 1984. [Google Scholar]
- Imsland, A.K.D.; Berg, M.S.; Haugland, G.T.; Eliasen, K. Comparing Body Density of Lumpfish (Cyclopterus lumpus) to Different Operational Welfare Indicators. Fishes 2022, 7, 284. [Google Scholar] [CrossRef]
- Imsland, A.K.D. Cleaner Fish in Aquaculture. Fishes 2023, 8, 83. [Google Scholar] [CrossRef]
- Hanssen, A.J. The Effect of Long-Term Stress on Basal Levels of Plasma Cortisol and Hypothalamic-Pituitary-Interrenal (HPI) Axis on Lumpsucker (Cyclopterus lumpus). Nord University. 2016. Available online: https://nordopen.nord.no/nord-xmlui/bitstream/handle/11250/2425596/Hanssen.pdf?sequence=1&isAllowed=y (accessed on 6 September 2022).
- Hvas, M.; Oppedal, F. Physiological responses of farmed Atlantic salmon and two cohabitant species of cleaner fish to progressive hypoxia. Aquaculture 2019, 512, 734353. [Google Scholar] [CrossRef]
- Foss, A.; Imsland, A.K.D. Physiological Effects of Recapture and Transport from Net-Cages in Lumpfish. Fishes 2022, 7, 242. [Google Scholar] [CrossRef]
- Smith, A.J.; Clutton, R.E.; Lilley, E.; Hansen, K.E.A.; Brattelid, T. PREPARE: Guidelines for planning animal research and testing. Lab. Anim. 2017, 52, 135–141. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique. Med. J. Aust. 1960, 1, 500. [Google Scholar] [CrossRef]
- Barton, B.A.; Iwama, G.K. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu. Rev. Fish Dis. 1991, 1, 3–26. [Google Scholar] [CrossRef]
- Barton, B.A. Stress in Fishes: A Diversity of Responses with Particular Reference to Changes in Circulating Corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Jentoft, S.; Aastveit, A.H.; Torjesen, P.A.; Andersen, Ø. Effects of stress on growth, cortisol and glucose levels in non-domesticated Eurasian perch (Perca fluviatilis) and domesticated rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 141, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Henderson, R.J.; Tocher, D.R. The lipid composition and biochemistry of freshwater fish. Prog. Lipid Res. 1987, 26, 281–347. [Google Scholar] [CrossRef]
- Gracey, A.Y.; Lee, T.-H.; Higashi, R.M.; Fan, T. Hypoxia-induced mobilization of stored triglycerides in the euryoxic goby Gillichthys mirabilis. J. Exp. Biol. 2011, 214, 3005–3012. [Google Scholar] [CrossRef]
- van Raaij, M.T.; Breukel, B.-J.; Thillart, G.E.v.D.; Addink, A.D. Lipid metabolism of goldfish, Carassius auratus (L.) during normoxia and anoxia. Indications for fatty acid chain elongation. Comp. Biochem. Physiol. Part B Comp. Biochem. 1994, 107, 75–84. [Google Scholar] [CrossRef]
- Liu, H.; Fu, Z.; Yu, G.; Ma, Z.; Zong, H. Effects of Acute High-Temperature Stress on Physical Responses of Yellowfin Tuna (Thunnus albacares). J. Mar. Sci. Eng. 2022, 10, 1857. [Google Scholar] [CrossRef]
- Han, B.; Meng, Y.; Tian, H.; Li, C.; Li, Y.; Gongbao, C.; Fan, W.; Ma, R. Effects of Acute Hypoxic Stress on Physiological and Hepatic Metabolic Responses of Triploid Rainbow Trout (Oncorhynchus mykiss). Front. Physiol. 2022, 13, 921709. [Google Scholar] [CrossRef]
- Naour, S.; Espinoza, B.M.; Aedo, J.E.; Zuloaga, R.; Maldonado, J.; Bastias-Molina, M.; Silva, H.; Meneses, C.; Gallardo-Escarate, C.; Molina, A.; et al. Transcriptomic analysis of the hepatic response to stress in the red cusk-eel (Genypterus chilensis): Insights into lipid metabolism, oxidative stress and liver steatosis. PLoS ONE 2017, 12, e0176447. [Google Scholar] [CrossRef]
- Welker, T.L.; Congleton, J.L. Oxidative stress in juvenile chinook salmon, Oncorhynchus tshawytscha (Walbaum). Aquac. Res. 2004, 35, 881–887. [Google Scholar] [CrossRef]
- Neyrão, I.M.; Biller, J.D.; Takahashi, L.S.; Urbinati, E.C. Modulation of immunity and hepatic antioxidant defense by corticosteroids in pacu (Piaractus mesopotamicus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 260, 111025. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Reactive oxygen species and cellular defense system. In Free Radicals in Human Health and Disease; Springer: Berlin/Heidelberg, Germany, 2015; pp. 17–29. [Google Scholar] [CrossRef]
- Magnoni, L.J.; Novais, S.C.; Eding, E.; Leguen, I.; Lemos, M.F.L.; Ozório, R.O.A.; Geurden, I.; Prunet, P.; Schrama, J.W. Acute Stress and an Electrolyte- Imbalanced Diet, but Not Chronic Hypoxia, Increase Oxidative Stress and Hamper Innate Immune Status in a Rainbow Trout (Oncorhynchus mykiss) Isogenic Line. Front. Physiol. 2019, 10, 453. [Google Scholar] [CrossRef] [PubMed]




| Oxidative Stress | 30minC | 30minS | 1hC | 1hS | 2hC | 2hS | 4hC | 4hS | 24hC | 24hS |
|---|---|---|---|---|---|---|---|---|---|---|
| CAT | 56.2 ± 4.3 ab | 55.2 ± 4.8 | 59.9 ± 2.7 ab | 64.4 ± 5.3 | 69.4 ± 7.1 b | 50.8 ± 2.4 | 63.9 ± 4.7 b | 52.0 ± 3.3 | 42.3 ± 2.4 a | 60.8 ± 4.5 |
| SOD | 2.0 ± 0.2 b | 2.0 ± 0.1 | 1.8 ± 0.1 ab | 1.7 ± 0.1 | 1.7 ± 0.2 ab | 1.4 ± 0.1 | 1.9 ± 0.1 b | 1.8 ± 0.1 | 1.3 ± 0.1 a | 1.5 ± 0.1 |
| LPO | 25.3 ± 1.5 b | 24.9 ± 1.2 bc | 20.7 ± 2.4 ab | 18.7 ± 1.4 a | 16.7 ± 0.8 a | 17.2 ± 1.2 a | 22.8 ± 1.3 ab | 26.0 ± 1.0 c | 32.5 ± 1.8 c | 29.7 ± 1.2 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Santa Lopes, T.; Costas, B.; Ramos-Pinto, L.; Reynolds, P.; Imsland, A.K.D.; Fernandes, J.M.O. Exploring the Effects of Acute Stress Exposure on Lumpfish Plasma and Liver Biomarkers. Animals 2023, 13, 3623. https://doi.org/10.3390/ani13233623
da Santa Lopes T, Costas B, Ramos-Pinto L, Reynolds P, Imsland AKD, Fernandes JMO. Exploring the Effects of Acute Stress Exposure on Lumpfish Plasma and Liver Biomarkers. Animals. 2023; 13(23):3623. https://doi.org/10.3390/ani13233623
Chicago/Turabian Styleda Santa Lopes, Tiago, Benjamin Costas, Lourenço Ramos-Pinto, Patrick Reynolds, Albert K. D. Imsland, and Jorge M. O. Fernandes. 2023. "Exploring the Effects of Acute Stress Exposure on Lumpfish Plasma and Liver Biomarkers" Animals 13, no. 23: 3623. https://doi.org/10.3390/ani13233623
APA Styleda Santa Lopes, T., Costas, B., Ramos-Pinto, L., Reynolds, P., Imsland, A. K. D., & Fernandes, J. M. O. (2023). Exploring the Effects of Acute Stress Exposure on Lumpfish Plasma and Liver Biomarkers. Animals, 13(23), 3623. https://doi.org/10.3390/ani13233623








