Pharmacokinetics, Tissue Residues, and Withdrawal Times of Oxytetracycline in Rainbow Trout (Oncorhynchus mykiss) after Single- and Multiple-Dose Oral Administration
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Experimental Design
2.3.1. Single-Dose Pharmacokinetic Study
2.3.2. Multiple-Dose Pharmacokinetic Study
2.4. Oxytetracycline Analysis
2.5. Pharmacokinetic Analysis
2.6. Withdrawal Time Analysis
2.7. Statistical Analysis
3. Results
3.1. Single-Dose Pharmacokinetic Study
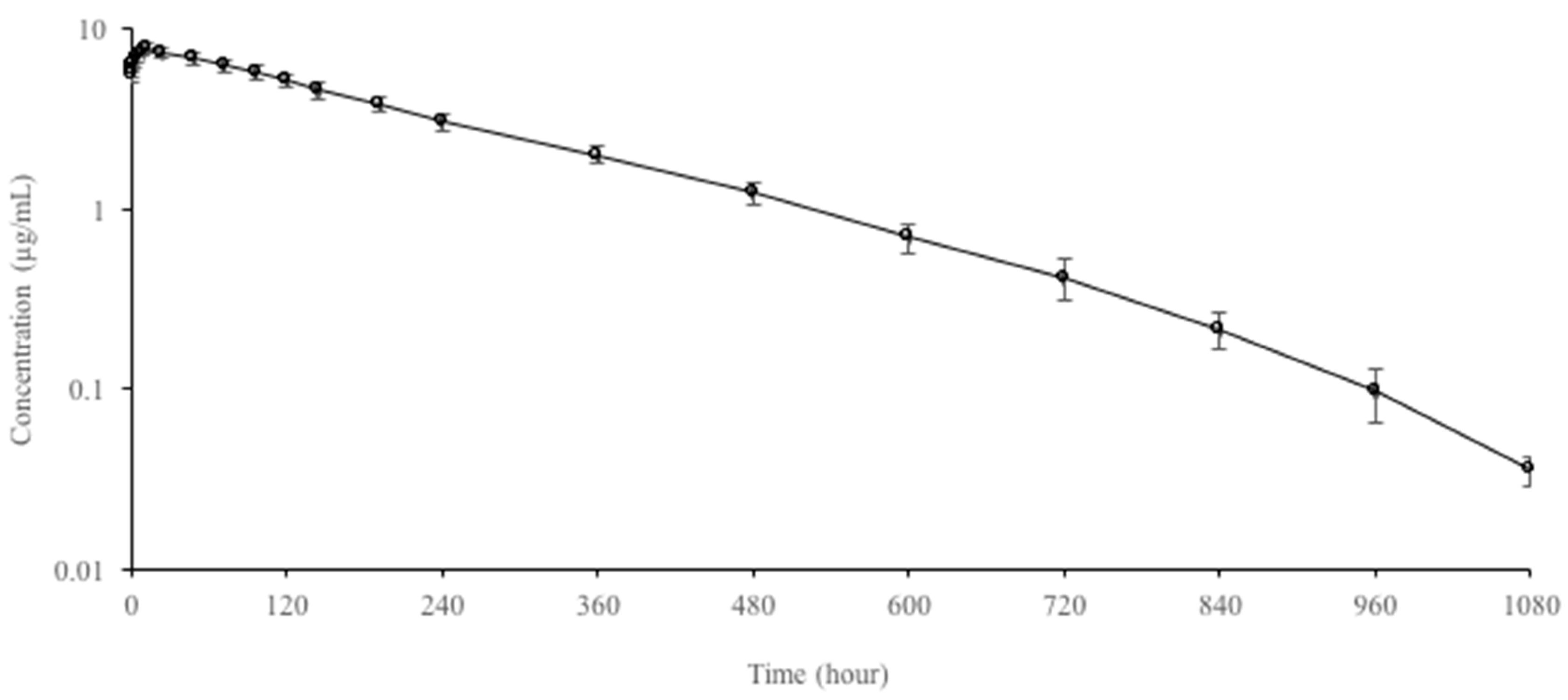
3.2. Multiple-Dose Pharmacokinetic Study
3.3. Residue Depletion Study and Withdrawal Time Estimation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quesada, S.P.; Paschoal, J.A.; Reyes, F.G. Considerations on the aquaculture development and on the use of veterinary drugs: Special issue for fluoroquinolones—A review. J. Food Sci. 2013, 78, 1321–1333. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Stanković, D.; Crivelli, A.J.; Snoj, A. Rainbow trout in Europe: Introduction, naturalization, and impacts. Rev. Fish. Sci. Aquac. 2015, 23, 39–71. [Google Scholar] [CrossRef]
- Verner-Jeffreys, D.; Taylor, N. Review of Freshwater Treatments Used in the Scottish Freshwater Rainbow Trout Aquaculture Industry; Scottish Aquaculture Research Forum: Pitlochry, Scotland, 2015. [Google Scholar]
- Okocha, R.C.; Olatoye, I.O.; Adedeji, O.B. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. 2018, 39, 21. [Google Scholar] [CrossRef]
- Corum, O.; Uney, K.; Terzi, E.; Durna Corum, D.; Coskun, D.; Altan, F.; Elmas, M. Effects of temperature on the pharmacokinetics, tissue residues, and withdrawal times of doxycycline in rainbow trout (Oncorhynchus mykiss) following oral administration. Vet. Sci. 2023, 10, 401. [Google Scholar] [CrossRef] [PubMed]
- Corum, O.; Er, A.; Durna Corum, D.; Atik, O.; Uney, K. Pharmacokinetics and bioavailability of ceftriaxone in brown trout (Salmo trutta fario) after intravenous and intramuscular administration. Aquaculture 2019, 500, 272–277. [Google Scholar] [CrossRef]
- Pinto, N.; Naik, M.G.; Kumar, B.N.; Shankar, K.M.; Rakesh, K.; Abhiman, P.B.; Ramesh, K.S. Oxytetracycline efficacy and preliminary establishment of pharmacokinetic residues in tropical fish, Catla catla (Hamilton, 1822). Aquaculture 2023, 571, 739481. [Google Scholar] [CrossRef]
- Leal, J.F.; Santos, E.B.; Esteves, V.I. Oxytetracycline in intensive aquaculture: Water quality during and after its administration, environmental fate, toxicity and bacterial resistance. Rev. Aquac. 2019, 11, 1176–1194. [Google Scholar] [CrossRef]
- Manna, S.K.; Das, N.; Sarkar, D.J.; Bera, A.K.; Baitha, R.; Nag, S.K.; Das, B.K.; Kumar, S.; Ravindran, R.; Krishna, N.; et al. Pharmacokinetics, bioavailability and withdrawal period of antibiotic oxytetracycline in catfish Pangasianodon hypophthalmus. Environ. Toxicol. 2022, 89, 103778. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin. 2010. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:015:0001:0072:en:PDF (accessed on 25 September 2023).
- Codex Alimentarius. Codex Veterinary Drug Residue in Food Online Database. 2021. Available online: http://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/vetdrugs/en/ (accessed on 25 September 2023).
- United Stated Department of Agriculture (USDA). China: China Publishes Maximum Residue Limits for Veterinary Drugs in Food. 2021. Available online: https://www.fas.usda.gov/data/china-china-publishes-maximum-residue-limits-veterinary-drugs-food (accessed on 25 September 2023).
- Li, R.Q.; Ren, Y.W.; Li, J.; Huang, C.; Shao, J.H.; Chen, X.X.; Wu, Z.X. Comparative pharmacokinetics of oxytetracycline in blunt-snout bream (Megalobrama amblycephala) with single and multiple-dose oral administration. Fish Physiol. Biochem. 2015, 41, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Wang, Y.; Zou, X.; Fu, G.; Li, C.; Fan, P.; Fang, W. Pharmacokinetics of oxytetracycline in Pacific white shrimp, Penaeus vannamei, after oral administration of a single-dose and multiple-doses. Aquaculture 2019, 512, 734348. [Google Scholar] [CrossRef]
- Rigos, G.; Smith, P. A critical approach on pharmacokinetics, pharmacodynamics, dose optimisation and withdrawal times of oxytetracycline in aquaculture. Rev. Aquac. 2015, 7, 77–106. [Google Scholar] [CrossRef]
- Björklund, H.V.; Bylund, G. Comparative pharmacokinetics and bioavailability of oxolinic acid and oxytetracycline in rainbow trout (Oncorhynchus mykiss). Xenobiotica 1991, 21, 1511–1520. [Google Scholar] [CrossRef]
- Turk, E.; Corum, O.; Tekeli, I.O.; Sakin, F.; Uney, K. Effects of single and repeated doses on disposition and kinetics of doxycycline hyclate in goats. Animals 2020, 10, 1088. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Guideline on Bioanalytical Method Validation. 2011. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 15 April 2023).
- Corum, O.; Terzi, E.; Durna Corum, D.; Tastan, Y.; Gonzales, R.C.; Kenanoglu, O.N.; Arriesgado, D.M.; Navarro, V.R.; Bilen, S.; Sonmez, A.Y.; et al. Plasma and muscle tissue disposition of enrofloxacin in Nile tilapia (Oreochromis niloticus) after intravascular, intraperitoneal, and oral administrations. Food Addit Contam Part A 2022, 39, 1806–1817. [Google Scholar] [CrossRef] [PubMed]
- Durna Corum, D.; Corum, O.; Terzi, E.; Coskun, D.; Bilen, S.; Cetin, G.; Uney, K. Pharmacokinetics of cefquinome in rainbow trout (Oncorhynchus mykiss) after intravascular, intraperitoneal, and oral administrations. J. Vet. Pharmacol. Ther. 2022, 45, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Colburn, W.A. Estimating the accumulation of drugs. J. Pharm. Sci. 1983, 72, 833–834. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Guideline on Determination of Withdrawal Periods for Edible Tissues. 2022. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/adopted-guideline-determination-withdrawal-periods-edible-tissues-revision-2_en.pdf (accessed on 15 September 2023).
- Corum, O.; Terzi, E.; Corum, D.D.; Kenanoglu, O.N.; Bilen, S.; Uney, K. Pharmacokinetic/pharmacodynamic integration of marbofloxacin after oral and intravenous administration in rainbow trout (Oncorhynchus mykiss). Aquaculture 2020, 514, 734510. [Google Scholar] [CrossRef]
- Elema, M.O.; Hoff, K.A.; Kristensen, H.G. Bioavailability of oxytetracycline from medicated feed administered to Atlantic salmon (Salmo salar L.) in seawater. Aquaculture 1996, 143, 7–14. [Google Scholar] [CrossRef]
- Yanong, R.P.E. Use of Antibiotics in Ornamental Fish Aquaculture. UF/IFAS Ext. 2006, 84. Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.581.6949&rep=rep1&type=pdf (accessed on 25 September 2023). [CrossRef]
- Turel, I.; Dagoglu, G.; Yılmaz, O.; Kankaya, E.; Sen, F. Pharmacokinetics of oxytetracycline in rainbow trout (Oncorhynchus mykiss) following administration of medicated feed. Turk. J. Vet. Anim. Sci. 2003, 27, 223–227. [Google Scholar]
- Uno, K.; Aoki, T.; Ueno, R.; Maeda, I. Pharmacokinetics of oxytetracycline in rainbow trout Oncorhynchus mykiss following bolus intravenous administration. Fish Sci. 1997, 63, 90–93. [Google Scholar] [CrossRef]
- Abedini, S.; Namdari, R.; Law, F.C.P. Comparative pharmacokinetics and bioavailability of oxytetracycline in rainbow trout and chinook salmon. Aquaculture 1998, 162, 23–32. [Google Scholar] [CrossRef]
- Grondel, J.L.; Nouws, J.F.M.; Schutte, A.R.; Driessens, F. Comparative pharmacokinetics of oxytetracycline in rainbow trout (Salmo gairdneri) and African catfish (Clarias gariepinus). J. Vet. Pharmacol. Ther. 1989, 12, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Black, W.D.; Ferguson, H.W.; Byrne, P.; Claxton, M.J. Pharmacokinetic and tissue distribution study of oxytetracycline in rainbow trout following bolus intravenous administration. J. Vet. Pharmacol. Ther. 1991, 14, 351–358. [Google Scholar] [CrossRef]
- Björklund, H.; Bylund, G. Temperature-related absorption and excretion of oxytetracycline in rainbow trout (Salmo gairdneri R.). Aquaculture 1990, 84, 363–372. [Google Scholar] [CrossRef]
- Cravedi, J.P.; Choubert, G.; Delous, G. Digestibility of chloramphenicol, oxolinic acid and oxytetracycline in rainbow trout and influence of these antibiotics on lipid digestibility. Aquaculture 1987, 60, 133–141. [Google Scholar] [CrossRef]
- Ueno, R.; Kinoshita, A.; Wakabayashi, J. Comparative pharmacokinetics of oxytetracycline in eel and its fate in a closed aquatic environment. Aquaculture 2004, 235, 53–63. [Google Scholar] [CrossRef]
- Dauble, D.D.; Curtis, L.R. Influence of digestive processes on the absorption and fate of quinoline ingested by rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem. 1990, 9, 505–512. [Google Scholar] [CrossRef]
- Van Rossum, J.M. Pharmacokinetics of accumulation. J. Pharm. Sci. 1968, 57, 2162–2165. [Google Scholar] [CrossRef]
- Shan, Q.; Huang, H.; Zheng, G.; Yin, Y.; Zhu, X.; Ma, L.; Zhou, H.; Xie, W.; Li, L.; Liu, S.; et al. Pharmacokinetics and tissue residue profiles of enrofloxacin in crucian carp (Carassius auratus gibelio) following single and multiple oral administration. Front. Vet. Sci 2022, 9, 872828. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Xu, L.; Sheng, Y.; Huang, J.; Zheng, Q. Systematic evaluation of dose accumulation studies in clinical pharmacokinetics. Curr. Drug Metab. 2013, 14, 605–615. [Google Scholar] [CrossRef]
- Barza, M.; Brown, R.B.; Shanks, C.; Gamble, C.; Weinstein, L. Relation between lipophilicity and pharmacological behavior of minocycline, doxycycline, tetracycline, and oxytetracycline in dogs. Antimicrob. Agents Chemother. 1975, 8, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Rogstad, A.; Hormazabal, V.; Ellingsen, O.F.; Rasmussen, K.E. Pharmacokinetic study of oxytetracycline in fish. I. Absorption, distribution and accumulation in rainbow trout in freshwater. Aquaculture 1991, 96, 219–226. [Google Scholar] [CrossRef]
- Uno, K.; Aoki, T.; Ueno, R. Pharmacokinetic study of oxytetracycline in cultured rainbow trout, amago salmon, and yellowtail. Nippon Suisan Gakkaishi 1992, 58, 1151–1156. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Q.; Li, J. Tissue distribution and elimination of oxytetracycline in perch Lateolabras janopicus and black seabream (Sparus macrocephalus) following oral administration. Aquaculture 2004, 237, 31–40. [Google Scholar] [CrossRef]
- Dyer, D.C. Pharmacokinetics of oxytetracycline in the turkey: Evaluation of biliary and urinary excretion. Am. J. Vet. Res. 1989, 50, 522–524. [Google Scholar]
- Anonymous. Oxytetracycline. 2023. Available online: https://inchem.org/documents/jecfa/jecmono/v27je06.htm (accessed on 10 September 2023).
- Toutain, P.L.; Pelligand, L.; Lees, P.; Bousquet-Mélou, A.; Ferran, A.A.; Turnidge, J.D. The pharmacokinetic/pharmacodynamic paradigm for antimicrobial drugs in veterinary medicine: Recent advances and critical appraisal. J. Vet. Pharmacol. Ther. 2021, 44, 172–200. [Google Scholar] [CrossRef]
- CLSI 2020; Performance Standards for Antimicrobial Susceptibility Testing of Bacteria Isolated from Aquatic Animals. VET04, 3rd ed. Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2020.
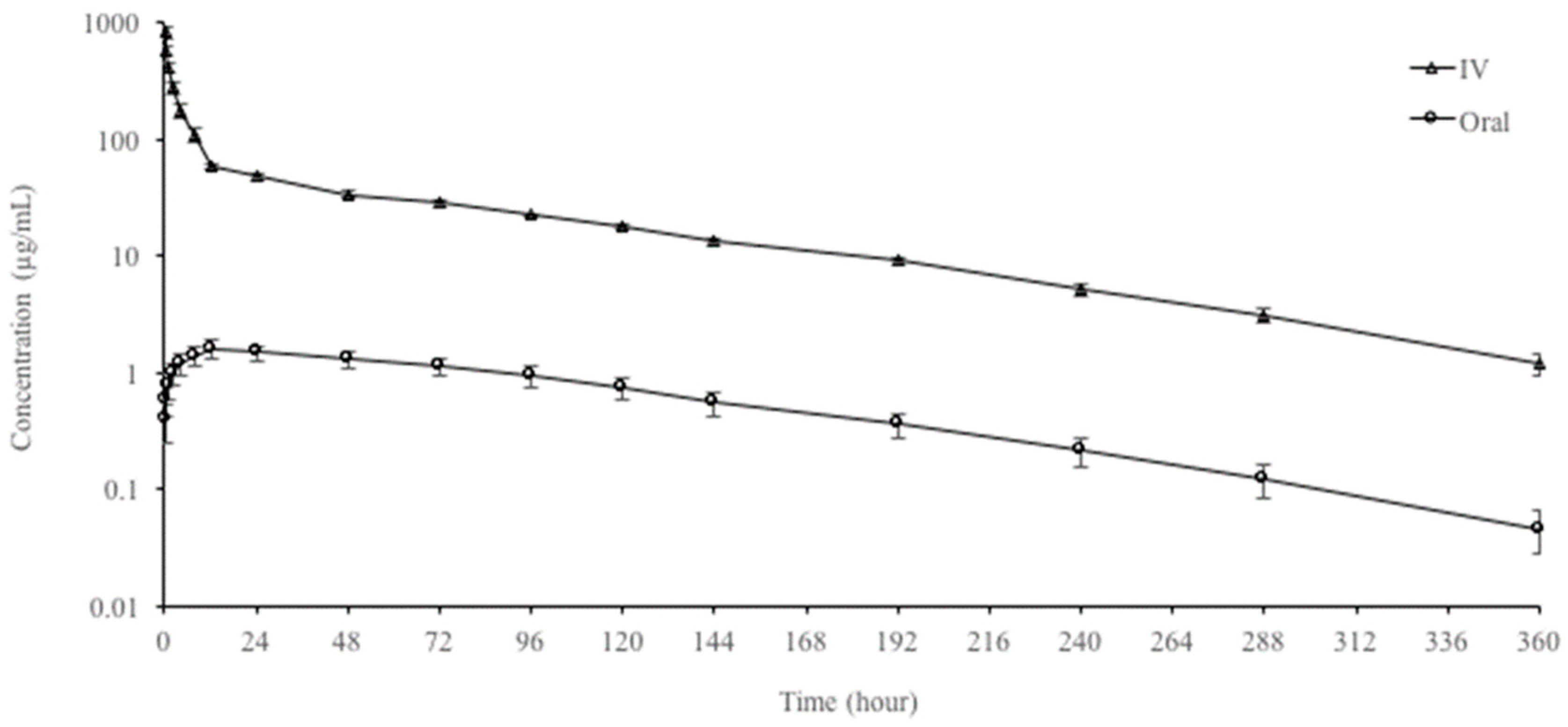

| Parameters | IV | Oral | %GD * |
|---|---|---|---|
| t1/2ʎz (h) | 65.05 | 68.94 | 5.98 |
| AUC0–last (h·µg/mL) | 7309.79 | 203.53 | −97.22 |
| AUC0–∞ (h·µg/mL) | 7421.10 | 208.11 | −97.20 |
| AUCextrap (%) | 1.50 | 2.20 | - |
| MRT0-∞ (h) | 74.61 | 105.62 | 41.56 |
| ClT (mL/h/kg) | 8.09 | - | - |
| Vdss (mL/kg) | 603.23 | - | - |
| Vdarea (mL/kg) | 758.74 | - | - |
| C0.25h (µg/mL) | 825.39 ± 96.75 | - | - |
| Cmax (µg/mL) | - | 1.61 ± 0.25 | - |
| Tmax (h) | - | 12 | |
| F (%) | - | 2.80 | - |
| Parameters | Oral |
|---|---|
| t1/2ʎz (h) | 147.26 |
| AUC0–1080 (h·µg/mL) | 1978.98 |
| AUC0–∞ (h·µg/mL) | 1986.02 |
| AUCextrap (%) | 0.35 |
| MRT0–∞ (h) | 230.20 |
| Cmax (µg/mL) | 7.82 ± 0.42 |
| Tmax (h) | 12 |
| Parameters | First Dose | Last Dose | %GD * |
|---|---|---|---|
| AUC0–24 (h·µg/mL) | 32.71 | 174.38 | 433.06 |
| Cmax (µg/mL) | 1.60 ± 0.18 | 7.82 ± 0.42 | 390.15 |
| C24h (µg/mL) | 1.46 ± 0.16 | 7.28 ± 0.43 | 397.53 |
| R | - | 5.33 |
| Time (hour) | Muscle+Skin (µg/g) | Liver (µg/g) | Kidney (µg/g) | Plasma (µg/mL) |
|---|---|---|---|---|
| 0.25 | 10.65 ± 1.23 | 87.33 ± 12.98 | 12.82 ± 1.44 | 5.50 ± 0.48 |
| 0.5 | 12.52 ± 1.31 | 105.40 ± 11.96 | 14.55 ± 1.49 | 5.86 ± 0.46 |
| 1 | 14.03 ± 1.58 | 135.77 ± 11.22 | 16.52 ± 1.10 | 6.18 ± 0.44 |
| 2 | 15.59 ± 1.60 | 151.19 ± 16.50 | 18.70 ± 1.16 | 6.56 ± 0.39 |
| 4 | 17.14 ± 1.71 | 172.66 ± 23.41 | 21.00 ± 1.41 | 6.91 ± 0.37 |
| 8 | 18.23 ± 1.41 | 162.36 ± 15.19 | 24.40 ± 1.59 | 7.34 ± 0.34 |
| 12 | 17.43 ± 0.78 | 152.96 ± 12.84 | 23.20 ± 1.61 | 7.82 ± 0.42 |
| 24 | 15.83 ± 0.61 | 128.54 ± 12.96 | 21.45 ± 1.97 | 7.28 ± 0.43 |
| 48 | 13.81 ± 0.68 | 108.39 ± 10.15 | 19.16 ± 1.81 | 6.79 ± 0.46 |
| 72 | 12.42 ± 0.68 | 87.92 ± 9.65 | 17.17 ± 1.95 | 6.26 ± 0.51 |
| 96 | 10.94 ± 0.78 | 67.36 ± 8.43 | 15.13 ± 1.79 | 5.69 ± 0.49 |
| 120 | 9.38 ± 0.75 | 54.68 ± 8.67 | 13.20 ± 1.68 | 5.12 ± 0.46 |
| 144 | 7.96 ± 0.69 | 41.37 ± 4.99 | 11.30 ± 1.23 | 4.55 ± 0.45 |
| 192 | 6.30 ± 0.60 | 33.18 ± 3.40 | 9.45 ± 0.95 | 3.83 ± 0.34 |
| 240 | 4.75 ± 0.56 | 25.13 ± 2.91 | 7.62 ± 0.71 | 3.05 ± 0.33 |
| 360 | 3.25 ± 0.43 | 17.84 ± 2.03 | 6.09 ± 0.67 | 1.98 ± 0.22 |
| 480 | 2.40 ± 0.25 | 11.77 ± 0.59 | 4.52 ± 0.48 | 1.23 ± 0.16 |
| 600 | 1.73 ± 0.13 | 6.33 ± 0.60 | 3.26 ± 0.39 | 0.69 ± 0.12 |
| 720 | 1.13 ± 0.12 | 3.68 ± 0.51 | 2.21 ± 0.35 | 0.42 ± 0.11 |
| 840 | 0.63 ± 0.08 | 2.24 ± 0.26 | 1.45 ± 0.26 | 0.21 ± 0.05 |
| 960 | 0.32 ± 0.06 | 1.27 ± 0.17 | 0.91 ± 0.13 | 0.10 ± 0.03 |
| 1080 | 0.15 ± 0.03 | 0.79 ± 0.13 | 0.49 ± 0.07 | 0.04 ± 0.01 |
| 1200 | 0.04 ± 0.02 | 0.37 ± 0.06 | 0.23 ± 0.04 | <LLOQ |
| 1440 | <LLOQ | 0.12 ± 0.02 | 0.09 ± 0.03 | <LLOQ |
| 1920 | <LLOQ | <LLOQ | <LLOQ | <LLOQ |
| Tissues | Europe | China |
|---|---|---|
| Muscle+skin | 55.87 (56) | 49.32 (50) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corum, O.; Durna Corum, D.; Terzi, E.; Uney, K. Pharmacokinetics, Tissue Residues, and Withdrawal Times of Oxytetracycline in Rainbow Trout (Oncorhynchus mykiss) after Single- and Multiple-Dose Oral Administration. Animals 2023, 13, 3845. https://doi.org/10.3390/ani13243845
Corum O, Durna Corum D, Terzi E, Uney K. Pharmacokinetics, Tissue Residues, and Withdrawal Times of Oxytetracycline in Rainbow Trout (Oncorhynchus mykiss) after Single- and Multiple-Dose Oral Administration. Animals. 2023; 13(24):3845. https://doi.org/10.3390/ani13243845
Chicago/Turabian StyleCorum, Orhan, Duygu Durna Corum, Ertugrul Terzi, and Kamil Uney. 2023. "Pharmacokinetics, Tissue Residues, and Withdrawal Times of Oxytetracycline in Rainbow Trout (Oncorhynchus mykiss) after Single- and Multiple-Dose Oral Administration" Animals 13, no. 24: 3845. https://doi.org/10.3390/ani13243845






