Transcriptome Analysis of Marbled Rockfish Sebastiscus marmoratus under Salinity Stress
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Salinity Stress Experiment
2.2. Total RNA Extraction, Library Construction, and High-Throughput Sequencing
2.3. Reference Genome Alignment and De Novo Transcriptome Transfer, Annotation
2.4. Quantitative Analysis of Differential Expression Levels and Differential Gene Enrichment Analysis
2.5. RT-qPCR Analysis
3. Results
3.1. Transcriptome Sequencing Data and Reference Genome Alignment Analysis
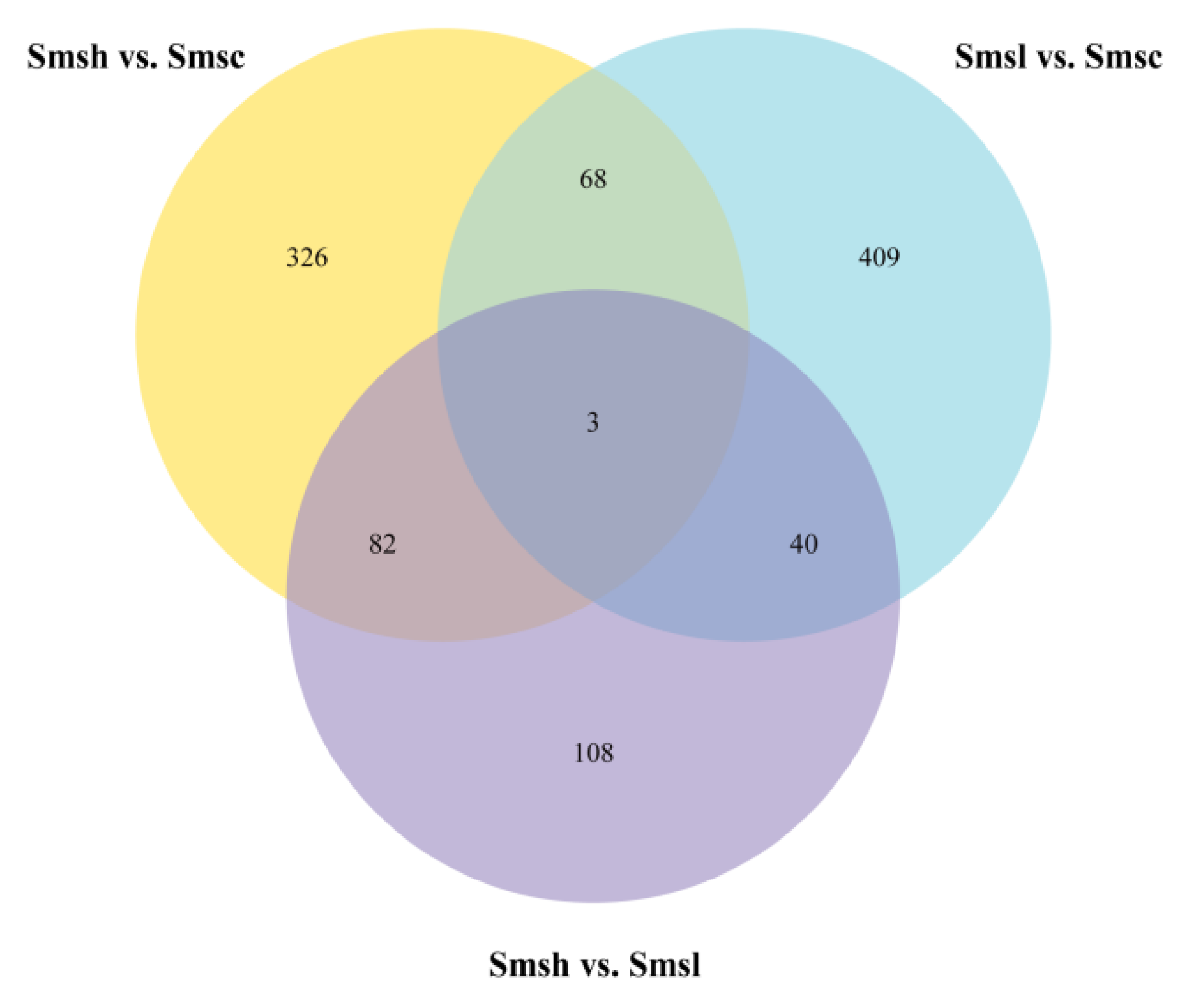
3.2. Differential Gene Expression
3.3. GO Functional Enrichment of Differentially Expressed Genes
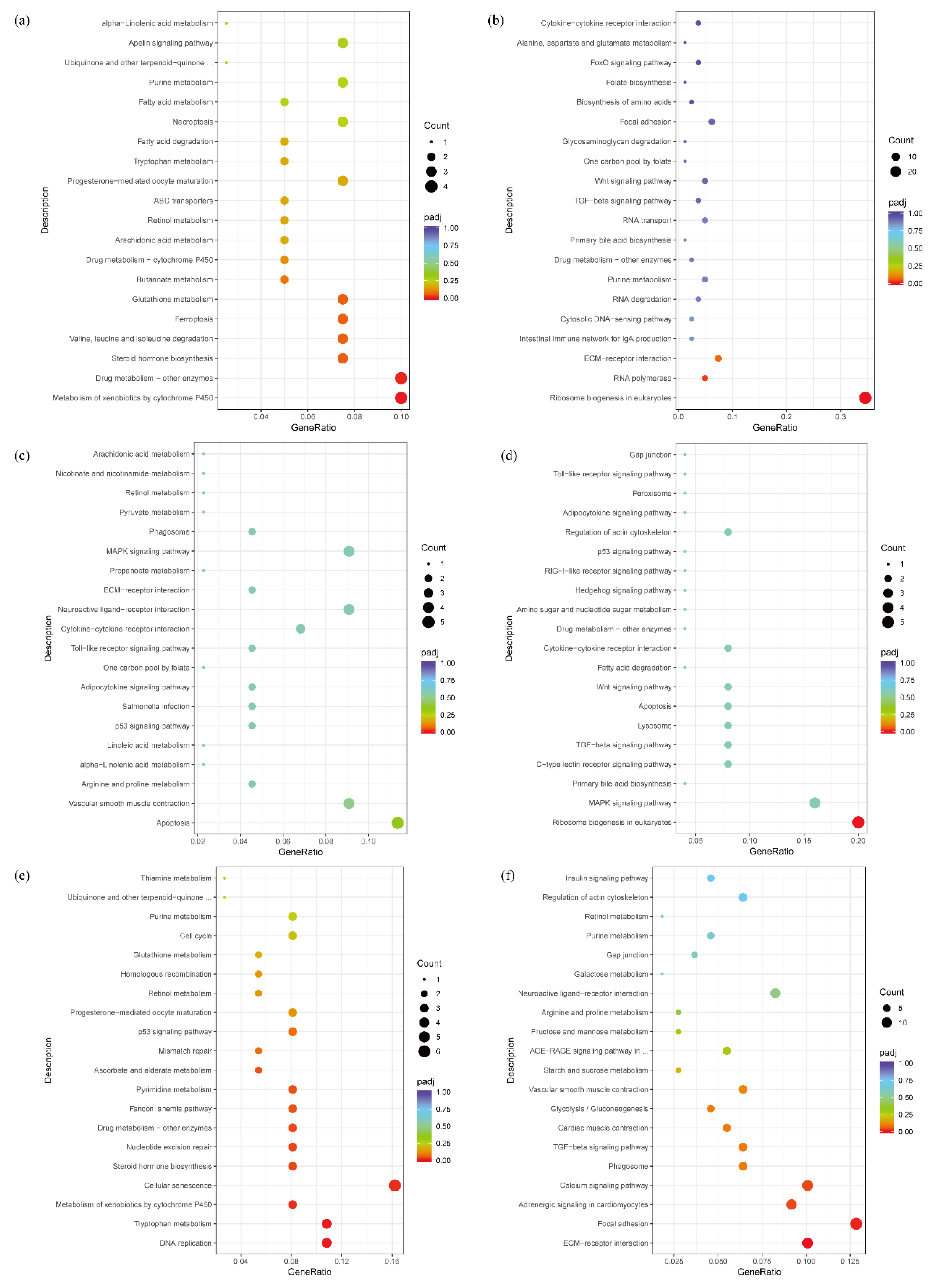
3.4. KEGG Pathway Analysis of Differentially Expressed Genes
3.5. Real-Time Quantitative PCR Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Doney, S.C.; Ruckelshaus, M.; Emmett Duffy, J.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N.; et al. Climate Change Impacts on Marine Ecosystems. Ann. Rev. Mar. Sci. 2012, 4, 11–37. [Google Scholar] [PubMed]
- Field, C.B.; Barros, V.R.; Mastrandrea, M.D.; Mach, K.J.; Abdrabo; Adger, W.N.; Anokhin, Y.A.; Anisimov, O.A.; Arent, D.J.; Barnett, J.; et al. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. CUP 2014, 6, 411–484. [Google Scholar]
- Held, I.M.; Soden, B.J. Robust responses of the hydrological cycle to global warming. J. Clim. 2006, 19, 5686–5699. [Google Scholar] [CrossRef]
- Wiltshire, K.H.; Kraberg, A.; Bartsch, I.; Boersma, M.; Franke, H.D.; Freund, J.; Gebühr, C.; Gerdts, G.; Stockmann, K.; Wichels, A. Helgoland roads, North Sea: 45 years of change. Estuaries Coasts 2010, 33, 295–310. [Google Scholar]
- Dasgupta, S.; Kamal, F.A.; Khan, Z.H.; Choudhury, S.; Nishat, A. River Salinity and Climate Change: Evidence from Coastal Bangladesh. World Sci. Ref. Asia World Econ. 2015, 1, 205–242. [Google Scholar]
- Ran, F.X.; Jin, W.J.; Huang, S.; Liu, C.X.; Li, Z.X.; Li, C.Z. Research progress on the effects of salinity change on fish. J. Northwest AF Univ. 2020, 48, 10–18. [Google Scholar]
- Cossins, A.R.; Crawford, D.L. Fish as models for environmental genomics. Nat. Rev. Genet 2005, 6, 324–333. [Google Scholar] [PubMed]
- Huni, A.A.; Aravindan, C.M. The effect of salinity on the oxygen consumption of two intertidal crustaceans. Comp. Biochem. Physiol. A Physiol. 1985, 81, 869–871. [Google Scholar]
- Buf, G.; Payan, P. How should salinity influence fish growth? Comp. Biochem. Phys. C 2001, 130, 411–423. [Google Scholar]
- Kültz, D. Physiological mechanisms used by fish to cope with salinity stress. J. Exp. Biol. 2015, 218, 1907–1914. [Google Scholar]
- Kang, Z.Q.; Deng, C.Z.; Yu, H.J.; Ling, X.R.; Huang, Y.C. Study advances in the effect of salinity change on fish. J. Fujian Fish. 2013, 35, 395–401. [Google Scholar]
- Romano, N.; Zeng, C. The effects of salinity on the survival, growth and haemolymph osmolality of early juvenile blue swimmer crabs, Portunus pelagicus. Aquaculture 2006, 260, 151–162. [Google Scholar] [CrossRef]
- Ye, L.; Jiang, S.; Zhu, X.; Yang, Q.; Wen, W.; Wu, K. Effects of salinity on growth and energy budget of juvenile Penaeus monodon. Aquaculture 2009, 290, 140–144. [Google Scholar] [CrossRef]
- Rubio, V.C.; Sánchez-Vázquez, F.J.; Madrid, J.A. Effects of salinity on food intake and macronutrient selection in European sea bass. Physiol. Behav. 2005, 85, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.G.; Kültz, D. The cellular stress response in fish exposed to salinity fluctuations. J. Exp. Zool. Part A 2020, 333, 421–435. [Google Scholar] [CrossRef]
- Fiol, D.F.; Kultz, D. Osmotic stress sensing and signaling in fishes. FEBS J. 2007, 274, 5790–5798. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.; Noreldin, A.E.; Sewilam, H. Long term salinity disrupts the hepatic function, intestinal health, and gills antioxidative status in Nile tilapia stressed with hypoxia. Ecotox. Environ. Saf. 2021, 220, 112412. [Google Scholar] [CrossRef]
- Meng, W.; Xu, K.D.; Li, Z.H.; Shi, H.L.; Zhou, Y.D. Transcriptome analysis of Nibea japonica under acute salinity stress. J. Fish China 2021, 45, 649–660. [Google Scholar]
- Xu, L.W.; Liu, G.F.; Wang, R.X.; Su, Y.L.; Guo, Z.X.; Feng, J. Effects of abrupt salinity stress onosmo regulation of juvenile Rachycentron canadum. J. Appl. Ecol. 2007, 18, 1596–1600. [Google Scholar]
- Jia, Q.Q.; Lv, W.Q. Effects of low salinity stress on plasma osmolality, cortisol, prolactin and growth hormone of Japanese flounder, Paralichthys olivaceus. J. Shanghai Ocean. Univ. 2016, 25, 71–77. [Google Scholar]
- Shui, B.; Wang, Y.; Lou, F.; Han, Z.Q. Salinity fluctuation on the genetic regulatory mechanisms of the crustacean, Charybdis japonica. Front. Mar. 2022, 9, 870891. [Google Scholar] [CrossRef]
- Bo, J.; Yang, Y.; Zheng, R.; Fang, C.; Jiang, Y.; Liu, J.; Chen, M.; Hong, F.; Bailey, C.; Segner, H.; et al. Antimicrobial activity and mechanisms of multiple antimicrobial peptides isolated from rockfish Sebastiscus marmoratus. Fish Shellfish Immunol. 2019, 93, 1007–1017. [Google Scholar] [CrossRef]
- Chen, D.G.; Zhang, M.Z. Marine Fishes of China; China Ocean University Press: Qingdao, China, 2015. [Google Scholar]
- Wu, C. The Effect of Several Environmental Factors on the Surival Rate of Larvae. J. Zhejiang Ocean. Univ. (Nat. Sci.) 2000, 12–16. [Google Scholar]
- Qian, X.; Ba, Y.; Zhuang, Q.; Zhong, G. RNA-Seq technology and its application in fish transcriptomics. OMICS 2014, 18, 98–110. [Google Scholar] [CrossRef]
- Smith, S.; Bernatchez, L.; Beheregaray, L.B. RNA-seq analysis reveals extensive transcriptional plasticity to temperature stress in a freshwater fish species. BMC Genom. 2013, 14, 375. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, A.; Yuan, C.; Zhao, T.; Chang, H.; Zhang, J. Transcriptome analysis of liver lipid metabolism disorders of the turbot Scophthalmus maximus in response to low salinity stress. Aquaculture 2020, 534, 736273. [Google Scholar] [CrossRef]
- Guo, B.; Tang, Z.; Wu, C.; Xu, K.; Qi, P. Transcriptomic analysis reveal an efficient osmoregulatory system in Siberian sturgeon Acipenser baeri in response to salinity stress. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Lou, F.; Gao, T.; Han, Z. Effect of salinity fluctuation on the transcriptome of the Japanese mantis shrimp Oratosquilla oratoria. Int. J. Biol. Macromol. 2019, 140, 1202–1213. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gao, T.; Han, Z. RNA-seq and Analysis of Argyrosomus japonicus Under Different Salinities. Front. Mar. 2021, 8, 790065. [Google Scholar] [CrossRef]
- Li, Q.H.; Li, Z.B.; Dai, G.; Cao, Y.; Chen, X.; Chen, L.; Shangguan, J.; Ning, Y. Isolation and characterization of eleven microsatellite loci in the marbled rockfish, Sebastiscus marmoratus (Scorpaenidae). Conserv. Genet. Resour. 2014, 6, 53–55. [Google Scholar] [CrossRef]
- He, C.; Zuo, Z.; Shi, X.; Li, R.; Chen, D.; Huang, X.; Chen, Y.; Wang, C. Effects of benzo(a)pyrene on the skeletal development of Sebastiscus marmoratus embryos and the molecular mechanism involved. Aquat. Toxicol. 2010, 101, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.; Li, W.; Liang, X.; Liu, J.; Ge, H.; Dong, Z. Gill transcriptome analysis reveals the molecular response to the acute low-salinity stress in Cyclina sinensis. Aquacult. Rep. 2021, 19, 100564. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yang, Y.; Li, X.; Yang, J.; Lian, J.; Lei, X.; Wang, D.; Zhang, J. Benthic community changes following the 2010 Hainan flood: Implications for reef resilience. Mar. Biol. Res. 2014, 10, 601–611. [Google Scholar] [CrossRef]
- Conte, F.P.; Lin, D. Kinetics of cellular morphogenesis in gill epithelium during sea water adaptation of oncorhynchus (walbaum). Comp. Biochem. Physiol. 1967, 23, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z. Fish osmoregulation. Bull. Biol. 2002, 37, 22–23. [Google Scholar]
- Marshall, W.S. Ion transport, osmoregulation, and acid-base balance. In The Physiology of Fishes; CRC Press: Boca Raton, FL, USA, 2005; pp. 177–230. [Google Scholar]
- Adachi, J.; Kishida, M.; Watanabe, S.; Hashimoto, Y.; Fukamizu, K.; Tomonaga, T. Proteome-wide discovery of unknown ATP-binding proteins and kinase inhibitor target proteins using an ATP probe. J. Proteome Res. 2014, 13, 5461–5470. [Google Scholar] [CrossRef]
- Shivkamat, P.; Roy, R. Regulation of membrane lipid bilayer structure during salinity adaptation: A study with the gill epithelial cell membranes of Oreochromis niloticus. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2005, 142, 28–36. [Google Scholar] [CrossRef]
- Evans, D.H.; Claiborne, J.B. Osmotic and ionic regulation in fishes. In Osmotic and Ionic Regulation; CRC Press: Boston, MA, USA, 2008; pp. 295–366. [Google Scholar]
- Wang, H.; Tang, L.; Wei, H.; Lu, J.; Mu, C.; Wang, C. Transcriptomic analysis of adaptive mechanisms in response to sudden salinity drop in the mud crab, Scylla paramamosain. BMC Genom. 2018, 19, 1–12. [Google Scholar] [CrossRef]
- Han, H.; Li, J.; Li, J.T.; Zhang, Z. Effects of baicalin on activity and mRNA expression of CYP1A in liver of flounder, Paralichthys olivaceus. J. Fish. Sci. China 2010, 17, 1121–1127. [Google Scholar]
- Sun, M.L. Revealing Osmotic Pressure Regulating Mechanism of Takifugu rubripes Based on Transcriptome Technology. Master’s Thesis, Dalian Ocean University, Da Lian, China.
- Evans, T.G.; Somero, G.N. A microarray-based transcriptomic time-course of hyper- and hypo-osmotic stress signaling events in the euryhaline fish Gillichthys mirabilis: Osmosensors to effectors. J. Exp. Biol. 2008, 211, 3636–3649. [Google Scholar] [CrossRef]
- Lempiäinen, H.; Shore, D. Growth control and ribosome biogenesis. Curr. Opin. Cell Biol. 2009, 21, 855–863. [Google Scholar] [CrossRef]
- Thomson, E.; Ferreira-Cerca, S.; Hurt, E. Eukaryotic ribosome biogenesis at a glance. J. Cell 2013, 126, 4815–4821. [Google Scholar] [CrossRef]
- Jastrzebski, K.; Hannan, K.M.; Tchoubrieva, E.B.; Hannan, R.D.; Pearson, R.B. Coordinate regulation of ribosome biogenesis and function by the ribosomal protein S6 kinase, a key mediator of mTOR function. Growth Factors 2007, 25, 209–226. [Google Scholar] [CrossRef]
- Sangiao-Alvarellos, S.; Laiz-Carrion, R.; Guzman, J.M.; Martín del Río, M.P.; Miguez, J.M.; Mancera, J.M.; Soengas, J.L. Acclimation of S. aurata to various salinities alters energy metabolism of osmoregulatory and nonosmoregulatory organs. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2003, 285, R897–R907. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Shang, Y.; Guo, R.; Chang, Y.; Jiang, Y. Salinity stress-induced differentially expressed miRNAs and target genes in sea cucumbers Apostichopus japonicus. Cell Stress Chaperones 2019, 24, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Soengas, J.L.; Aldegunde, M.; Andrés, M.D. Gradual transfer to sea water of rainbow trout: Effects on liver carbohydrate metabolism. J. Fish Biol. 1995, 47, 466–478. [Google Scholar] [CrossRef]
- Pattanagul, W.; Thitisaksakul, M. Effect of salinity stress on growth and carbohydrate metabolism in three rice (Oryza sativa L.) cultivars differing in salinity tolerance. Indian J. Exp. Biol. 2008, 46, 736–742. [Google Scholar]
- Jiahuaihan, T. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005, 15, 11–18. [Google Scholar]
- Gaitanaki, C.; Kefaloyianni, E.; Marmari, A.; Beis, I. Various stressors rapidly activate the p38-MAPK signaling pathway in Mytilus galloprovincialis (Lam.). Mol. Cell Biochem. 2004, 260, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Burhans, W.C.; Martin, W. DNA replication stress, genome instability and aging. Nucleic Acids Res. 2007, 35, 7545–7556. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.M.; Brown-Clay, J.D.; Fornace, A.J., Jr. Gadd45 in stress signaling, cell cycle control, and apoptosis. Adv. Exp. Med. Biol. 2013, 793, 1–19. [Google Scholar]
- Maryoung, L.A.; Lavado, R.; Bammler, T.K.; Gallagher, E.P.; Stapleton, P.L.; Beyer, R.P.; Schlenk, D. Differential gene expression in liver, gill, and olfactory rosettes of coho salmon (Oncorhynchus kisutch) after acclimation to salinity. Mar. Biotechnol. 2015, 17, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Manishankar, P.; Wang, N.; Köster, P.; Alatar, A.A.; Kudla, J. Calcium signaling during salt stress and in the regulation of ion homeostasis. J. Exp. Bot. 2018, 69, 4215–4226. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, J.; Liu, H.; Zhu, H.; Xu, G.; Xu, J. Adaptive evolution of low-salinity tolerance and hypoosmotic regulation in a euryhaline teleost, Takifugu obscurus. Mar. Biol. 2020, 167, 1–12. [Google Scholar] [CrossRef]
- Kim, J.J.; Lee, S.B.; Jang, J.; Yi, S.Y.; Kim, S.H.; Han, S.A.; Lee, J.M.; Tong, S.Y.; Vincelette, N.D.; Gao, B.; et al. WSB1 promotes tumor metastasis by inducing pVHL degradation. Genes Dev. 2015, 29, 2244–2257. [Google Scholar] [CrossRef]
- Lv, W.; Zhang, Y.; Wu, Z.; Chu, L.; Koide, S.S.; Chen, Y.; Yan, Y.; Li, Y. Identification of WSB1 gene as an important regulator in the development of zebrafish embryo during midblastula transition. Acta Biochim. Biophys. Sin. 2008, 40, 478–488. [Google Scholar] [CrossRef]
- Haque, M.; Kendal, J.K.; MacIsaac, R.M.; Demetrick, D.J. WSB1: From homeostasis to hypoxia. J. Biomed. Sci. 2016, 23, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Akinleye, A.; Avvaru, P.; Furqan, M.; Song, Y.; Liu, D. Phosphatidylinositol 3-kinase (PI3K) inhibitors as cancer therapeutics. J. Hematol. Oncol. 2013, 6, 88. [Google Scholar] [CrossRef]
- Li, D.; Wenger, T.; Seiler, C.; March, M.; Tian, L.; Kao, C.; Pandey, R.; Nguyen, K.; Chiavacci, R.; Sleiman, P.; et al. Whole Exome Sequencing Identifies EPHB4 and PIk3R6 as Causes of Generalized Lymphatic Anomaly. In Proceedings of the 55th Annual ESPE, Paris, France, 10–12 September 2016; p. 86. [Google Scholar]
- He, L.Y.; Shi, X.L.; Zhou, F.F.; Chen, M.X.; Han, K.H. Effect of low salinity stress on non-specific immunity enzymetic activity of Larimichthys crocea. J. Appl. Oceanogr. 2022, 41, 347–354. [Google Scholar]
- Li, X.Q.; Li, X.X.; Leng, X.J.; Liu, X.M.; Wang, X.C.; Li, J.L. Effect of different salinities on growth and flesh quality of Ctenopharyngodon idellus. J. Fish China 2007, 66, 343–348. [Google Scholar] [CrossRef]
- Huang, W.Q. Effects of low salinity culture on blood physiological and biochemical indexes of Larimichthys crocea. J. Ningde Norm. Univ. (Nat. Sci.) 2020, 32, 195–200. [Google Scholar]
- Asai, K. Human Group IVC Phospholipase A2(cPLA2γ) Roles in The Membrane Remodeling and Activation Induced by Oxidative Stress. J. Biol. Chem. 2003, 278, 8809–8814. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Kamata, R.; Kawagishi, N.; Nakanishi, H.; Suzuki, H.; Sugiura, T.; Waku, K. Roles of C-Terminal Processing, and Involvement in Transacylation Reaction of Human Group IVC Phospholipase A2 (cPLA2γ). J. Biochem. 2005, 137, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Kuang, F.W.; Luo, Z.G.; D, B.; Wu, C.; Wang, X.Y. Study on the relationship between cytosolic phospholipase A2 -γ activation and myocardial cell injury during cardiopulmonary bypass. Chin. Crit. Care Med. 2005, 17, 417–420. [Google Scholar]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Phys. 2000, 51, 463–499. [Google Scholar] [CrossRef]





| Gene | Primer Sequences (5′–3′) | Product Length |
|---|---|---|
| cPLA2γ-like | F: GCTGAGGTGCCGTCTTGTATC R: GGGAGGGTAGGGAGTGTTGAG | 84 |
| novel.1593 | F: ACCGATCAACTGAAGAACCAA R: ATTATTGCGAGAACATCTGGAGG | 85 |
| cyp1a1 | F: TGGCACCGAAGTCAACAAGC R: TGCTTCATTGTGAGACCGTATT | 195 |
| fgf10a | F: CCAACGGCAAGCCAATGAG R: CCAAAGAAGTCCGATACCCCG | 143 |
| pik3r6b | F: CCCAGAGTTCAGACACCTTA R: CTCCCTTTACAACTTCTCGT | 140 |
| dlgap1-like | F: CGTCAGCCGTCAATTTCCAGA | 229 |
| R: CACCACCATTAACGCTCCCA | ||
| β-actin | F: AGGGAAATCGTGCGTG R: ATGATGCTGTTGTAGGTGGT | 233 |
| Sample | Raw Reads | Clean Reads | Clean Bases | Error Rate | Q20 | Q30 | GC pct |
|---|---|---|---|---|---|---|---|
| Smsc1 | 55,156,824 | 54,422,314 | 8.16G | 0.03% | 97.06% | 91.99% | 48.64% |
| Smsc2 | 55,986,586 | 55,328,268 | 8.30G | 0.03% | 97.46% | 93.04% | 48.84% |
| Smsc3 | 48,404,256 | 47,888,456 | 7.18G | 0.03% | 97.30% | 92.66% | 48.80% |
| Smsh1 | 47,342,722 | 46,811,828 | 7.02G | 0.03% | 97.00% | 92.04% | 48.62% |
| Smsh2 | 52,238,798 | 51,609,812 | 7.74G | 0.03% | 97.21% | 92.46% | 48.62% |
| Smsh3 | 64,941,598 | 63,948,600 | 9.59G | 0.03% | 97.14% | 92.36% | 48.59% |
| Smsl1 | 56,895,700 | 56,030,266 | 8.40G | 0.03% | 97.51% | 93.17% | 49.18% |
| Smsl2 | 46,197,774 | 45,712,684 | 6.86G | 0.03% | 97.22% | 92.50% | 49.27% |
| Smsl3 | 59,582,084 | 58,994,516 | 8.85G | 0.03% | 97.59% | 93.28% | 48.65% |
| Sample | Total Reads | Total Mapped | Unique Mapped | Multi Mapped | Positive Mapped | Negative Mapped |
|---|---|---|---|---|---|---|
| Smsc1 | 54,422,314 | 50,263,680(92.36%) | 47,275,717(86.87%) | 2,987,963(5.49%) | 23,609,908(43.38%) | 23,665,809(43.49%) |
| Smsc2 | 55,328,268 | 51,090,961(92.34%) | 47,972,123(86.70%) | 3,118,838(5.64%) | 23,973,749(43.33%) | 23,998,374(43.37%) |
| Smsc3 | 47,888,456 | 44,027,917(91.94%) | 41,325,882(86.30%) | 2,702,035(5.64%) | 20,641,365(43.10%) | 20,684,517(43.19%) |
| Smsh1 | 46,811,828 | 43,545,299(93.02%) | 41,078,484(87.75%) | 2,466,815(5.27%) | 20,509,674(43.81%) | 20,568,810(43.94%) |
| Smsh2 | 51,609,812 | 48,037,088(93.08%) | 45,181,209(87.54%) | 2,855,879(5.53%) | 22,574,962(43.74%) | 22,606,247(43.80%) |
| Smsh3 | 63,948,600 | 58,998,754(92.26%) | 55,076,438(86.13%) | 3,922,316(6.13%) | 27,502,529(43.01%) | 27,573,909(43.12%) |
| Smsl1 | 56,030,266 | 48,358,037(86.31%) | 45,255,002(80.77%) | 3,103,035(5.54%) | 22,617,737(40.37%) | 22,637,265(40.40%) |
| Smsl2 | 45,712,684 | 41,128,026(89.97%) | 38,567,615(84.37%) | 2,560,411(5.60%) | 19,264,471(42.14%) | 19,303,144(42.23%) |
| Smsl3 | 58,994,516 | 54,979,662(93.19%) | 51,426,000(87.17%) | 3,553,662(6.02%) | 25,706,629(43.57%) | 25,719,371(43.60%) |
| Common DEGs | Expression Of Experimental Group | ||
|---|---|---|---|
| Smsc | Smsh | Smsl | |
| pik3r6b | 6.25 | 26.64 | 11.71 |
| cPLA2γ-like | 17.58 | 44.99 | 2.84 |
| WSB1 | 89.76 | 350.29 | 73.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Shou, C.; Han, Z. Transcriptome Analysis of Marbled Rockfish Sebastiscus marmoratus under Salinity Stress. Animals 2023, 13, 400. https://doi.org/10.3390/ani13030400
He Z, Shou C, Han Z. Transcriptome Analysis of Marbled Rockfish Sebastiscus marmoratus under Salinity Stress. Animals. 2023; 13(3):400. https://doi.org/10.3390/ani13030400
Chicago/Turabian StyleHe, Zhiqi, Chenyan Shou, and Zhiqiang Han. 2023. "Transcriptome Analysis of Marbled Rockfish Sebastiscus marmoratus under Salinity Stress" Animals 13, no. 3: 400. https://doi.org/10.3390/ani13030400
APA StyleHe, Z., Shou, C., & Han, Z. (2023). Transcriptome Analysis of Marbled Rockfish Sebastiscus marmoratus under Salinity Stress. Animals, 13(3), 400. https://doi.org/10.3390/ani13030400






