In Vitro Evaluation of Winged Bean (Psophocarpus tetragonolobus) Tubers as an Alternative Feed for Ruminants
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Treatments and Experimental Design
2.3. Rumen Liquid Sampling
2.4. In Vitro Rumen Incubation and Gas Determination
2.5. Chemical Analysis and Calculations
2.6. Statistical Analysis
3. Results
3.1. Ingredient Composition and Chemical Composition
3.2. Cumulation and Kinetics of Gas
3.3. Dry Matter, Organic Matter, and Fiber Degradability
3.4. Ammonia–N and Volatile Fatty Acid Concentrations
3.5. Rumen Microbial Count
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, G.; Zhou, Y.; Zhang, J.; Han, S.; Liu, X.; Yuan, C.; Ndayisenga, F.; Shi, J.; Zhang, B. Optimized strategy valorizing unautoclaved cottonseed hull as ruminant alternative feeds via solid-state fermentation: Detoxifying polyphenols, restraining hazardous microflora and antibiotic-resistance gene hosts. Environ. Technol. Innov. 2022, 28, 102937. [Google Scholar] [CrossRef]
- Bakr, M.; Ali, R.; Mahmoud, A.; Rahmy, H. Nutritional effects of feeding date pits to Barki lambs on their growth performance, rumen and blood parameters, and economic efficiency. Egypt. J. Nutr. Health. 2022, 25, 211–222. [Google Scholar] [CrossRef]
- Mbah, R.E.; Wasum, D.F. Russian-Ukraine 2022 War: A review of the economic impact of Russian-Ukraine crisis on the USA, UK, Canada, and Europe. Adv. Soc. Sci. Res. 2022, 9, 144–153. [Google Scholar] [CrossRef]
- Thai Feed Mill Association (TFM). Monthly Raw Materials Price. Home Page. Available online: https://www.thaifeedmill.com (accessed on 27 November 2022).
- Tollefson, J. What the war in Ukraine means for energy, climate and food. Nature 2022, 604, 232–233. [Google Scholar] [CrossRef] [PubMed]
- Wanapat, M.; Kang, S. Cassava chip (Manihot esculenta Crantz) as an energy source for ruminant feeding. Anim. Nutr. 2015, 1, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Office of Agricultural Economics (OAE). Home Page. Available online: https://www.oae.go.th (accessed on 14 November 2022).
- Singvejsakul, J.; Chaovanapoonphol, Y.; Limnirankul, B. Modeling the price volatility of cassava chips in Thailand: Evidence from Bayesian GARCH-X Estimates. Economies 2021, 9, 132. [Google Scholar] [CrossRef]
- Kant, A.; Nandan, R.; Kole, P. Agronomic performance for tuber characters in winged bean [Psophocarpus tetragonolobus (L.) DC.]. Int. J. Econ. Plants. 2018, 5, 86–89. [Google Scholar] [CrossRef]
- Sriwichai, S.; Monkham, T.; Sanitchon, J.; Jogloy, S.; Chankaew, S. Dual-purpose of the winged bean (Psophocarpus tetragonolobus (L.) DC.), the neglected Tropical legume, based on pod and tuber yields. Plants 2021, 10, 1746. [Google Scholar] [CrossRef]
- Jakrawatana, N.; Pingmuangleka, P.; Gheewala, S.H. Material flow management and cleaner production of cassava processing for future food, feed and fuel in Thailand. J. Clean. Prod. 2016, 134, 633–641. [Google Scholar] [CrossRef]
- Suntara, C.; Wanapat, M.; Chankaew, S.; Khonkhaeng, B.; Supapong, C.; Chanjula, P.; Gunun, P.; Gunun, N.; Foiklang, S.; Phesatcha, K. Improvement of the nutritional quality of psophocarpus tetragonolobus tubers by fermentation with ruminal crabtree-negative yeasts on the in vitro degradability and fermentation in rumen fluid. Fermentation 2022, 8, 209. [Google Scholar] [CrossRef]
- Adegboyega, T.T.; Abberton, M.T.; AbdelGadir, A.H.; Dianda, M.; Maziya-Dixon, B.; Oyatomi, O.A.; Ofodile, S.; Babalola, O.O. Nutrient and antinutrient composition of winged bean (Psophocarpus tetragonolobus (L.) DC.) seeds and tubers. J. Food Qual. 2019, 2019, 3075208. [Google Scholar] [CrossRef] [Green Version]
- Kant, A.; Saha, S.; Sarkar, S.; Lagoriya, D.S.; Paul, S.K.; Nandan, R. D2 Statistical Analysis of Tuber Yield and its Contributing Traits in Winged Bean [Psophocarpus tetragonolobus (L.) Dc.]. Biol. Int. J. 2022, 14, 1010–1015. [Google Scholar]
- National Research Council (NRC). Nutrient Requirements of Dairy Cattle, 7th ed.; The National Academies Press: Washington, DC, USA, 2001.
- Menke, K.H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Association of Official Analytical Chemist (AOAC). The Official Methods of Analysis of the Association of Official Analytical Chemist, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1998. [Google Scholar]
- Karlsson, J.; Spörndly, R.; Lindberg, M.; Holtenius, K. Replacing human-edible feed ingredients with by-products increases net food production efficiency in dairy cows. J. Dairy Sci. 2018, 101, 7146–7155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Soest, P.V.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Rinne, M.; Jaakkola, S.; Huhtanen, P. Grass maturity effects on cattle fed silage-based diets. 1. Organic matter digestion, rumen fermentation and nitrogen utilization. Anim. Feed Sci. Technol. 1997, 67, 1–17. [Google Scholar] [CrossRef]
- Goering, H.K. Forage Fiber Analyses (Apparatus, Reagents, Procedures, and Some Applications); US Agricultural Research Service: Washington, DC, USA, 1970.
- Fawcett, J.K.; Scott, J.E. A rapid and precise method for the determination of urea. J. Clin. Pathol. 1960, 13, 156–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozaki, M.U.; Okada, S.T. Experimental Manual of Lactic Acid Bacteria; Asakurasyoten: Tokyo, Japan, 1992; pp. 34–37. [Google Scholar]
- Ørskov, E.-R.; McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. Technol. 1979, 92, 499–503. [Google Scholar] [CrossRef] [Green Version]
- Statistical Analysis Systems (SAS). SAS/STAT User’s Guide. In Statistical Analysis Systems Institute, 2nd ed.; Version 9; SAS Institute Inc.: Cary, NC, USA, 2002. [Google Scholar]
- Steel, R.G.; Torrie, J.H. Principles and Procedures of Statistics; McGraw-Hill Book Co., Inc.: New York, NY, USA, 1980; p. 633. [Google Scholar]
- Getachew, G.; Blümmel, M.; Makkar, H.; Becker, K. In vitro gas measuring techniques for assessment of nutritional quality of feeds: A review. Anim. Feed Sci. Technol. 1998, 72, 261–281. [Google Scholar] [CrossRef]
- Blümmel, M.; Makkar, H.; Becker, K. In vitro gas production: A technique revisited. J. Anim. Physiol. Anim. 1997, 77, 24–34. [Google Scholar] [CrossRef]
- Elghandour, M.M.; Salem, A.Z.; Castañeda, J.S.M.; Camacho, L.M.; Kholif, A.E.; Chagoyán, J.C.V. Direct-fed microbes: A tool for improving the utilization of low-quality roughages in ruminants. J. Integr. Agric. 2015, 14, 526–533. [Google Scholar] [CrossRef]
- Ørskov, E. Manipulation of fibre digestion in the rumen. Proc. Nutr. Soc. 1991, 50, 187–196. [Google Scholar] [CrossRef] [PubMed]
- McAllister, T.; Bae, H.; Jones, G.; Cheng, K.-J. Microbial attachment and feed digestion in the rumen. J. Anim. Sci. 1994, 72, 3004–3018. [Google Scholar] [CrossRef] [Green Version]
- Chumpawadee, S.; Sommart, K.; Vongpralub, T.; Pattarajinda, V. Nutritional evaluation of non-forage high fibrous tropical feeds for ruminant using in vitro gas production technique. Walailak J. Sci. Technol. 2005, 2, 209–218. [Google Scholar]
- Sommart, K.; Parker, D.; Rowlinson, P.; Wanapat, M. Fermentation characteristics and microbial protein synthesis in an in vitro system using cassava, rice straw and dried ruzi grass as substrates. Asian-Australas. J. Anim. Sci. 2000, 13, 1084–1093. [Google Scholar] [CrossRef]
- Olivera, R.M.P. Use of in vitro gas production technique to assess the contribution of both soluble and insoluble fractions on the nutritive value of forages. Master’s Thesis, University of Aberdeen, Aberdeen, Scotland, 1998. [Google Scholar]
- Suntara, C.; Cherdthong, A.; Uriyapongson, S.; Wanapat, M.; Chanjula, P. Comparison effects of ruminal Crabtree-negative yeasts and Crabtree-positive yeasts for improving ensiled rice straw quality and ruminal digestion using in vitro gas production. J. Fungi. 2020, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Akinfemi, A.; Adua, M.; Adu, O. Evaluation of nutritive values of tropical feed sources and by-products using in vitro gas production technique in ruminant animals. Emir. J. Food Agric. 2012, 24, 348–353. [Google Scholar]
- Olfaz, M.; Kilic, U.; Boga, M.; Abdi, A.M. Determination of the in vitro gas production and potential feed value of olive, mulberry and sour orange tree leaves. Open Life Sci. 2018, 13, 269–278. [Google Scholar] [CrossRef]
- Chesson, A.; Forsberg, C. Polysaccharide degradation by rumen microorganisms. In The Rumen Microbial Ecosystem; Springer: Berlin/Heidelberg, Germany, 1997; pp. 329–381. [Google Scholar]
- Cherdthong, A.; Suntara, C.; Khota, W. Lactobacillus casei TH14 and additives could modulate the quality, gas kinetics and the in vitro degradability of ensilaged rice straw. J. Anim. Physiol. Anim. 2020, 104, 1690–1703. [Google Scholar] [CrossRef]
- Ekani, N.; Wahyono, T. Effect of different level of urea addition for rice straw fermentation application: In vitro evaluation. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; p. 012016. [Google Scholar]
- Yulistiani, D.; Puastuti, W.; Widiawati, Y. In Vitro Degradability and Rumen Fermentation of Grass or Rice Straw Basal Diet with or without Complete Rumen Modifier Supplementation. In International Seminar on Livestock Production and Veterinary Technology; Indonesian Research Institute for Animal Production: Bogor, Indonesia, 2016; pp. 310–317. [Google Scholar]
- Han, I.K.; Ha, J.; Garrett, W. Utilization of rice straw by ruminants as influenced by grass hay supplementation. Asian-Australas. J. Anim. Sci. 1993, 6, 561–567. [Google Scholar] [CrossRef]
- Wanapat, M.; Polyorach, S.; Boonnop, K.; Mapato, C.; Cherdthong, A. Effects of treating rice straw with urea or urea and calcium hydroxide upon intake, degradability, rumen fermentation and milk yield of dairy cows. Livest. Sci. 2009, 125, 238–243. [Google Scholar] [CrossRef]
- Polyorach, S.; Wanapat, M. Improving the quality of rice straw by urea and calcium hydroxide on rumen ecology, microbial protein synthesis in beef cattle. J. Anim. Physiol. Anim. 2015, 99, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Aquino, D.; Barrio, A.D.; Trach, N.X.; Hai, N.T.; Khang, D.N.; Toan, N.T.; Hung, N.V. Rice straw-based fodder for ruminants. In Sustainable Rice Straw Management; Springer: Berlin/Heidelberg, Germany, 2020; pp. 111–129. [Google Scholar]
- Cherdthong, A.; Wanapat, M. Manipulation of in vitro ruminal fermentation and degradability by dried rumen digesta. Livest. Sci. 2013, 153, 94–100. [Google Scholar] [CrossRef]
- Gunun, P.; Wanapat, M.; Anantasook, N.; Cherdthong, A. Effects of condensed tannins in Mao (Antidesma thwaitesianum Muell. Arg.) seed meal on rumen fermentation charecteristics and nitrogen utilization in goats. Asian-Australas. J Anim. Sci. 2016, 29, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Zhang, S.; Vo, H.N.P.; Bui, X.T.; Hoang, B.N. Volatile fatty acids production from waste streams by anaerobic digestion: A critical review of the roles and application of enzymes. Bioresour. Technol. 2022, 359, 127420. [Google Scholar] [CrossRef]
- Wanapat, M.; Gunun, P.; Anantasook, N.; Kang, S. Changes of rumen pH, fermentation and microbial population as influenced by different ratios of roughage (rice straw) to concentrate in dairy steers. J. Agric. Sci. Technol. 2014, 152, 675–685. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Li, Y.; Zhang, Y. Effects of high forage/concentrate diet on volatile fatty acid production and the microorganisms involved in VFA production in cow rumen. Animals 2020, 10, 223. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Harstad, O.M.; McAllister, T.; Dörsch, P.; Holo, H. Propionic acid bacteria enhance ruminal feed degradation and reduce methane production in vitro. Acta Agric. Scand. A Anim. Sci. 2020, 69, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Supapong, C.; Cherdthong, A.; Seankamsorn, A.; Khonkhaeng, B.; Wanapat, M.; Uriyapongson, S.; Gunun, N.; Gunun, P.; Chanjula, P.; Polyorach, S. In vitro fermentation, degradability and methane production as influenced by Delonix regia seed meal containing tannins and saponins. J. Anim. Feed Sci. 2017, 26, 123–130. [Google Scholar] [CrossRef]

| Chemical Compositions | Winged Bean Tuber | Rice Straw | Ruzi Grass | Napier Grass |
|---|---|---|---|---|
| Dry matter, g kg−1 fresh matter | 435 | 861 | 234 | 192 |
| g kg−1 dry matter | ||||
| Organic matter | 963 | 859 | 893 | 886 |
| Crude protein | 189.8 | 33.7 | 131.8 | 112.3 |
| Ether extract | 4.5 | 7.5 | 19.2 | 20.5 |
| Neutral detergent fiber (NDF) | 168.5 | 672.1 | 592.6 | 538.3 |
| Acid detergent fiber (ADF) | 54.2 | 458.7 | 305.1 | 301.1 |
| Acid detergent lignin (ADL) | 5.1 | 90.5 | 31.6 | 35.2 |
| Hemicellulose 1 | 113.8 | 214.2 | 287.3 | 237.9 |
| Cellulose 2 | 49.1 | 368 | 273 | 265.8 |
| Calcium | 11.5 | 4.2 | 4.3 | 5.3 |
| Phosphorus | 7.9 | 2.3 | 1.7 | 1.8 |
| Item | Rice Straw | Ruzi Grass | Napier Grass | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBT 0 | WBT 33 | WBT 66 | WBT 100 | WBT 0 | WBT 33 | WBT 66 | WBT 100 | WBT 0 | WBT 33 | WBT 66 | WBT 100 | |
| Ingredients, g kg−1 | ||||||||||||
| Roughage | ||||||||||||
| Rice straw | 200 | 200 | 200 | 200 | - | - | - | - | - | - | - | - |
| Ruzi grass | - | - | - | - | 200 | 200 | 200 | 200 | - | - | - | - |
| Napier grass | - | - | - | - | - | - | - | - | 200 | 200 | 200 | 200 |
| Concentrate | ||||||||||||
| Cassava chip | 500 | 335 | 170 | 0 | 500 | 335 | 170 | 0 | 500 | 335 | 170 | 0 |
| Winged bean tuber | 0 | 165 | 330 | 500 | 0 | 165 | 330 | 500 | 0 | 165 | 330 | 500 |
| Soybean meal | 95 | 95 | 95 | 95 | 95 | 95 | 95 | 95 | 95 | 95 | 95 | 95 |
| Palm kernel meal | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| Rice bran | 78 | 87 | 97 | 107 | 82 | 89 | 99 | 107 | 81 | 87 | 98 | 107 |
| Palm oil | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Salt | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Dicalcium phosphate | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Urea | 29 | 20 | 10 | 0 | 25 | 18 | 8 | 0 | 26 | 20 | 9 | 0 |
| Mineral and vitamin mix 1 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Chemical composition | ||||||||||||
| Dry matter, g/kg−1 | 967 | 966 | 953 | 958 | 962 | 967 | 965 | 961 | 962 | 959 | 964 | 967 |
| g kg−1 dry matter | ||||||||||||
| Organic matter | 932 | 930 | 925 | 921 | 945 | 947 | 943 | 952 | 941 | 945 | 942 | 949 |
| Crude protein | 158 | 159 | 159 | 160 | 160 | 161 | 162 | 160 | 160 | 159 | 160 | 161 |
| Ether extract | 38.9 | 39.1 | 39.4 | 39.5 | 40.2 | 42.1 | 41.5 | 42.3 | 41.4 | 41.3 | 41.9 | 41.2 |
| Neutral detergent fiber | 389 | 392 | 393 | 395 | 373 | 375 | 376 | 379 | 369 | 372 | 377 | 378 |
| Acid detergent fiber | 145 | 147 | 150 | 155 | 138 | 142 | 139 | 140 | 137 | 140 | 136 | 139 |
| Roughage Source | WBT Inclusion | Gas Kinetics 1 | |||
|---|---|---|---|---|---|
| a | b | c | |a| + b | ||
| Rice straw, RS (Oryza sativa) | 0 | −18.33 | 137.3 b | 0.076 | 155.7 b |
| 33 | −14.94 | 130.9 b | 0.064 | 146.3 b | |
| 66 | −13.19 | 125.4 b | 0.067 | 138.9 b | |
| 100 | −12.33 | 130.6 b | 0.066 | 142.9 b | |
| Ruzi grass, RZ (Brachiaria ruziziensis) | 0 | −18.64 | 139.9 b | 0.077 | 157.5 b |
| 33 | −17.27 | 151.3 b | 0.077 | 168.9 b | |
| 66 | −21.08 | 183.9 a | 0.074 | 204.9 a | |
| 100 | −23.88 | 196.2 a | 0.079 | 220.1 a | |
| Napier grass, NP (Cenchrus purpureus) | 0 | −19.85 | 149.9 b | 0.076 | 168.7 b |
| 33 | −18.88 | 142.7 b | 0.087 | 162.2 b | |
| 66 | −16.14 | 136.8 b | 0.081 | 152.9 b | |
| 100 | −13.36 | 128.9 b | 0.076 | 142.3 b | |
| SEM | 2.13 | 9.75 | 0.003 | 11.81 | |
| Roughage source | RS | −14.69 a | 131.1 | 0.068 b | 146.0 |
| RZ | −20.22 b | 145.6 | 0.077 a | 163.2 | |
| NP | −17.06 a | 139.6 | 0.079 a | 156.5 | |
| Level of WBT | 0 | −18.94 | 142.4 | 0.077 | 160.6 |
| 33 | −17.03 | 141.7 | 0.076 | 159.2 | |
| 66 | −16.80 | 131.1 | 0.074 | 145.9 | |
| 100 | −16.53 | 129.8 | 0.074 | 142.6 | |
| Interaction | Roughage source × WBT level | 0.07 | p < 0.01 | 0.054 | 0.0113 |
| Contrast | Rice straw | −14.69 b | 130.5 b | 0.068 b | 145.9 b |
| Grass | −18.63 a | 141.6 a | 0.078 a | 172.2 a | |
| Polynomial | WTB (Linear) | 0.050 | 0.562 | 0.525 | 0.397 |
| WTB (Quadratic) | 0.558 | 0.558 | 0.024 | 0.575 | |
| WTB (Cubic) | 0.937 | 0.823 | 0.027 | 0.863 | |
| Roughage Source | WBT Inclusion | Degradability at 12 h (g/kg Dry Matter) | Degradability at 24 h (g/kg Dry Matter) | ||||
|---|---|---|---|---|---|---|---|
| IVDMD | IVOMD | IVNDFD | IVDMD | IVOMD | IVNDFD | ||
| Rice straw, RS (Oryza sativa) | 0 | 560.1 | 623.3 abcde | 623.3 | 604.5 | 630.2 de | 632.2 |
| 33 | 582.8 | 593.3 bcde | 599.9 | 579.5 | 611.2 e | 625.1 | |
| 66 | 554.4 | 629.9 abcde | 606.8 | 572.4 | 630.8 de | 620.0 | |
| 100 | 546.5 | 546.4 e | 579.4 | 636.6 | 643.6 cde | 619.9 | |
| Ruzi grass, RZ (Brachiaria ruziziensis) | 0 | 639.6 | 582.2 cde | 631.5 | 672.2 | 694.8 ab | 653.5 |
| 33 | 618.5 | 625.1 abcde | 633.0 | 661.5 | 719.3 ab | 649.9 | |
| 66 | 616.1 | 675.6 ab | 617.9 | 664.9 | 681.7 bc | 663.3 | |
| 100 | 613.2 | 682.9 a | 636.8 | 638.8 | 693.2 ab | 635.9 | |
| Napier grass, NP (Cenchrus purpureus) | 0 | 602.6 | 674.5 ab | 630.0 | 665.5 | 727.9 a | 651.6 |
| 33 | 586.9 | 648.3 abc | 625.1 | 690.4 | 653.2 cd | 633.0 | |
| 66 | 598.7 | 552.2 de | 620.2 | 663.1 | 642.2 cde | 614.5 | |
| 100 | 604.2 | 639.9 abcd | 624.8 | 621.0 | 623.7 de | 610.8 | |
| SEM | 21.1 | 25.7 | 15.1 | 20.7 | 12.3 | 16.6 | |
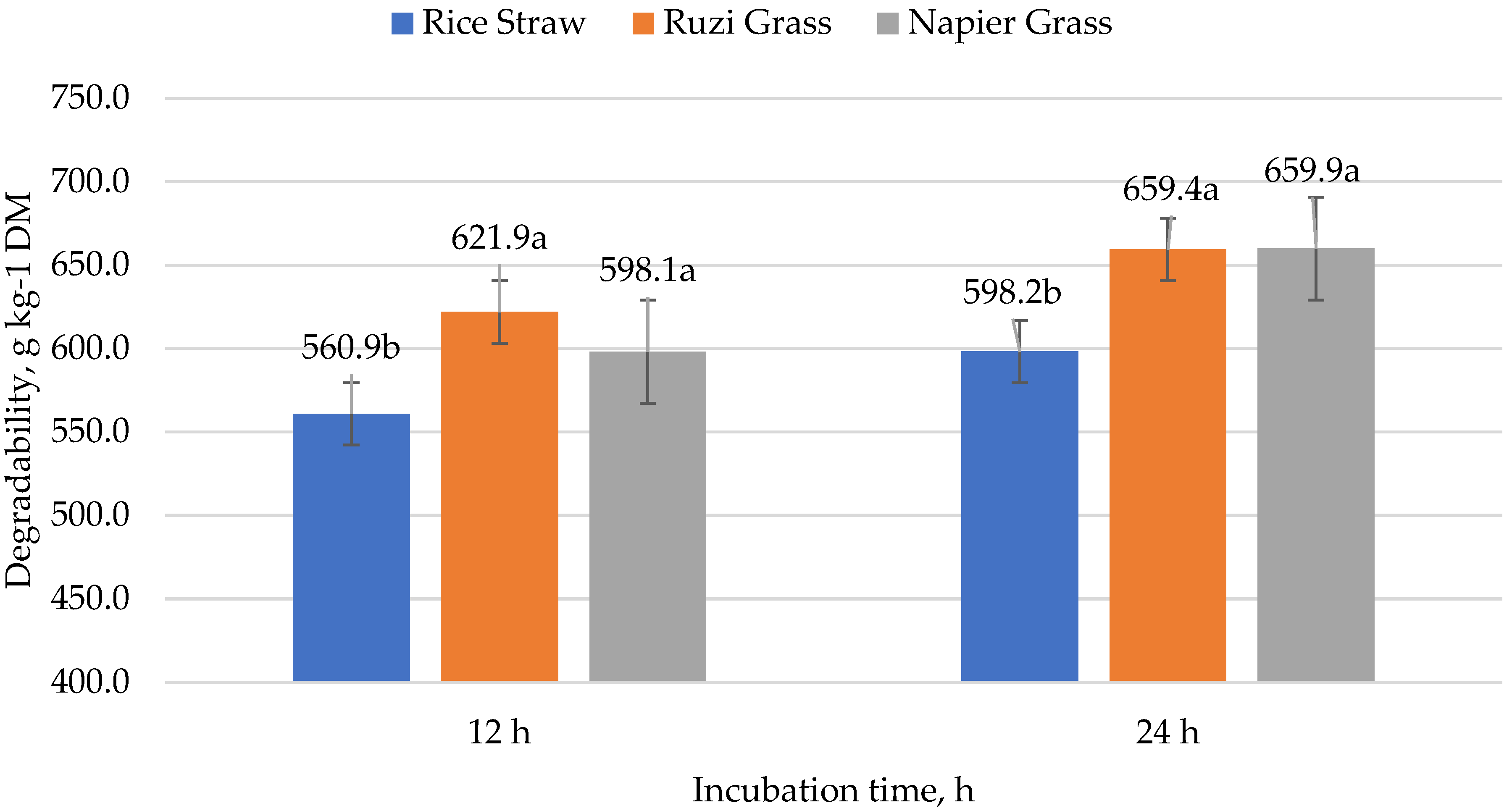
| Roughage source | RS | 560.9 b | 598.2 b | 602.4 | 560.9 b | 628.9 | 624.3 |
| RZ | 621.9 a | 641.5 a | 629.8 | 621.9 a | 697.2 | 650.6 | |
| NP | 598.1 a | 628.8 a | 625.0 | 598.1 a | 661.7 | 627.5 | |
| Level of WBT | 0 | 600.8 | 626.7 | 628.3 | 647.4 | 684.3 | 645.7 |
| 33 | 596.1 | 622.3 | 619.3 | 643.8 | 661.2 | 636.0 | |
| 66 | 589.7 | 619.2 | 614.9 | 633.5 | 651.6 | 632.6 | |
| 100 | 588.0 | 623.1 | 613.7 | 632.1 | 653.5 | 622.2 | |
| Interaction | Roughage source × WBT | 0.87 | <0.01 | 0.71 | 0.14 | <0.01 | 0.17 |
| Contrast | Rice straw | 560.9 b | 598.2 b | 602.4 b | 598.2 | 628.9 | 624.3 |
| Forage | 609.9 a | 635.1 a | 627.4 a | 659.7 | 679.5 | 639.1 | |
| Polynomial | WBT (Linear) | 0.48 | 0.12 | 0.09 | 0.35 | 0.03 | 0.58 |
| WBT (Quadratic) | 0.48 | 0.32 | 0.90 | 0.05 | 0.21 | 0.84 | |
| WBT (Cubic) | 0.46 | 0.13 | 0.36 | 0.57 | 0.41 | 0.97 | |
| Roughage | WBT Inclusion | NH3-N | TVFA | ||||
|---|---|---|---|---|---|---|---|
| 4 h | 8 h | Mean | 4 h | 8 h | Mean | ||
| Rice straw, RS (Oryza sativa) | 0 | 16.6 | 19.1 | 17.9 | 65.4 | 80.0 | 72.7 |
| 33 | 15.8 | 20.7 | 18.2 | 54.8 | 71.4 | 63.1 | |
| 66 | 15.8 | 21.2 | 18.5 | 72.8 | 66.8 | 69.8 | |
| 100 | 15.3 | 21.0 | 18.1 | 61.9 | 66.2 | 64.0 | |
| Ruzi grass, RZ (Brachiaria ruziziensis) | 0 | 15.7 | 23.2 | 19.5 | 77.5 | 80.7 | 79.1 |
| 33 | 15.2 | 19.7 | 17.5 | 77.3 | 85.1 | 81.2 | |
| 66 | 15.6 | 20.7 | 18.2 | 63.7 | 82.3 | 73.0 | |
| 100 | 17.2 | 21.3 | 19.2 | 66.9 | 80.3 | 73.6 | |
| Napier grass, NP (Cenchrus purpureus) | 0 | 15.7 | 19.0 | 17.4 | 74.4 | 89.0 | 81.7 |
| 33 | 15.2 | 20.6 | 17.9 | 71.8 | 81.8 | 76.8 | |
| 66 | 15.6 | 22.0 | 18.8 | 70.1 | 86.5 | 78.3 | |
| 100 | 15.7 | 21.7 | 18.7 | 67.8 | 72.0 | 69.9 | |
| SEM | 2.34 | 1.88 | 1.25 | 4.99 | 3.77 | 3.03 | |
| Roughage source | RS | 15.9 | 20.5 | 18.2 | 63.7 | 71.1 b | 67.4 b |
| RZ | 15.9 | 21.2 | 18.6 | 71.3 | 82.1 a | 76.7 a | |
| NP | 15.6 | 20.8 | 18.2 | 71.0 | 82.3 a | 76.7 a | |
| Level of WBT | 0 | 16.0 | 20.5 | 18.2 | 72.4 | 83.2 a | 77.8 a |
| 33 | 15.4 | 20.3 | 17.9 | 68.0 | 79.4 a | 73.7 ab | |
| 66 | 15.7 | 21.3 | 18.5 | 68.8 | 78.5 ab | 73.7 ab | |
| 100 | 16.1 | 21.3 | 18.7 | 65.5 | 72.8 b | 69.2 b | |
| Interaction | Roughage source × WBT | 1.00 | 0.96 | 0.91 | 0.18 | 0.16 | 0.31 |
| Contrast | Rice straw | 15.9 | 20.5 | 18.2 | 63.7 b | 71.1 b | 67.4 b |
| Grass | 15.8 | 21.0 | 18.4 | 71.2 a | 82.2 a | 76.7 a | |
| Polynomial | WTB (Linear) | 0.71 | 0.76 | 0.86 | 0.74 | 0.01 | 0.18 |
| WTB (Quadratic) | 0.94 | 0.97 | 0.79 | 0.98 | 0.27 | 0.53 | |
| WTB (Cubic) | 0.89 | 0.56 | 0.92 | 0.02 | 1.00 | 0.06 | |
| Roughage | WBT Inclusion | Acetate (mol/100 mol) | Propionate (mol/100 mol) | Acetate/Propionate Ratio | Butyrate (mol/100 mol) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 h | 8 h | Mean | 4 h | 8 h | Mean | 4 h | 8 h | Mean | 4 h | 8 h | Mean | ||
| Rice straw, RS (Oryza sativa) | 0 | 66.5 | 64.2 | 65.4 | 24.1 | 27.6 | 25.8 | 2.8 | 2.3 | 2.5 | 9.5 | 8.2 ab | 8.8 |
| 33 | 67.0 | 65.9 | 66.4 | 23.3 | 25.9 | 24.6 | 2.9 | 2.6 | 2.7 | 9.7 | 8.2 ab | 8.9 | |
| 66 | 67.8 | 66.5 | 67.1 | 22.5 | 24.7 | 23.6 | 3.0 | 2.7 | 2.8 | 9.7 | 8.9 a | 9.3 | |
| 100 | 67.4 | 68.0 | 67.7 | 22.3 | 23.2 | 22.7 | 3.0 | 2.9 | 3.0 | 10.3 | 8.9 a | 9.6 | |
| Ruzi grass, RZ (Brachiaria ruziziensis) | 0 | 63.3 | 60.8 | 62.1 | 27.6 | 32.7 | 30.1 | 2.3 | 1.9 | 2.1 | 9.0 | 6.5 c | 7.8 |
| 33 | 60.5 | 60.8 | 60.6 | 29.5 | 32.4 | 30.9 | 2.1 | 1.9 | 2.0 | 10.0 | 6.9 bc | 8.4 | |
| 66 | 64.0 | 63.0 | 63.5 | 27.3 | 29.0 | 28.1 | 2.3 | 2.2 | 2.3 | 8.7 | 8.0 ab | 8.4 | |
| 100 | 64.2 | 63.8 | 64.0 | 27.1 | 27.5 | 27.3 | 2.4 | 2.3 | 2.3 | 8.7 | 8.7 a | 8.7 | |
| Napier grass, NP (Cenchrus purpureus) | 0 | 65.9 | 60.8 | 63.3 | 26.2 | 31.3 | 28.7 | 2.5 | 2.0 | 2.2 | 8.0 | 8.0 ab | 8.0 |
| 33 | 65.0 | 61.5 | 63.2 | 27.2 | 30.6 | 28.9 | 2.4 | 2.0 | 2.2 | 7.8 | 7.9 abc | 7.8 | |
| 66 | 64.5 | 62.0 | 63.3 | 28.2 | 30.8 | 29.5 | 2.3 | 2.0 | 2.1 | 7.3 | 7.2 bc | 7.2 | |
| 100 | 63.3 | 64.9 | 64.1 | 29.1 | 28.0 | 28.6 | 2.2 | 2.3 | 2.3 | 7.7 | 7.0 bc | 7.3 | |
| SEM | 1.17 | 1.09 | 0.68 | 1.31 | 1.27 | 0.74 | 0.19 | 0.14 | 0.10 | 0.43 | 0.41 | 0.31 | |
| Roughage source | RS | 67.2 a | 66.1 a | 66.7 a | 23.1 b | 25.3 b | 24.2 b | 2.9 a | 2.6 a | 2.8 a | 9.8 a | 8.5 a | 9.2 a |
| RZ | 63.0 b | 62.1 b | 62.6 b | 27.9 a | 30.4 a | 29.1 a | 2.3 b | 2.1 b | 2.2 b | 9.1 b | 7.5 b | 8.3 b | |
| NP | 64.7 b | 62.3 b | 63.5 b | 27.6 a | 30.2 a | 28.9 a | 2.4 b | 2.1 b | 2.2 b | 7.7 c | 7.5 b | 7.6 c | |
| Level of WBT | 0 | 65.2 | 62.0 b | 63.6 b | 26.0 | 30.5 a | 28.2 a | 2.6 | 2.1 b | 2.3 b | 8.8 | 7.5 | 8.2 |
| 33 | 64.2 | 62.7 b | 63.4 b | 26.7 | 29.6 a | 28.2 a | 2.4 | 2.2 b | 2.3 b | 9.2 | 7.6 | 8.4 | |
| 66 | 65.4 | 63.8 ab | 64.6 ab | 26.0 | 28.1 ab | 27.1 ab | 2.6 | 2.3 ab | 2.4 ab | 8.6 | 8.0 | 8.3 | |
| 100 | 65.0 | 65.6 a | 65.3 a | 26.1 | 26.2 b | 26.2 b | 2.5 | 2.5 a | 2.5 a | 8.9 | 8.2 | 8.5 | |
| Interaction | Roughage source × WBT | 0.33 | 0.95 | 0.33 | 0.52 | 0.80 | 0.18 | 0.77 | 0.83 | 0.34 | 0.39 | 0.04 | 0.20 |
| Contrast | Rice straw | 67.2 a | 66.1 a | 66.7 a | 23.1 b | 25.3 b | 24.2 b | 2.9 a | 2.6 a | 2.8 a | 9.8 a | 8.5 a | 9.2 a |
| Grass | 63.8 b | 62.2 b | 63.1 b | 27.8 a | 30.3 a | 29.2 a | 2.3 b | 2.1 b | 2.2 b | 8.4 b | 7.5 b | 8.0 b | |
| Polynomial | WTB (Linear) | 0.50 | 0.03 | 0.03 | 0.31 | 0.03 | 0.01 | 0.52 | <0.01 | <0.01 | 0.23 | 0.14 | 0.08 |
| WTB (Quadratic) | 0.70 | 0.95 | 0.72 | 0.82 | 0.97 | 0.77 | 0.90 | 0.86 | 0.80 | 0.64 | 0.90 | 0.75 | |
| WTB (Cubic) | 0.80 | 0.73 | 0.90 | 0.92 | 0.89 | 0.99 | 0.86 | 0.81 | 0.91 | 0.71 | 0.52 | 0.94 | |
| Roughage | WBT Inclusion | Bacteria, Log 10 Cell/mL | Protozoa, Log 10 Cell/mL | Fungal Zoospore, Log 10 Cell/mL | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 h | 8 h | Mean | 4 h | 8 h | Mean | 4 h | 8 h | Mean | ||
| Rice straw, RS (Oryza sativa) | 0 | 7.49 | 8.47 | 7.98 | 4.70 | 4.99 | 4.84 | 4.03 | 4.79 | 4.41 |
| 33 | 7.72 | 8.08 | 7.90 | 4.45 | 4.82 | 4.64 | 4.21 | 5.00 | 4.60 | |
| 66 | 7.72 | 8.00 | 7.86 | 4.58 | 5.15 | 4.87 | 3.97 | 5.09 | 4.53 | |
| 100 | 7.74 | 7.99 | 7.86 | 4.81 | 5.04 | 4.92 | 4.09 | 4.57 | 4.33 | |
| Ruzi grass, RZ (Brachiaria ruziziensis) | 0 | 7.84 | 8.40 | 8.12 | 4.40 | 5.06 | 4.73 | 3.90 | 4.81 | 4.36 |
| 33 | 7.82 | 8.40 | 8.11 | 4.58 | 5.03 | 4.80 | 3.94 | 5.30 | 4.62 | |
| 66 | 7.86 | 8.07 | 7.97 | 4.49 | 4.99 | 4.74 | 3.85 | 5.12 | 4.48 | |
| 100 | 7.93 | 8.06 | 8.00 | 4.29 | 4.92 | 4.60 | 4.03 | 4.80 | 4.41 | |
| Napier grass, NP (Cenchrus purpureus) | 0 | 7.86 | 8.21 | 8.04 | 4.41 | 5.16 | 4.79 | 4.07 | 4.82 | 4.44 |
| 33 | 7.46 | 8.14 | 7.80 | 4.65 | 4.90 | 4.77 | 4.03 | 4.84 | 4.43 | |
| 66 | 7.87 | 8.21 | 8.04 | 4.62 | 5.14 | 4.88 | 4.02 | 5.17 | 4.60 | |
| 100 | 7.88 | 8.18 | 8.03 | 4.32 | 5.12 | 4.72 | 4.07 | 4.92 | 4.50 | |
| SEM | 0.146 | 0.099 | 0.091 | 0.171 | 0.127 | 0.082 | 0.184 | 0.141 | 0.108 | |
| Roughage source | RS | 7.67 | 8.13 | 7.90 | 4.64 | 5.00 | 4.82 | 4.08 | 4.86 | 4.47 |
| RZ | 7.86 | 8.23 | 8.05 | 4.44 | 5.00 | 4.72 | 3.93 | 5.01 | 4.47 | |
| NP | 7.77 | 8.18 | 7.97 | 4.50 | 5.08 | 4.79 | 4.05 | 4.94 | 4.49 | |
| Level of WBT | 0 | 7.73 | 8.36 a | 8.05 | 4.51 | 5.07 | 4.79 | 4.00 | 4.81 bc | 4.40 |
| 33 | 7.67 | 8.20 ab | 7.93 | 4.56 | 4.91 | 4.74 | 4.06 | 5.04 ab | 4.55 | |
| 66 | 7.82 | 8.09 b | 7.95 | 4.56 | 5.09 | 4.83 | 3.95 | 5.13 a | 4.54 | |
| 100 | 7.85 | 8.08 b | 7.96 | 4.47 | 5.03 | 4.75 | 4.06 | 4.76 b | 4.41 | |
| Interaction | Roughage source × WBT level | 0.53 | 0.39 | 0.47 | 0.47 | 0.76 | 0.23 | 0.99 | 0.40 | 0.72 |
| Contrast | Rice straw | 7.67 | 8.13 | 7.90 | 4.64 | 5.00 | 4.82 | 4.08 | 4.86 | 4.47 |
| Grass | 7.81 | 8.21 | 8.01 | 4.47 | 5.04 | 4.75 | 3.99 | 4.97 | 4.48 | |
| Polynomial | WTB (Linear) | 0.28 | 0.01 | 0.36 | 0.56 | 0.42 | 0.21 | 0.92 | 0.39 | 0.53 |
| WTB (Quadratic) | 0.48 | 0.08 | 0.65 | 0.19 | 0.80 | 0.14 | 0.87 | 0.02 | 0.10 | |
| WTB (Cubic) | 0.71 | 0.58 | 0.99 | 0.72 | 0.13 | 0.12 | 0.36 | 0.44 | 0.79 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suntara, C.; Sombuddee, N.; Lukbun, S.; Kanakai, N.; Srichompoo, P.; Chankaew, S.; Khonkhaeng, B.; Gunun, P.; Gunun, N.; Polyorach, S.; et al. In Vitro Evaluation of Winged Bean (Psophocarpus tetragonolobus) Tubers as an Alternative Feed for Ruminants. Animals 2023, 13, 677. https://doi.org/10.3390/ani13040677
Suntara C, Sombuddee N, Lukbun S, Kanakai N, Srichompoo P, Chankaew S, Khonkhaeng B, Gunun P, Gunun N, Polyorach S, et al. In Vitro Evaluation of Winged Bean (Psophocarpus tetragonolobus) Tubers as an Alternative Feed for Ruminants. Animals. 2023; 13(4):677. https://doi.org/10.3390/ani13040677
Chicago/Turabian StyleSuntara, Chanon, Napudsawun Sombuddee, Saowalak Lukbun, Natdanai Kanakai, Pachara Srichompoo, Sompong Chankaew, Benjamad Khonkhaeng, Pongsatorn Gunun, Nirawan Gunun, Sineenart Polyorach, and et al. 2023. "In Vitro Evaluation of Winged Bean (Psophocarpus tetragonolobus) Tubers as an Alternative Feed for Ruminants" Animals 13, no. 4: 677. https://doi.org/10.3390/ani13040677








