Ungulates’ Behavioral Responses to Humans as an Apex Predator in a Hunting-Prohibited Area of China
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study System
2.2. Playback Experiment
2.3. Statistical Analysis
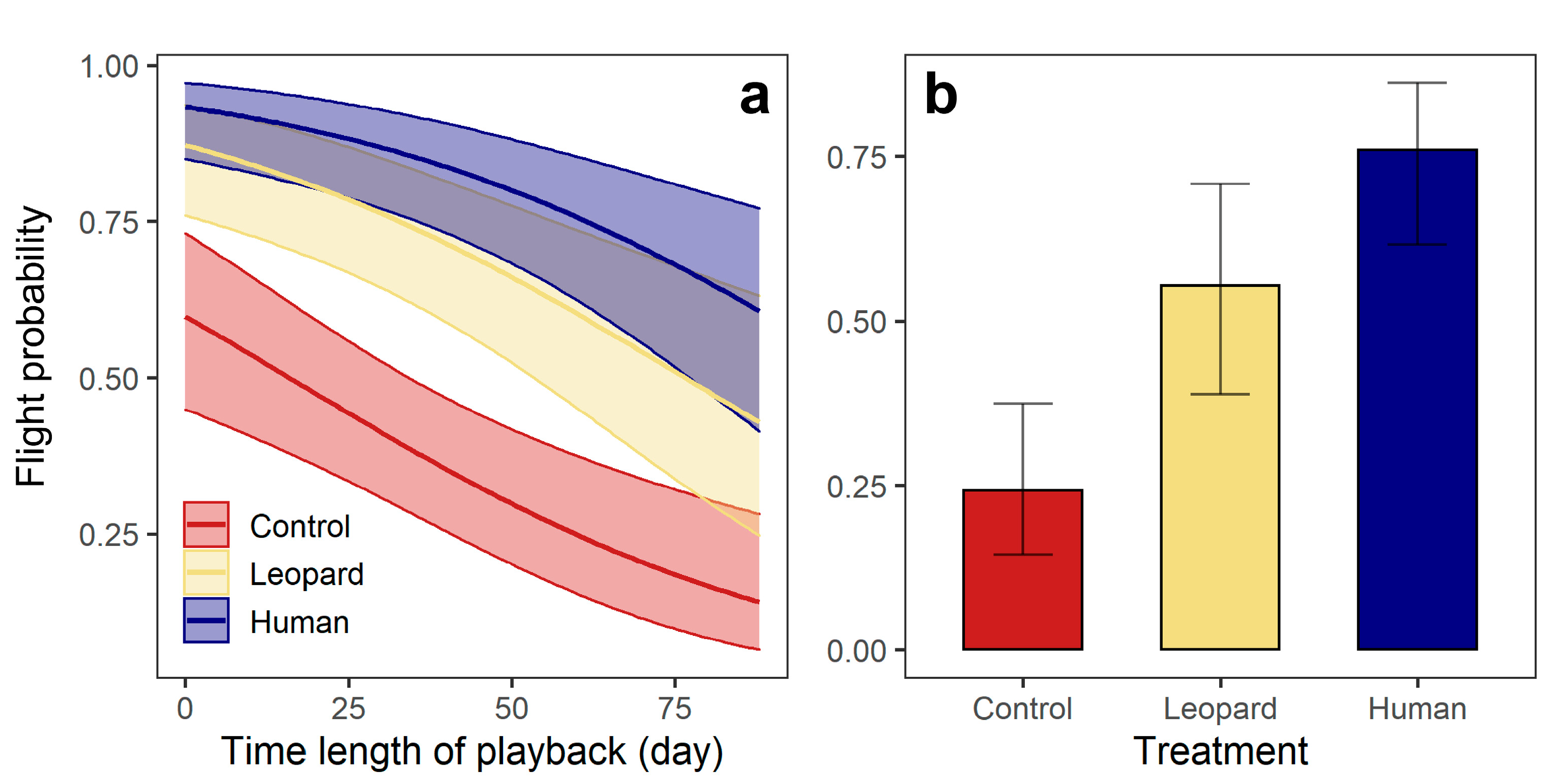
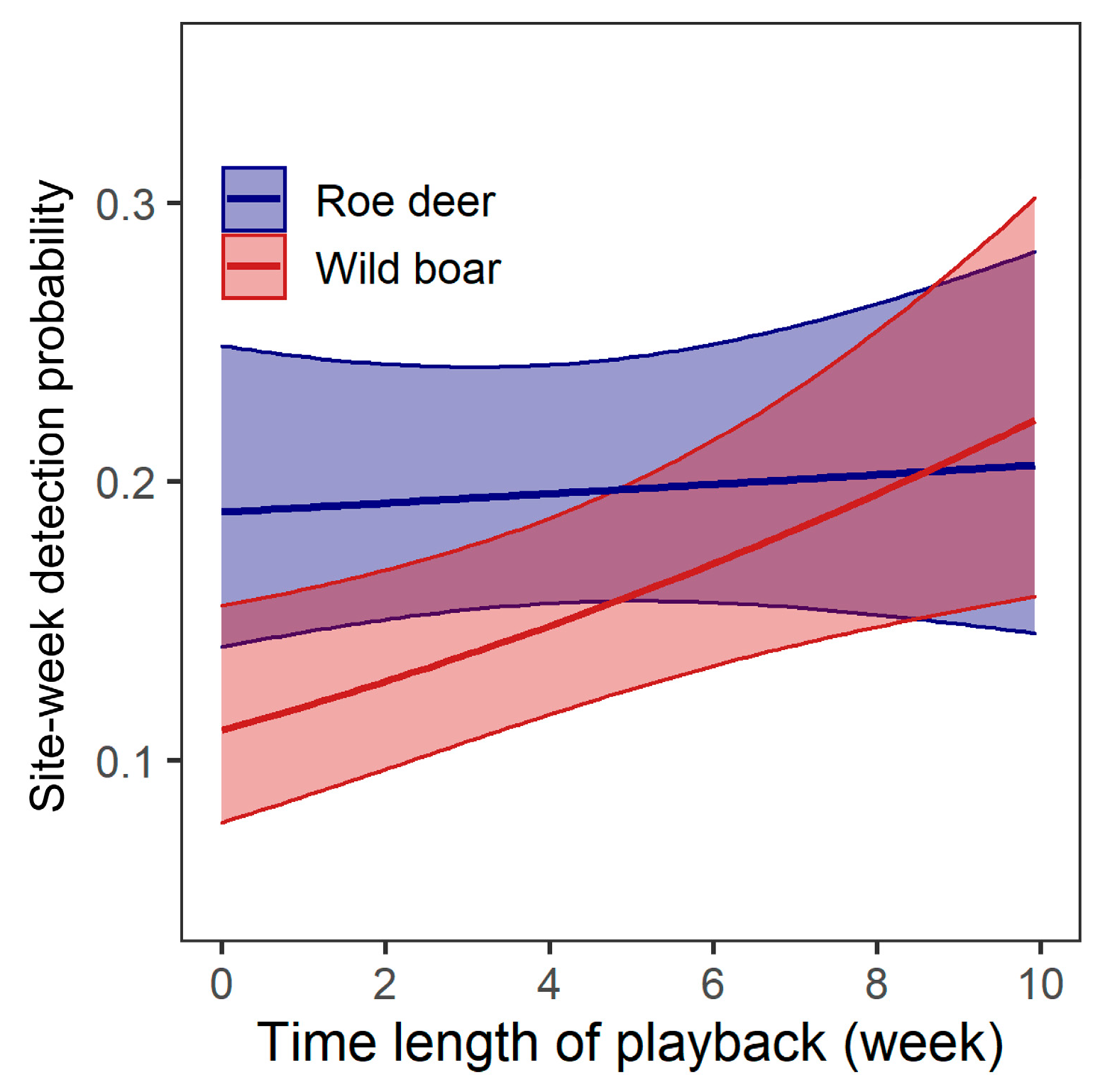
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darimont, C.; Fox, C.; Bryan, H.; Reimchen, T. The unique ecology of human predators. Science 2015, 349, 858–860. [Google Scholar] [CrossRef]
- Ciuti, S.; Northrup, J.; Muhly, T.; Simi, S.; Musiani, M.; Pitt, J.; Boyce, M. Effects of humans on behaviour of wildlife exceed those of natural predators in a landscape of fear. PLoS ONE 2012, 7, e50611. [Google Scholar] [CrossRef]
- Tucker, M.; Böhning-Gaese, K.; Fagan, W.; Fryxell, J.; Moorter, B.; Alberts, S.; Ali, A.; Allen, A.; Attias, N.; Avgar, T.; et al. Moving in the Anthropocene: Global reductions in terrestrial mammalian movements. Science 2018, 359, 466–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaynor, K.M.; Hojnowski, C.; Carte, N.; Brashares, J. The influence of human disturbance on wildlife nocturnality. Science 2018, 360, 1232–1235. [Google Scholar] [CrossRef] [Green Version]
- Mehlhoop, A.C.; Van Moorter, B.; Rolandsen, C.; Hagen, D.; Granhus, A.; Eriksen, R.; Ringsby, T.; Solberg, E.J. Moose in our neighborhood: Does perceived hunting risk have cascading effects on tree performance in vicinity of roads and houses? Ecol. Evol. 2022, 12, e8795. [Google Scholar] [CrossRef] [PubMed]
- Suraci, J.P.; Clinchy, M.; Zanette, L.; Wilmers, C. Fear of humans as apex predators has landscape-scale impacts from mountain lions to mice. Ecol. Lett. 2019, 22, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Yovovich, V.; Thomsen, M.; Wilmers, C. Pumas’ fear of humans precipitates changes in plant architecture. Ecosphere 2021, 12, e03309. [Google Scholar] [CrossRef]
- Nickel, B.A.; Suraci, J.; Allen, M.; Wilmers, C. Human presence and human footprint have non-equivalent effects on wildlife spatiotemporal habitat use. Biol. Conserv. 2020, 241, 108383. [Google Scholar] [CrossRef]
- Wilmers, C.C.; Wang, Y.; Nickel, B.; Houghtaling, P.; Shakeri, Y.; Allen, M.; Kermish-Wells, J.; Yovovich, V.; Williams, T. Scale dependent behavioral responses to human development by a large predator, the puma. PLoS ONE 2013, 8, e60590. [Google Scholar] [CrossRef] [Green Version]
- Suraci, J.P.; Frank, L.; Oriol-Cotterill, A.; Ekwanga, S.; Williams, T.; Wilmers, C. Behavior-specific habitat selection by African lions may promote their persistence in a human-dominated landscape. Ecology 2019, 100, e02644. [Google Scholar] [CrossRef]
- Ordiz, A.; Støen, O.-G.; Sæbø, S.; Kindberg, J.; Delibes, M.; Swenson, J. Do bears know they are being hunted? Biol. Conserv. 2012, 152, 21–28. [Google Scholar] [CrossRef]
- Wang, Y.; Allen, M.; Wilmers, C. Mesopredator spatial and temporal responses to large predators and human development in the Santa Cruz Mountains of California. Biol. Conserv. 2015, 190, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Mols, B.; Lambers, E.; Cromsigt, J.; Kuijper, D.; Smit, C. Recreation and hunting differentially affect deer behaviour and sapling performance. Oikos 2022, 2022, e08448. [Google Scholar] [CrossRef]
- Frid, A.; Dill, L. Human-caused disturbance stimuli as a form of predation risk. Conserv. Ecol. 2002, 6, 11. [Google Scholar] [CrossRef]
- Stankowich, T. Ungulate flight responses to human disturbance: A review and meta-analysis. Biol. Conserv. 2008, 141, 2159–2173. [Google Scholar] [CrossRef]
- Palmer, M.S.; Wang, C.; Plucinski, J.; Pringle, R. BoomBox: An Automated Behavioural Response (ABR) camera trap module for wildlife playback experiments. Methods Ecol. Evol. 2022, 13, 611–618. [Google Scholar] [CrossRef]
- Suraci, J.P.; Clinchy, M.; Mugerwa, B.; Delsey, M.; Macdonald, D.; Smith, J.; Wilmers, C.; Zanette, L.; Golding, N. A new Automated Behavioural Response system to integrate playback experiments into camera trap studies. Methods Ecol. Evol. 2017, 8, 957–964. [Google Scholar] [CrossRef] [Green Version]
- Hettena, A.M.; Munoz, N.; Blumstein, D.; Ebensperger, L. Prey responses to predator’s sounds: A review and empirical study. Ethology 2014, 120, 427–452. [Google Scholar] [CrossRef]
- McComb, K.; Shannon, G.; Sayialel, K.; Moss, C. Elephants can determine ethnicity, gender, and age from acoustic cues in human voices. Proc. Natl. Acad. Sci. USA 2014, 111, 5433–5438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yorzinski, J.L.; Ziegler, T. Do naïve primates recognize the vocalizations of felid predators? Ethology 2007, 113, 1219–1227. [Google Scholar] [CrossRef]
- Smith, J.A.; Suraci, J.; Clinchy, M.; Crawford, A.; Roberts, D.; Zanette, L.; Wilmers, C. Fear of the human ’super predator’ reduces feeding time in large carnivores. Proc. Biol. Sci. 2017, 284, 20170433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinchy, M.; Zanette, L.; Roberts, D.; Suraci, J.; Buesching, C.; Newman, C.; Macdonald, D. Fear of the human “super predator” far exceeds the fear of large carnivores in a model mesocarnivore. Behav. Ecol. 2016, 27, 1826–1832. [Google Scholar] [CrossRef] [Green Version]
- Reilly, C.M.; Suraci, J.; Smith, J.; Wang, Y.; Wilmers, C.; Candolin, U. Mesopredators retain their fear of humans across a development gradient. Behav. Ecol. 2022, 33, 428–435. [Google Scholar] [CrossRef]
- Crawford, D.A.; Conner, L.; Clinchy, M.; Zanette, L.; Cherry, M. Prey tells, large herbivores fear the human ‘super predator’. Oecologia 2022, 198, 91–98. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Lodnert, D.; Olsson, M.; Winsvold, A.; Eilertsen, S.; Kjellander, P.; Seiler, A. Inducing fear using acoustic stimuli—A behavioral experiment on moose (Alces alces) in Sweden. Ecol. and Evol. 2022, 12, e9492. [Google Scholar] [CrossRef] [PubMed]
- Laundré, J.W.; Hernández, L.; Altendorf, K. Wolves, elk, and bison: Reestablishing the “landscape of fear” in Yellowstone National Park, U.S.A. Can. J. Zool. 2001, 79, 1401–1409. [Google Scholar] [CrossRef]
- Tablado, Z.; Jenni, L. Determinants of uncertainty in wildlife responses to human disturbance. Biol. Rev. 2017, 92, 216–233. [Google Scholar] [CrossRef]
- Hansen, B.B.; Aanes, R. Habituation to humans in a predator-free wild ungulate. Polar Biol. 2014, 38, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Marino, A.; Johnson, A. Behavioural response of free-ranging guanacos (Lama guanicoe) to land-use change: Habituation to motorised vehicles in a recently created reserve. Wildl. Res. 2012, 39, 503–511. [Google Scholar] [CrossRef]
- Reimers, E.; Loe, L.; Eftestøl, S.; Colman, J.; Dahle, B. Effects of hunting on response behaviors of wild reindeer. J. Wildl. Manag. 2009, 73, 844–851. [Google Scholar] [CrossRef]
- Zhu, M.; Zaman, M.; Wang, M.; Vitekere, K.; Ma, J.; Jiang, G. Population density and driving factors of North China leopards in Tie Qiao Shan Nature Reserve. Animals 2021, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Seasonal Difference in Prey Abundance and Food Composition of the North China Leopard (Panthera pardus fontanierii) in Tieqiaoshan Nature Reserve, Shanxi. Master’s Thesis, Northeast Forestry University, Harbin, China, 2020. [Google Scholar]
- Liu, M.; Wang, Y.; Xia, F.; Bu, H.; Liu, Y.; Shen, X.; Li, S. Free-ranging livestock altered the spatiotemporal behavior of the endangered North Chinese leopard (Panthera pardus japonensis) and its prey and intensified human–leopard conflicts. Integr. Zool. 2023, 18, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.M.; Oakleaf, J.; Theobald, D.; Baruch-Mordo, S.; Kiesecker, J. Managing the middle: A shift in conservation priorities based on the global human modification gradient. Glob. Chang. Biol. 2019, 25, 811–826. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, D.; Liu, B.; Xia, F.; Chen, Y.; Wang, Y.; Huang, Q. Overview of the camera-trapping platform for felid species in China: Data integration by a conservation NGO. Biodivers. Sci. 2020, 28, 1067–1074. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Ma, J.; Liu, H. Analysis on wild boars home ranges in South Lesser Khingan Mountains. Acta Theriol. Sin. 2007, 27, 257–262. [Google Scholar]
- Teng, Y.; Zhang, S.; Saihan; Han, Z.; Bao, W. Dynamic analysis of Capreolus pygargus home range in Saihanwula Nature Reserve, Inner Mongolia of northern China. J. Beijing For. Univ. 2021, 43, 73–82. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environemnt for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 1 January 2022).
- Arnold, T.W. Uninformative parameters and model selection using Akaike’s Information Criterion. J. Wildl. Manage. 2010, 74, 1175–1178. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 1st ed.; Springer Science and Business Media: New York, NY, USA, 2002. [Google Scholar]
- Zuur, A.F.; Leno, E.; Walker, N.; Saveliev, A.; Smith, G. Mixed Effects Models and Extensions in Ecology with R, 1st ed.; Springer Science and Business Media: New York, NY, USA, 2009. [Google Scholar]
- van Beeck Calkoen, S.T.S.; Deis, M.; Oeser, J.; Kuijper, D.; Heurich, M. Humans rather than Eurasian lynx (Lynx lynx) shape ungulate browsing patterns in a temperate forest. Ecosphere 2022, 13, e3931. [Google Scholar] [CrossRef]
- Widén, A.; Clinchy, M.; Felton, A.; Hofmeester, T.; Kuijper, D.; Singh, N.; Widemo, F.; Zanette, L.; Cromsigt, J. Playbacks of predator vocalizations reduce crop damage by ungulates. Agric. Ecosyst. Environ. 2022, 328, 107853. [Google Scholar] [CrossRef]
- Zanette, L.Y.; Clinchy, M. Ecology and neurobiology of fear in free-living wildlife. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 297–318. [Google Scholar] [CrossRef]
- Kuijper, D.P.; Sahlen, E.; Elmhagen, B.; Chamaille-Jammes, S.; Sand, H.; Lone, K.; Cromsigt, J. Paws without claws? Ecological effects of large carnivores in anthropogenic landscapes. Proc. Biol. Sci. 2016, 283, 20161625. [Google Scholar] [CrossRef] [Green Version]
- Oriol-Cotterill, A.; Valeix, M.; Frank, L.; Riginos, C.; Macdonald, D. Landscapes of coexistence for terrestrial carnivores: The ecological consequences of being downgraded from ultimate to penultimate predator by humans. Oikos 2015, 124, 1263–1273. [Google Scholar] [CrossRef]
- Brown, J.S.; Kotler, B. Hazardous duty pay and the foraging cost of predation. Ecol. Lett. 2004, 7, 999–1014. [Google Scholar] [CrossRef]
- Lamb, C.T.; Mowat, G.; McLellan, B.; Nielsen, S.; Boutin, S. Forbidden fruit: Human settlement and abundant fruit create an ecological trap for an apex omnivore. J. Anim.Ecol. 2017, 86, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Lamb, C.T.; Ford, A.; McLellan, B.; Proctor, M.; Mowat, G.; Ciarniello, L.; Nielsen, S.; Boutin, S. The ecology of human-carnivore coexistence. Proc. Natl. Acad. Sci. USA 2020, 117, 17876–17883. [Google Scholar] [CrossRef]
- Hayward, M.W.; Henschel, P.; O’Brien, J.; Hofmeyr, M.; Balme, G.; Kerley, G. Prey preferences of the leopard (Panthera pardus). J. Zool. 2006, 270, 298–313. [Google Scholar] [CrossRef]
- Kuijper, D.P.; Verwijmeren, M.; Churski, M.; Zbyryt, A.; Schmidt, K.; Jedrzejewska, B.; Smit, C. What cues do ungulates use to assess predation risk in dense temperate forests? PLoS ONE 2014, 9, e84607. [Google Scholar] [CrossRef] [Green Version]
- Bejder, L.; Samuels, A.; Whitehead, H.; Finn, H.; Allen, S. Impact assessment research: Use and misuse of habituation, sensitisation and tolerance in describing wildlife responses to anthropogenic stimuli. Mar. Ecol. Prog. Ser. 2009, 395, 177–185. [Google Scholar] [CrossRef]
- Suraci, J.P.; Clinchy, M.; Dill, L.; Roberts, D.; Zanette, L. Fear of large carnivores causes a trophic cascade. Nat. Commun. 2016, 7, 10698. [Google Scholar] [CrossRef] [Green Version]
- Venter, O.; Sanderson, E.; Magrach, A.; Allan, J.; Beher, J.; Jones, K.; Possingham, H.; Laurance, W.; Wood, P.; Fekete, B.M.; et al. Sixteen years of change in the global terrestrial human footprint and implications for biodiversity conservation. Nat. Commun. 2016, 7, 12558. [Google Scholar] [CrossRef] [Green Version]

| ID | Formula | Hypothesis |
|---|---|---|
| 1 | ~Treatment | Ungulates exhibit different behavior in response to sounds of human, leopard and wind. |
| 2 | ~Length | Ungulates exhibit progressive changes in their intensity of response to the sounds with increased exposure. |
| 3 | ~Treatment + Length | 1 + 2; The intensity of the response does not vary among type of sounds with increased exposure. |
| 4 | ~Treatment × Length | 1 + 2; The intensity of response varies among type of sounds with increased exposure. |
| 5 | ~Treatment × Period | 1; The intensity of response varies among experimental periods. |
| 6 | ~1 | Null model |
| ID | Formula | df | logLik | AICc | Delta | Weight |
|---|---|---|---|---|---|---|
| Roe deer | ||||||
| 3 | Treatment + Length | 5 | −141.51 | 293.25 | 0.00 | 0.80 |
| 4 | Treatment × Length | 7 | −140.81 | 296.07 | 2.81 | 0.20 |
| 1 | Treatment | 4 | −149.69 | 307.54 | 14.28 | 0.00 |
| 5 | Treatment × Period | 10 | −146.53 | 313.96 | 20.70 | 0.00 |
| 2 | Length | 3 | −162.40 | 330.89 | 37.63 | 0.00 |
| 6 | Null | 2 | −168.05 | 340.14 | 46.88 | 0.00 |
| Wild boar | ||||||
| 1 | Treatment | 4 | −128.90 | 265.99 | 0.00 | 0.46 |
| 4 | Treatment × Length | 7 | −126.30 | 267.11 | 1.13 | 0.26 |
| 3 | Treatment + Length | 5 | −128.74 | 267.76 | 1.77 | 0.19 |
| 5 | Treatment × Period | 10 | −124.16 | 269.35 | 3.36 | 0.09 |
| 6 | Null | 2 | −144.16 | 292.37 | 26.39 | 0.00 |
| 2 | Length | 3 | −144.16 | 294.42 | 28.43 | 0.00 |
| df | logLik | AICc | Delta | Weight | ||
|---|---|---|---|---|---|---|
| Roe deer | ||||||
| 6 | Null | 2 | −412.02 | 828.06 | 0.00 | 0.53 |
| 2 | Length | 3 | −411.95 | 829.93 | 1.87 | 0.21 |
| 1 | Treatment | 4 | −411.24 | 830.52 | 2.47 | 0.15 |
| 3 | Treatment + Length | 5 | −411.13 | 832.34 | 4.28 | 0.06 |
| 5 | Treatment × Period | 10 | −406.59 | 833.46 | 5.41 | 0.04 |
| 4 | Treatment × Length | 7 | −410.58 | 835.30 | 7.24 | 0.01 |
| Wild boar | ||||||
| 2 | Length | 3 | −354.57 | 715.18 | 0.00 | 0.59 |
| 3 | Treatment + Length | 5 | −353.34 | 716.76 | 1.58 | 0.27 |
| 4 | Treatment × Length | 7 | −352.43 | 718.99 | 3.82 | 0.09 |
| 6 | Null | 2 | −358.36 | 720.73 | 5.55 | 0.04 |
| 1 | Treatment | 4 | −357.25 | 722.55 | 7.37 | 0.01 |
| 5 | Treatment × Period | 10 | −353.07 | 726.42 | 11.24 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; McShea, W.J.; Wang, Y.; Xia, F.; Shen, X.; Li, S. Ungulates’ Behavioral Responses to Humans as an Apex Predator in a Hunting-Prohibited Area of China. Animals 2023, 13, 845. https://doi.org/10.3390/ani13050845
Liu M, McShea WJ, Wang Y, Xia F, Shen X, Li S. Ungulates’ Behavioral Responses to Humans as an Apex Predator in a Hunting-Prohibited Area of China. Animals. 2023; 13(5):845. https://doi.org/10.3390/ani13050845
Chicago/Turabian StyleLiu, Mingzhang, William J. McShea, Yidan Wang, Fan Xia, Xiaoli Shen, and Sheng Li. 2023. "Ungulates’ Behavioral Responses to Humans as an Apex Predator in a Hunting-Prohibited Area of China" Animals 13, no. 5: 845. https://doi.org/10.3390/ani13050845





