Exploring the Effect of Functional Diets Containing Phytobiotic Compounds in Whiteleg Shrimp Health: Resistance to Acute Hepatopancreatic Necrotic Disease Caused by Vibrio parahaemolyticus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Growth Conditions
2.2. Additives: In Vitro Study
2.3. Functional Diets
2.4. Animal Maintenance and Feeding Schedules
2.5. Optimization of the Infection Model and Challenge Dose Validation
2.6. Bacterial Challenge
2.7. Sample Collection
2.8. DNA Extraction from Shrimp and Water Samples
2.9. qPCR Specific for V. parahaemolyticus (Vp qPCR) Detection and Quantification
2.10. Statistical Analysis
3. Results
3.1. In Vitro Characterization of the Antibacterial Activity of the Additives
3.2. Infection Model and Challenge Dose
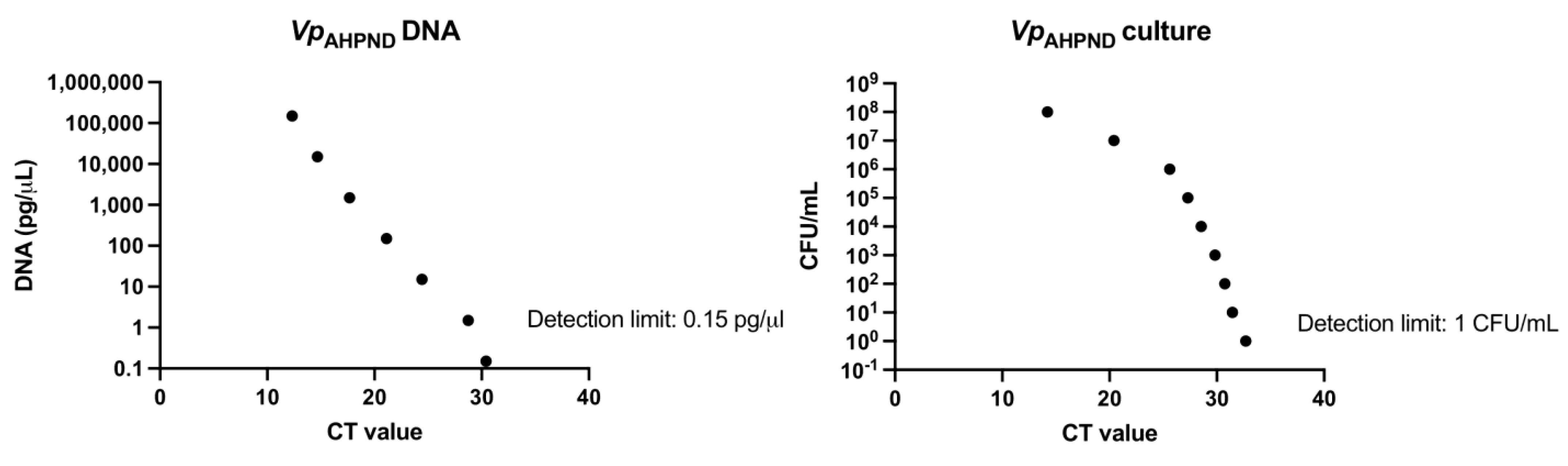
3.3. Optimization of the Vp qPCR
3.4. Effect of the Functional Diets in Protection against VpAHPND
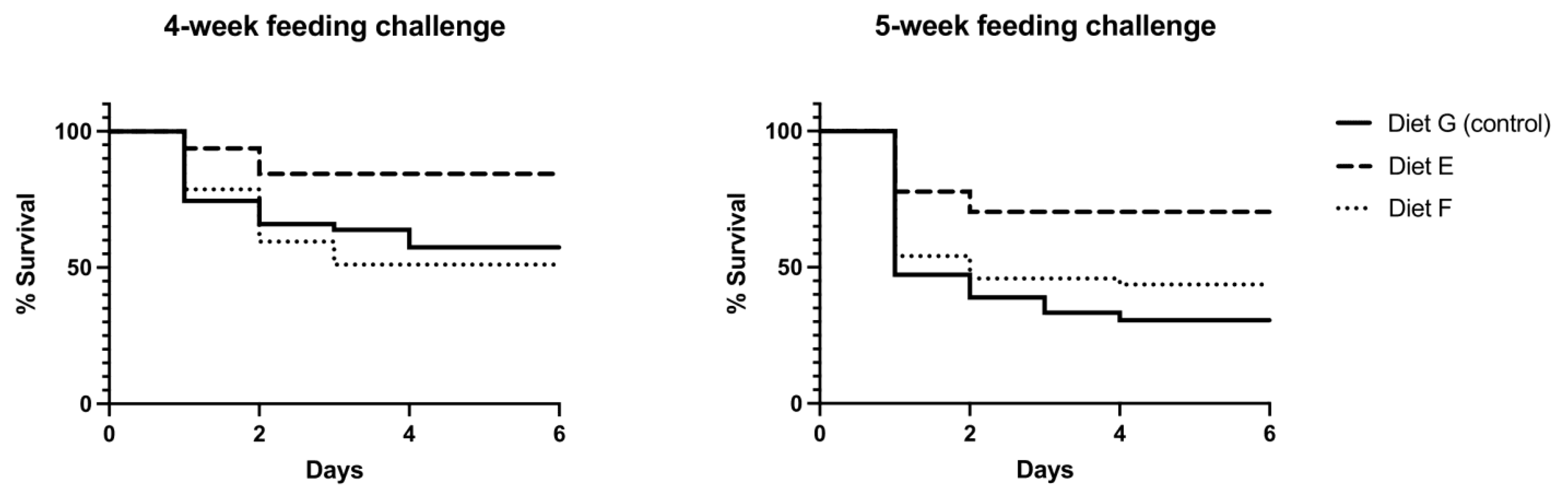
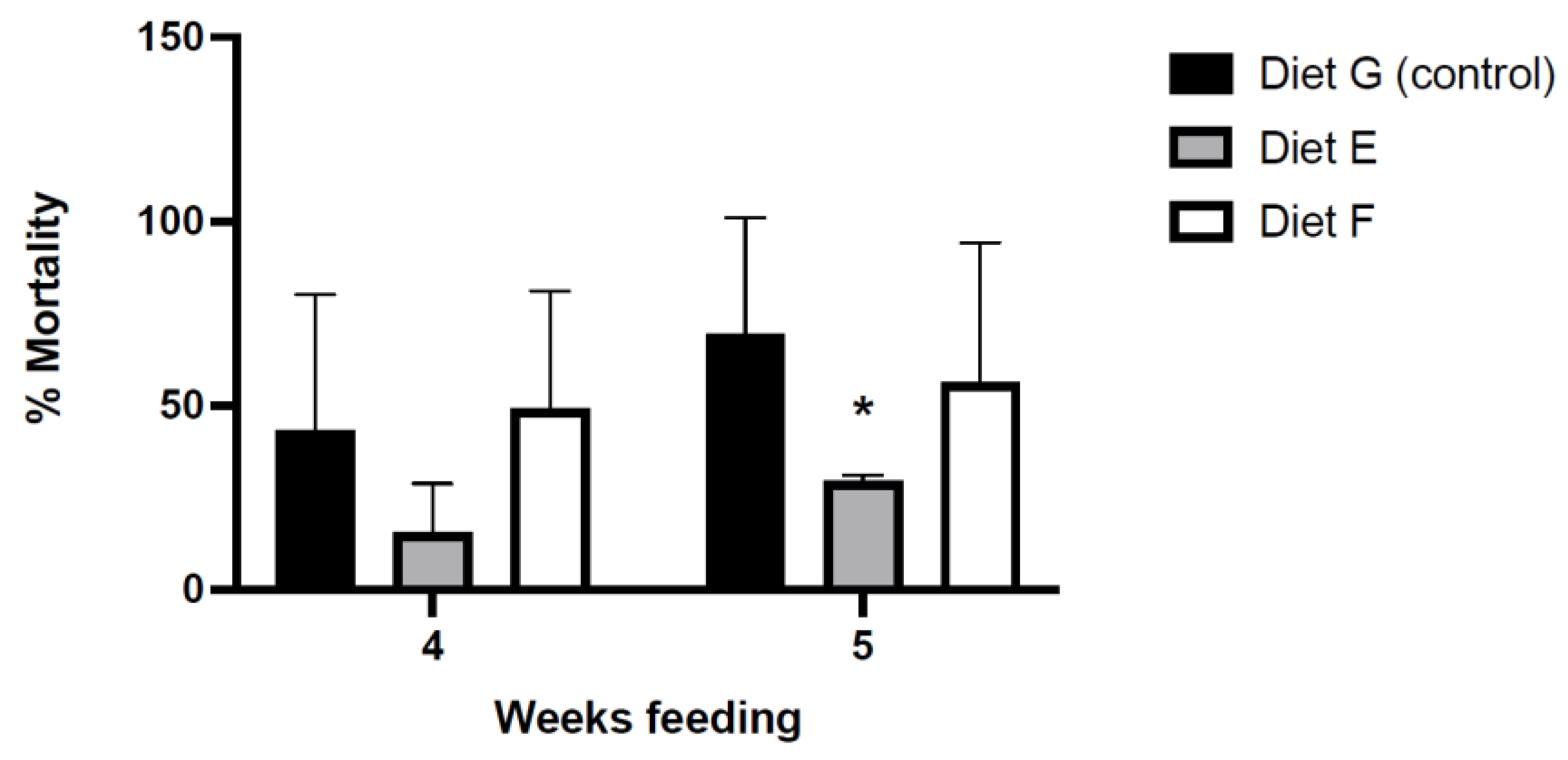
3.4.1. Mortality in Bacterial Challenges
3.4.2. Bacterial Loads in Survivors
3.5. Bacterial Loads in Water
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Chen, S.J.; Guo, Y.C.; Espe, M.; Yang, F.; Fang, W.P.; Wan, M.G.; Niu, J.; Liu, Y.J.; Tian, L.X. Growth performance, haematological parameters, antioxidant status and salinity stress tolerance of juvenile Pacific white shrimp (Litopenaeus vannamei) fed different levels of dietary myo-inositol. Aquac. Nutr. 2018, 24, 1527–1539. [Google Scholar] [CrossRef]
- FAO. Penaeus vannamei. In Cultured Aquatic Species Fact Sheets; FAO: Rome, Italy, 2009; Available online: https://www.fao.org/fishery/docs/DOCUMENT/aquaculture/CulturedSpecies/file/en/en_whitelegshrimp.htm (accessed on 6 March 2023).
- FAO. The state of world fisheries and aquaculture. In Nature and Resources; FAO: Rome, Italy, 2020; Volume 35. [Google Scholar]
- Tang, K.F.J.; Bondad-Reantaso, M.G.; Arthur, J.R.; MacKinnon, B.; Hao, B.; Alday-Sanz, V. Shrimp Acute Hepatopancreatic Necrosis Disease Strategy Manual. In FAO Fisheries and Aquaculture Circular; FAO: Rome, Italy, 2020; Volume 1190. [Google Scholar] [CrossRef]
- Dabu, I.M.; Lim, J.J.; Arabit, P.M.T.; Orense, S.J.A.B.; Tabardillo, J.A., Jr.; Corre, V.L., Jr.; Maningas, M.B.B. The first record of acute hepatopancreatic necrosis disease in the Philippines. Aquac. Res. 2017, 48, 792–799. [Google Scholar] [CrossRef]
- de la Peña, L.D.; Cabillon, N.A.R.; Catedral, D.D.; Amar, E.C.; Usero, R.C.; Monotilla, W.D.; Calpe, A.T.; Fernandez, D.D.; Saloma, C.P. Acute hepatopancreatic necrosis disease (AHPND) outbreaks in Penaeus vannamei and P. monodon cultured in the Philippines. Dis. Aquat. Organ. 2015, 116, 251–254. [Google Scholar] [CrossRef]
- Gomez-Jimenez, S.; Noriega-Orozco, L.; Sotelo-Mundo, R.R.; Cantu-Robles, V.A.; Cobian-Guemes, A.G.; Cota-Verdugo, R.G.; Gamez-Alejo, L.A.; Del Pozo-Yauner, L.; Guevara-Hernandez, E.; Garcia-Orozco, K.D.; et al. High-quality draft genomes of two Vibrio parahaemolyticus strains aid in understanding acute hepatopancreatic necrosis disease of cultured shrimps in Mexico. Genome Announc. 2014, 2, e00800-14. [Google Scholar] [CrossRef] [PubMed]
- Han, J.E.; Choi, S.K.; Han, S.H.; Lee, S.C.; Jeon, H.J.; Lee, C.; Kim, K.Y.; Lee, Y.S.; Park, S.C.; Rhee, G.; et al. Genomic and histopathological characteristics of Vibrio parahaemolyticus isolated from an acute hepatopancreatic necrosis disease outbreak in Pacific white shrimp (Penaeus vannamei) cultured in Korea. Aquaculture 2020, 24, 735284. [Google Scholar] [CrossRef]
- Kondo, H.; Tinwongger, S.; Proespraiwong, P.; Mavichak, R.; Unajak, S.; Nozaki, R.; Hirono, I. Draft genome sequences of six strains of Vibrio parahaemolyticus isolated from early mortality syndrome/acute hepatopancreatic necrosis disease shrimp in Thailand. Genome Announc. 2014, 2, e00221-14. [Google Scholar] [CrossRef]
- Kumar, R.; Chang, C.C.; Ng, T.H.; Ding, J.Y.; Tseng, T.C.; Lo, C.F.; Wang, H.C. Draft Genome Sequence of Vibrio parahaemolyticus Strain M1-1, Which Causes Acute Hepatopancreatic Necrosis Disease in Shrimp in Vietnam. Genome Announc. 2018, 6, 5–6. [Google Scholar] [CrossRef] [PubMed]
- World Organisation for Animal Health. Acute Hepatopancreatic Necrosis Disease. In Manual of Diagnostic Tests for Aquatic Animals; World Organisation for Animal Health: Paris, France, 2019; pp. 1–12. [Google Scholar]
- Restrepo, L.; Bayot, B.; Betancourt, I.; Pinzón, A. Genomics Data Draft genome sequence of pathogenic bacteria Vibrio parahaemolyticus strain Ba94C2, associated with acute hepatopancreatic necrosis disease isolate from South America. Genom. Data 2016, 9, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Soto-Rodriguez, S.A.; Gomez-Gil, B.; Lozano-Olvera, R.; Bolanmejia, C.; Aguilar-Rendon, K.G.; Enciso-Ibarra, J. Pathological, genomic and phenotypical characterization of Vibrio parahaemolyticus, causative agent of acute hepatopancreatic necrosis disease (AHPND) in Mexico. Asian Fish Sci. 2018, 31, 102–111. [Google Scholar] [CrossRef]
- Yang, Y.T.; Chen, I.T.; Lee, C.T.; Chen, C.Y.; Lin, S.S.; Hor, L.I.; Tseng, T.C.; Huang, Y.T.; Sritunyalucksana, K.; Thitamadee, S.; et al. Draft Genome Sequences of Four Strains of Vibrio parahaemolyticus, Three of Which Cause Early Mortality Syndrome/Acute Hepatopancreatic Necrosis Disease in Shrimp in China and Thailand. Genome Announc. 2014, 2, 2169–8287. [Google Scholar] [CrossRef]
- Soto-Rodriguez, S.A.; Gomez-Gil, B.; Lozano-Olvera, R.; Betancourt-Lozano, M.; Morales-Covarrubias, M.S. Field and Experimental Evidence of Vibrio parahaemolyticus as the Causative Agent of Acute Hepatopancreatic Necrosis Disease of Cultured Shrimp (Litopenaeus vannamei) in Northwestern Mexico. Appl. Environ. Microbiol. 2014, 81, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Thompson, F.L.; Iida, T.; Swings, J. Biodiversity of Vibrios. Microbiol. Mol. Biol. Rev. 2004, 68, 403–431. [Google Scholar] [CrossRef]
- Lin, S.J.; Hsu, K.C.; Wang, H.C. Structural insights into the cytotoxic mechanism of Vibrio parahaemolyticus PirAvp and PirBvp toxins. Marine Drugs 2017, 15, 373. [Google Scholar] [CrossRef]
- Lee, C.; Chen, I.T.; Yang, Y.T.; Ko, T.P.; Huang, Y.T.; Huang, J.Y.; Huang, M.F.; Lin, S.J.; Chen, C.Y.; Lin, S.S.; et al. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc. Natl. Acad. Sci. USA 2015, 112, 10798–10803. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, T.F.R.; Vidal, M.R.; Ribeiro, K.; Vicentini, C.A.; Bastos, I.; Vicentini, F. Histology of the hepatopancreas and anterior intestine in the freshwater prawn Macrobrachium carcinus (Crustacea, Decapoda). Nauphilus 2020, 28, e2020023. [Google Scholar] [CrossRef]
- Kumar, V.; Roy, S.; Behera, B.K.; Bossier, P.; Das, B.K. Acute hepatopancreatic necrosis disease (AHPND): Virulence, pathogenesis and mitigation strategies in Shrimp aquaculture. Toxins 2021, 13, 524. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Van, P.T.; Dang, L.T. Draft Genome Sequence of Non-Vibrio parahaemolyticus Acute Diseased Shrimp in Vietnam. Genome Announc. 2015, 3, 2014–2015. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, J.; Xia, X.; Pan, Y.; Yan, S.; Wang, Y. Draft genome sequence of Vibrio owensii strain SH-14, which causes shrimp acute hepatopancreatic necrosis disease. Genome Announc. 2015, 3, 3354. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, J.; Zhang, M.; Zhu, W.; Xia, X.; Dai, X. A Vibrio owensii strain as the causative agent of AHPND in cultured shrimp, Litopenaeus vannamei. J. Inv. Path. 2018, 153, 156–164. [Google Scholar] [CrossRef]
- Dong, X.; Wang, H.; Xie, G.; Zou, P.; Guo, C.; Liang, Y.; Huang, J. An isolate of Vibrio campbellii carrying the pirVP gene causes acute hepatopancreatic necrosis disease. Emerg. Microb. Inf. 2017, 6, e2–e3. [Google Scholar] [CrossRef]
- Dong, X.; Wang, H.; Zou, P.; Chen, J.; Liu, Z.; Wang, X.; Huang, J. Complete genome sequence of Vibrio campbellii strain 20130629003S01 isolated from shrimp with acute hepatopancreatic necrosis disease. Gut Pathog. 2017, 9, 31. [Google Scholar] [CrossRef]
- Restrepo, L.; Bayot, B.; Arciniegas, S.; Bajaña, L.; Betancourt, I.; Panchana, F.; Reyes-Muñoz, A. PirVP genes causing AHPND identified in a new Vibrio species (Vibrio punensis) within the commensal Orientalis clade. Sci. Rep. 2018, 8, 13080. [Google Scholar] [CrossRef]
- Dong, X.; Song, J.; Chen, J.; Bi, D.; Wang, W.; Ren, Y.; Wang, H.; Wang, G.; Tang, K.F.J.; Wang, X.; et al. Conjugative transfer of the PVA1-type plasmid carrying the PirABVP genes results in the formation of new AHPND-causing Vibrio. Front. Cell Inf. Microb. 2019, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Lin, S.J.; Mohapatra, A.; Kumar, R.; Wang, H.C. A review of the functional annotations of important genes in the AHPND-causing pva1 plasmid. Microorganisms 2020, 8, 996. [Google Scholar] [CrossRef] [PubMed]
- Rowley, A.F.; Pope, E.C. Vaccines and crustacean aquaculture-A mechanistic exploration. Aquaculture 2012, 334–337, 1–11. [Google Scholar] [CrossRef]
- Nghia, N.H.; Phan, N.; Van-Pham, T.; Giang, T.; Thi, N.; Domingos, S.S.J.A. Control of Vibrio parahaemolyticus (AHPND strain) and improvement of water quality using nanobubble technology. Aquac. Res. 2021, 52, 2727–2739. [Google Scholar] [CrossRef]
- Jun, J.W.; Han, J.E.; Giri, S.S.; Tang, K.F.J.; Zhou, X.; Arenguren, L.F.; Kim, H.J.; Yun, S.; Chu, C.; Kim, S.G.; et al. Phage Application for the Protection from Acute Hepatopancreatic Necrosis Disease (AHPND) in Penaeus vannamei. Indian J. Microbiol. 2018, 58, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Kuo, W.; Wang, H.; Chen, Y. Biocontrol of acute hepatopancreatic necrosis disease (AHPND) in shrimp using a microalgal-bacterial consortium. Aquaculture 2020, 521, 734990. [Google Scholar] [CrossRef]
- Pinoargote, G.; Flores, G.; Cooper, K.; Ravishankar, S. Effects on survival and bacterial community composition of the aquaculture water and gastrointestinal tract of shrimp (Litopenaeus vannamei) exposed to probiotic treatments after an induced infection of acute hepatopancreatic necrosis disease. Aquac. Nut. 2018, 49, 3270–3288. [Google Scholar] [CrossRef]
- Torpee, S.; Kantachote, D.; Rattanachuay, P. Dietary supplementation with probiotic Rhodobacter sphaeroides SS15 extract to control acute hepatopancreatic necrosis disease (AHPND)-causing Vibrio parahaemolyticus in cultivated white shrimp. J. Invertebr. Pathol. 2021, 186, 107585. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Tang, Y.; Sun, B.; Huang, J.; Song, X. Pseudoalteromonas probiotics as potential biocontrol agents improve the survival of Penaeus vannamei challenged with acute hepatopancreatic necrosis disease (AHPND)-causing Vibrio parahaemolyticus. Aquaculture 2018, 494, 30–36. [Google Scholar] [CrossRef]
- Wu, Y.; Liau, S.; Huang, C.; Nan, F. Beta 1, 3/1,6-glucan and vitamin C immunostimulate the non-specific immune response of white shrimp (Litopenaeus vannamei). Fish Shellfish Immunol. 2016, 57, 269–277. [Google Scholar] [CrossRef]
- Lawhavinit, O.; Sincharoenpokai, P.; Sunthornandh, P. Effects of Ethanol Tumeric (Curcuma longa Linn.) Extract Against Shrimp Pathogenic Vibrio spp. and on Growth Performance and Immune Status of White Shrimp (Litopenaeus vannamei). Nat. Sci. 2011, 77, 70–77. [Google Scholar]
- Lawhavinit, O.; Kongkathip, N.; Kongkathip, B. Antimicrobial Activity of Curcuminoids from Curcuma longa L. on Pathogenic Bacteria of Shrimp and Chicken. Nat. Sci. 2010, 371, 364–371. [Google Scholar]
- Quiroz-Guzmán, E.; Cabrera-Stevens, M.; Sánchez-Paz, A.; Mendoza-Cano, F.; Encinas-García, T.; Barajas-Sandoval, D.; Gómez-Gil, B.; Peña-Rodríguez, A. Effect of functional diets on intestinal microbiota and resistance to Vibrio parahaemolyticus causing acute hepatopancreatic necrosis disease (AHPND) of Pacific white shrimp (Penaeus vannamei). J. Appl. Microb. 2022, 132, 2649–2660. [Google Scholar] [CrossRef]
- Soowannayan, T.; Boonmee, C.; Puckcharoen, S.; Yatip, S.P.; Munyoo, P. Inhibition of Vibrio film formation by ginger extracts. Aquac. Asia Pacific. 2019, 15, 12–15. [Google Scholar]
- Han, B.; Inder, V.; Baruah, K.; Dung, V.; Bossier, P. High doses of sodium ascorbate act as a prooxidant and protect gnotobiotic brine shrimp larvae (Artemia franciscana) against Vibrio harveyi infection coinciding with heat shock protein 70 activation. Dev. Comp. Immunol. 2019, 92, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Jana, P.; Karmakar, S.; Roy, U.; Paul, M. Phytobiotics in aquaculture health management: A review. J. Ent. Zool. Stud. 2018, 6, 1422–1429. [Google Scholar]
- Caipang, C.M.A. Phytogenics in Aquaculture: A Short Review of Their Effects on Gut Health and Microflora in Fish. Philipp. J. Fish. 2020, 27, 246–259. [Google Scholar] [CrossRef]
- Logambal, S.M.; Venkatalakshmi, S.; Michael, R.D. Immunostimulatory effect of leaf extract of Ocimum sanctum Linn. in Oreochromis mossambicus (Peters). Hydrobiologia 2000, 430, 113–120. [Google Scholar] [CrossRef]
- Olusola, S.E.; Emikpe, B.; Olaifa, F. The potentials of medicinal plant extracts as bio-antimicrobials in aquaculture. The potentials of medicinal plant extracts as bio-antimicrobials in aquaculture. Int. J. Med. Aromat. Plants 2013, 3, 404–412. [Google Scholar]
- Alderman, D.J.; Smith, P. Development of draft protocols of standard reference methods for antimicrobial agent susceptibility testing of bacteria associated with fish diseases. Aquaculture 2001, 196, 211–243. [Google Scholar] [CrossRef]
- Amaro, C.; Biosca, E.G.; Fouz, B.; Alcaide, E.; Esteve, C. Evidence that Water Transmits Vibrio vulnificus Biotype 2 Infections to Eels. Appl. Environ. Microbiol. 1995, 61, 1133–1137. [Google Scholar] [CrossRef]
- Tran, L.; Nunan, L.; Redman, R.M.; Mohney, L.L.; Pantoja, C.R.; Fitzsimmons, K.; Lightner, D.V. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis. Aquat. Organ. 2013, 105, 45–55. [Google Scholar] [CrossRef]
- Park, J.Y.; Jeon, S.; Kim, J.Y.; Park, M.; Kim, S. Multiplex Real-time Polymerase Chain Reaction Assays for Simultaneous Detection of Vibrio cholerae, Vibrio parahaemolyticus, and Vibrio vulnificus. Osong Public Health Res. Perspect. 2013, 4, 133–139. [Google Scholar] [CrossRef]
- Han, J.E.; Tang, K.F.J.; Tran, L.H.; Lightner, D.V. Photorhabdus insect-related (Pir) toxin-like genes in a plasmid of Vibrio parahaemolyticus, the causative agent of acute hepatopancreatic necrosis disease (AHPND) of shrimp. Dis. Aquat. Organ. 2015, 113, 33–40. [Google Scholar] [CrossRef]
- De Schryver, P.; Defoirdt, T.; Sorgeloos, P. Early Mortality Syndrome Outbreaks: A Microbial Management Issue in Shrimp Farming? PLoS Path 2014, 10, 10–11. [Google Scholar] [CrossRef]
- Liao, I.; Chien, Y.H. The Pacific White Shrimp, Litopenaeus vannamei, in Asia: The World’s Most Widely Cultured Alien Crustacean. In In the Wrong Place—Alien Marine Crustaceans: Distribution, Biology and Impacts; Springer: Berlin/Heidelberg, Germany, 2011; pp. 489–519. [Google Scholar]
- Muthukrishnan, S.; Defoirdt, T.; Ina-Salwany, M.Y.; Yusoff, F.M.; Shariff, M.; Ismail, S.I.; Natrah, I. Vibrio parahaemolyticus and Vibrio harveyi causeing AHPND in shrimp. Aquaculture 2019, 511, 734227. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, L.; Ke, Y.; Li, X.; Liu, Y.; Pan, Y.; Yan, S.; Wang, Y. Shrimp AHPND-causing plasmids encoding the PirAB toxins as mediated by pirAB -Tn903 are prevalent in various Vibrio species. Sci. Rep. 2016, 7, 42177. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, D.T. Bacterial zoonoses of fishes: A review and appraisal of evidence for linkages between fish and human infections. Vet. J. 2015, 203, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Lu, L.; Xu, D. Progress in research on acute hepatopancreatic necrosis disease (AHPND). Aquac. Int. 2016, 24, 577–593. [Google Scholar] [CrossRef]
- Santos, M.H.; Tsai, C.Y.; Maquiling, K.R.A.; Tayo, L.L.; Mariatulqabtiah, A.R.; Lee, C.W.; Chuang, K.P. Diagnosis and potential treatments for acute hepatopancreatic necrosis disease (AHPND): A review. Aquac. Int. 2020, 28, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Schar, D.; Klein, E.Y.; Laxminarayan, R.; Gilbert, M.; Van Boeckel, T. Global trends in antimicrobial use in aquaculture. Sci. Rep. 2020, 10, 21878. [Google Scholar] [CrossRef] [PubMed]
- Thornber, K.; Verner-Jeffreys, D.; Hinchliffe, S.; Rahman, M.M.; Bass, D.; Tyler, C.R. Evaluating antimicrobial resistance in the global shrimp industry. Rev. Aquac. 2020, 12, 966–986. [Google Scholar] [CrossRef]
- Butt, D.U.; Lin, N.; Akhter, N.; Siddiqui, T.; Li, S.; Wu, B. Overview of the latest developments in the role of probiotics, prebiotics and synbiotics in shrimp aquaculture. Fish Shell Immunol. 2021, 114, 263–281. [Google Scholar] [CrossRef]
- Direkbusarakom, S.; Ruangpan, L.; Ezura, Y.; Yoshimizu, M. Protective Efficacy of Clillacantlms nutans on Yellow-head Disease in Black Tiger Shrimp (Pellaells monodon). Fish Path. 1998, 33, 401–404. [Google Scholar] [CrossRef]
- Balasubramanian, G.; Sarathi, M.; Venkatesan, C.; Thomas, J.; Hameed, A.S.S. Oral administration of antiviral plant extract of Cynodon dactylon on a large scale production against White spot syndrome virus (WSSV) in Penaeus monodon. Aquaculture 2008, 279, 2–5. [Google Scholar] [CrossRef]
- Lim, S.; Loo, K.W.; Wong, W. Synergistic Antimicrobial Effect of a Seaweed-Probiotic Blend Against Acute Hepatopancreatic Necrosis Disease (AHPND)-Causing Vibrio parahaemolyticus. Probiotics Antimicrob. Proteins 2020, 12, 906–917. [Google Scholar] [CrossRef]
- Roque, A.; Turnbull, J.F.; Escalante, G.; Gomez-Gil, B.; Alday-Sanz, M.V. Development of a bath challenge for the marine shrimp Penaeus vannamei Boone, 1931. Aquaculture 1998, 169, 283–290. [Google Scholar] [CrossRef]
- Soto-Rodriguez, S.A.; Gomez-Gil, B.; Lozano, R.; del Rio-Rodríguez, R.; Diéguez, A.L.; Romalde, J.L. Virulence of Vibrio harveyi responsible for the "Bright-red" Syndrome in the Pacific white shrimp Litopenaeus vannamei. J. Invertebr. Pathol. 2012, 109, 307–317. [Google Scholar] [CrossRef]
- Lee, C.T.; Amaro, C.; Wu, K.M.; Valiente, E.; Chang, Y.F.; Tsai, S.F.; Chang, C.H.; Hor, L.I. A common virulence plasmid in biotype 2 Vibrio vulnificus and its dissemination aided by a conjugal plasmid. J. Bacteriol. 2008, 190, 1638–1648. [Google Scholar] [CrossRef]
- Wang, H.; Tang, X.; Su, Y.C.; Chen, J.; Yan, J. Characterization of clinical Vibrio parahaemolyticus strains in Zhoushan, China, from 2013 to 2014. PLoS ONE 2017, 12, e0180335. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, W.; Kumeda, Y.; Misawa, N.; Nakaguchi, Y.; Nishibuchi, M. Development of a loop-mediated isothermal amplification assay for sensitive and rapid detection of the tdh and trh genes of Vibrio parahaemolyticus and related Vibrio species. Appl. Environ. Microbiol. 2010, 76, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, G.E.; Blackstone, G.M.; Vickery, M.C.; Bej, A.K.; Bowers, J.; Bowen, M.D.; Meyer, R.F.; DePaola, A. Real-time PCR quantification of Vibrio parahaemolyticus in oysters using an alternative matrix. J. Food Prot. 2004, 67, 2424–2429. [Google Scholar] [CrossRef] [PubMed]


| Ingredient | g/Kg |
|---|---|
| Fish flour | 150 |
| Soybean | 250 |
| Gluten | 90 |
| Wheat | 375 |
| Soybean oil | 32.5 |
| Fish oil | 32.5 |
| Calcium phosphate | 25 |
| Lecithin | 15 |
| Maltodextrin | 20 |
| Vitamin supplements | 10 |
| Shrimp Average Weight (g) | Bacterial Dose (CFU/mL) | Bath Length (min) | Mortality (%) |
|---|---|---|---|
| 1 | 5 × 107 | 30 | 0 |
| 1 | 5 × 107 | 60 | 100 |
| 1 | 2 × 107 | 60 | 75 |
| 1 | 4 × 106 | 60 | 0 |
| 1 | None | 60 | 0 |
| 2 | 5 × 107 | 60 | 50 |
| 2 | 1 × 107 | 60 | 0 |
| 2 | 8 × 106 | 60 | 0 |
| 2 | None | 60 | 0 |
| DNA from | CT Value (Vp qPCR) |
|---|---|
| V. parahaemolyticus | 12.32 |
| V. harveyi | 35.56 |
| V. vulnificus | 34.48 |
| V. alginolyticus | 28.25 |
| Negative control (H2O) | 33.12 |
| Challenge with VpAHPND after | Diet | Tank | Total Animals (n) | Dead Animals (n) |
|---|---|---|---|---|
| 4-week feeding schedule | G (control) | G1 | 16 | 7 |
| G2 | 16 | 1 | ||
| G3 | 15 | 12 | ||
| E (functional) | E1 | 16 | 4 | |
| E2 | 16 | 1 | ||
| E3 | 16 | 16 | ||
| F (functional) | F1 | 16 | 2 | |
| F2 | 15 | 10 | ||
| F3 | 16 | 11 | ||
| 5-week feeding schedule | G (control) | G1 | 12 | 4 |
| G2 | 12 | 10 | ||
| G3 | 12 | 11 | ||
| E (functional) | E1 | 13 | 4 | |
| E2 | 14 | 4 | ||
| E3 | 14 | 14 | ||
| F (functional) | F1 | 16 | 12 | |
| F2 | 16 | 13 | ||
| F3 | 16 | 2 |
| Type of Animal | CT Range (Vp qPCR) * | Vibrio parahaemolyticus (CFU/g HP) * | Carrier Category |
|---|---|---|---|
| Dead (diseased) | 14–27 | 5 × 103–1 × 109 | |
| Survivors | 14–26 | 1 × 106–1 × 109 | High load |
| 26–29 | 1 × 103–1 × 106 | Medium load | |
| 29–33 | 10–1 × 103 | Low load | |
| 33–36 | <10 | Non-carrier |
| Challenge with VpAHPND after | Diet | CT Range (Vp qPCR) | Vibrio parahaemolyticus (CFU/g HP) | Carrier Category |
|---|---|---|---|---|
| 4-week feeding schedule | G (control) | 26.5–36.59 | 10–8 × 105 | Low-medium |
| E (functional) | 15.42–27.5 | 8 × 105–8 × 108 | Medium-high | |
| F (functional) | 25.5–32.8 | 10–1 × 107 | Low-medium | |
| 5-week feeding schedule | G (control) | 16.5–30.5 | 1 × 102–9 × 109 | Low-high |
| E (functional) | 13.5–30.18 | 10–1 × 109 | Low-high | |
| F (functional) | 26.2–33.1 | 10–1 × 106 | Low-medium |
| Days Post-Challenge | CT Range (Vp qPCR) | Vibrio parahaemolyticus (CFU/mL Water) |
|---|---|---|
| 0 (before infection) | 34–35 | 0 |
| 1 | 22–25 | 5 × 107–3 × 108 |
| 2 | 21–29 | 5 × 103–5 × 108 |
| 3 | 23–29 | 5 × 103–5 × 108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Cabanyero, C.; Carrascosa, E.; Jiménez, S.; Fouz, B. Exploring the Effect of Functional Diets Containing Phytobiotic Compounds in Whiteleg Shrimp Health: Resistance to Acute Hepatopancreatic Necrotic Disease Caused by Vibrio parahaemolyticus. Animals 2023, 13, 1354. https://doi.org/10.3390/ani13081354
Hernández-Cabanyero C, Carrascosa E, Jiménez S, Fouz B. Exploring the Effect of Functional Diets Containing Phytobiotic Compounds in Whiteleg Shrimp Health: Resistance to Acute Hepatopancreatic Necrotic Disease Caused by Vibrio parahaemolyticus. Animals. 2023; 13(8):1354. https://doi.org/10.3390/ani13081354
Chicago/Turabian StyleHernández-Cabanyero, Carla, Esther Carrascosa, Silvia Jiménez, and Belén Fouz. 2023. "Exploring the Effect of Functional Diets Containing Phytobiotic Compounds in Whiteleg Shrimp Health: Resistance to Acute Hepatopancreatic Necrotic Disease Caused by Vibrio parahaemolyticus" Animals 13, no. 8: 1354. https://doi.org/10.3390/ani13081354
APA StyleHernández-Cabanyero, C., Carrascosa, E., Jiménez, S., & Fouz, B. (2023). Exploring the Effect of Functional Diets Containing Phytobiotic Compounds in Whiteleg Shrimp Health: Resistance to Acute Hepatopancreatic Necrotic Disease Caused by Vibrio parahaemolyticus. Animals, 13(8), 1354. https://doi.org/10.3390/ani13081354







