Could Insect Products Provide a Safe and Sustainable Feed Alternative for the Poultry Industry? A Comprehensive Review
Abstract
:Simple Summary
Abstract
1. Introduction
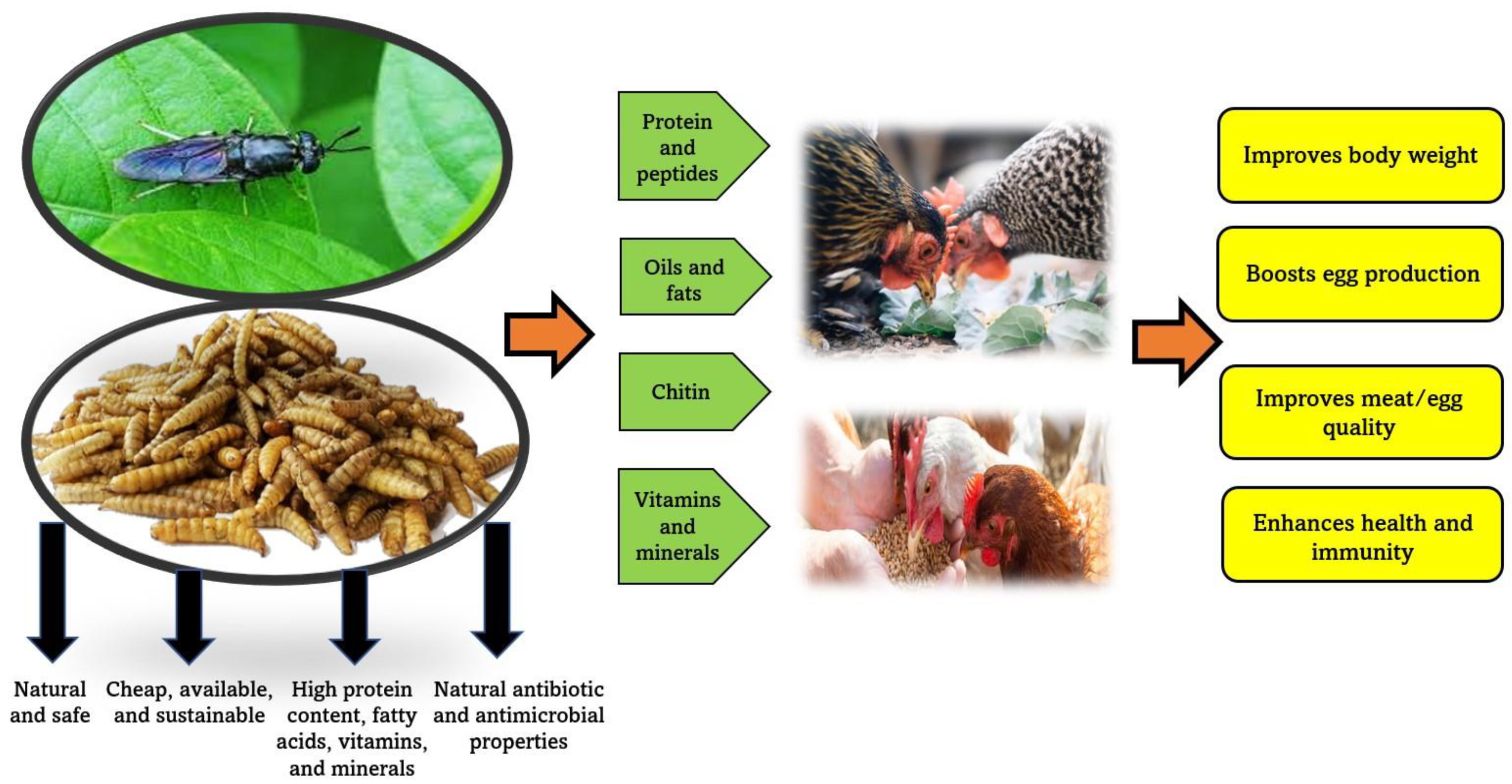
2. Insects’ Nutritional Value
2.1. Chemical Composition
2.2. Bioactive Components
2.2.1. Chitin
2.2.2. Antimicrobial Peptides and Lauric Acid
3. Use of Insects in Poultry Feed
3.1. Black Soldier Fly
3.2. Mealworm
3.3. Housefly
3.4. Grasshopper/Locust
3.5. Silkworm
3.6. Termite
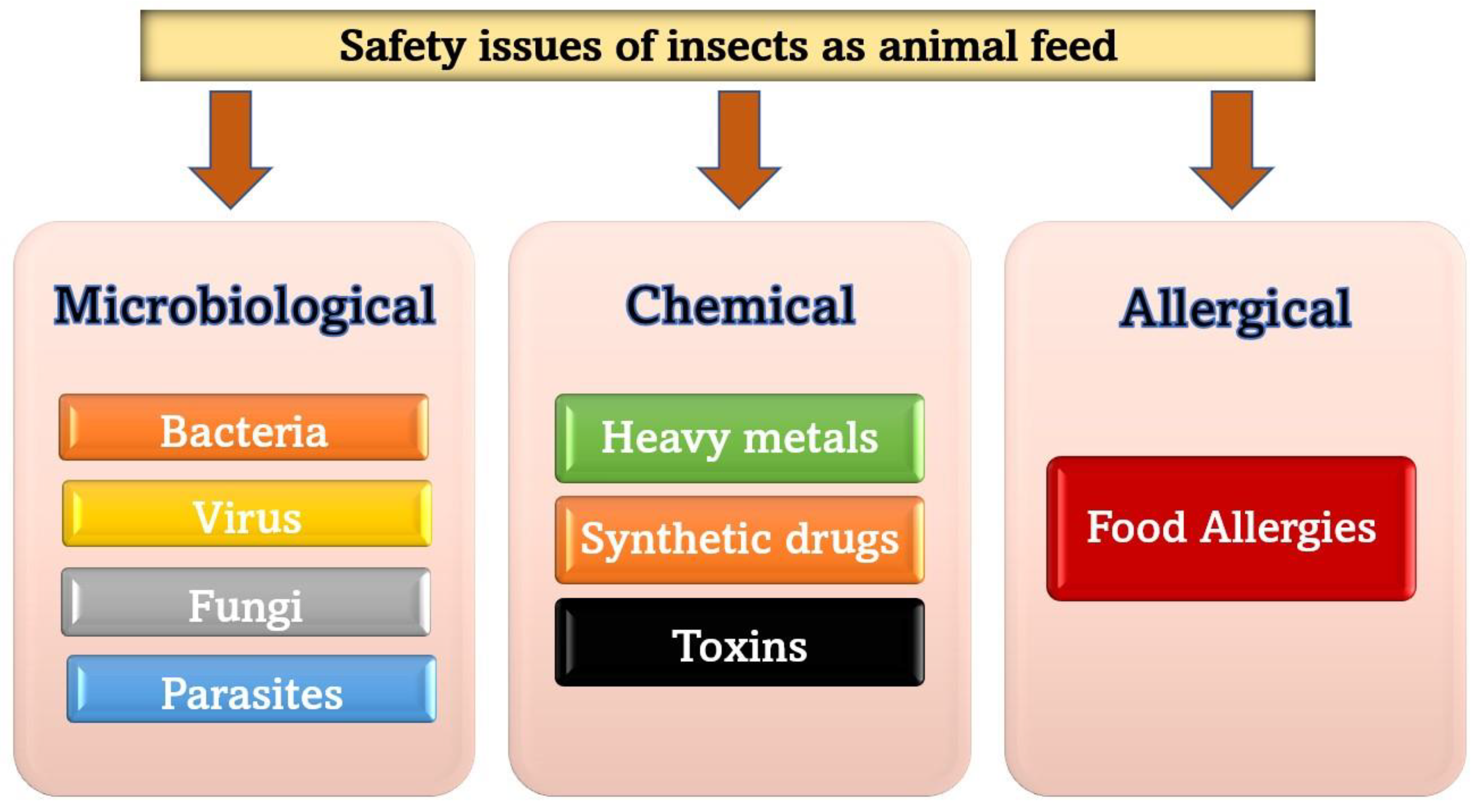
4. Safety and Guidelines of Using Insects as Animal Feed
4.1. Safety Issues of Insects as Feed
4.1.1. Microbiological Safety
4.1.2. Chemical Safety
4.1.3. Allergens
4.1.4. Antinutrients
5. Regulations for Insects as Animal Feed
6. Strengths and Weaknesses
6.1. Strength Points
6.1.1. Nutritional Composition
6.1.2. Bioactive Compounds
6.2. Weakness Points
6.2.1. Market Price
6.2.2. Polyunsaturated Fatty Acids and Minerals
6.2.3. Customer’s Acceptability
7. Future Prospects of Insects for the Animal Feed Industry
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yildiz, D. Global Poultry Industry and Trends. Available online: https://www.feedandadditive.com/global-poultry-industry-and-trends/ (accessed on 11 March 2021).
- Elkomy, A.; El-Hanoun, A.; Abdella, M.; El-Sabrout, K. Improving the reproductive, immunity and health status of rabbit does using honey bee venom. J. Anim. Physiol. Anim. Nutr. 2021, 105, 975–983. [Google Scholar] [CrossRef] [PubMed]
- El-Sabrout, K.; Khalifah, A.; Mishra, B. Application of botanical products as nutraceutical feed additives for improving poultry health and production. Vet. World 2023, 16, 369–379. [Google Scholar] [CrossRef] [PubMed]
- El-Deek, A.A.; Abdel-Wareth, A.A.A.; Osman, M.; El-Shafey, M.; Khalifah, A.M.; Elkomy, A.E.; Lohakare, J. Alternative Feed Ingredients in the Finisher Diets for Sustainable Broiler Production. Sci. Rep. 2020, 10, 17743. [Google Scholar] [CrossRef] [PubMed]
- Rodic, V.; Peric, L.; Djukic-Stojcic, M.; Vukelic, N. The Environmental Impact of Poultry Production. Biotechnol. Anim. Husb. 2011, 27, 1673–1679. [Google Scholar] [CrossRef]
- Hwangbo, J.; Hong, E.C.; Jang, A.; Kang, H.K.; Oh, J.S.; Kim, B.W.; Park, B.S. Utilization of House Fly-Maggots, a Feed Supplement in the Production of Broiler Chickens. J. Environ. Biol. 2009, 30, 609–614. [Google Scholar]
- Khan, S.H. Recent Advances in Role of Insects as Alternative Protein Source in Poultry Nutrition. J. Appl. Anim. Res. 2018, 46, 1144–1157. [Google Scholar] [CrossRef]
- Onsongo, V.O.; Osuga, I.M.; Gachuiri, C.K.; Wachira, A.M.; Miano, D.M.; Tanga, C.M.; Ekesi, S.; Nakimbugwe, D.; Fiaboe, K.K.M. Insects for Income Generation through Animal Feed: Effect of Dietary Replacement of Soybean and Fish Meal with Black Soldier Fly Meal on Broiler Growth and Economic Performance. J. Econ. Entomol. 2018, 111, 1966–1973. [Google Scholar] [CrossRef]
- Gómez, B.; Munekata, P.E.S.; Zhu, Z.; Barba, F.J.; Toldrá, F.; Putnik, P.; Bursać Kovačević, D.; Lorenzo, J.M. Challenges and Opportunities Regarding the Use of Alternative Protein Sources: Aquaculture and Insects. Adv. Food Nutr. Res. 2019, 89, 259–295. [Google Scholar] [CrossRef]
- Siddi, M. The European Green Deal: Assessing Its Current State and Future Implementation; UPI REPORT; Finnish Institute of International Affairs: Helsinki, Finland, 2020; p. 114. Available online: https://hdl.handle.net/11584/313484 (accessed on 1 January 2020).
- Camilleri, M.A. European Environment Policy for the Circular Economy: Implications for Business and Industry Stakeholders. Sustain. Dev. 2020, 28, 1804–1812. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-Art on Use of Insects as Animal Feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Miglietta, P.; De Leo, F.; Ruberti, M.; Massari, S. Mealworms for Food: A Water Footprint Perspective. Water 2015, 7, 6190–6203. [Google Scholar] [CrossRef]
- Kline, O.; Joshi, N.K. Mitigating the Effects of Habitat Loss on Solitary Bees in Agricultural Ecosystems. Agriculture 2020, 10, 115. [Google Scholar] [CrossRef]
- Van der Sluijs, J.P. Insect Decline, an Emerging Global Environmental Risk. Curr. Opin. Environ. Sustain. 2020, 46, 39–42. [Google Scholar] [CrossRef]
- Van Huis, A. Potential of Insects as Food and Feed in Assuring Food Security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Halloran, A.; Hanboonsong, Y.; Roos, N.; Bruun, S. Life Cycle Assessment of Cricket Farming in North-Eastern Thailand. J. Clean. Prod. 2017, 156, 83–94. [Google Scholar] [CrossRef]
- Nowak, V.; Persijn, D.; Rittenschober, D.; Charrondiere, U.R. Review of Food Composition Data for Edible Insects. Food Chem. 2016, 193, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Rochow, V.B.; Jung, C. Insects used as food and feed: Isn’t that what we all need? Foods 2020, 9, 1003. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B.; Gahukar, R.T.; Ghosh, S.; Jung, C. Chemical Composition, Nutrient Quality and Acceptability of Edible Insects Are Affected by Species, Developmental Stage, Gender, Diet, and Processing Method. Foods 2021, 10, 1036. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B. Can insects help to ease the problem of world food shortage? Search 1975, 6, 261–262. [Google Scholar]
- IPIFF (International Platform Insects for Food and Feed). An Overview of the European Market of Insects as Feed. Available online: https://ipiff.org/wp-content/uploads/2021/04/Apr-27-2021-IPIFF_The-European-market-of-insects-as-feed.pdf (accessed on 13 February 2023).
- Biasato, I.; De Marco, M.; Rotolo, L.; Renna, M.; Lussiana, C.; Dabbou, S.; Capucchio, M.T.; Biasibetti, E.; Costa, P.; Gai, F.; et al. Effects of Dietary Tenebrio Molitor Meal Inclusion in Free-Range Chickens. J. Anim. Physiol. Anim. Nutr. 2016, 100, 1104–1112. [Google Scholar] [CrossRef]
- Veldkamp, T.; Meijer, N.; Alleweldt, F.; Deruytter, D.; van Campenhout, L.; Gasco, L.; Roos, N.; Smetana, S.; Fernandes, A.; van der Fels-Klerx, H.J. Overcoming Technical and Market Barriers to Enable Sustainable Large-Scale Production and Consumption of Insect Proteins in Europe: A SUSINCHAIN Perspective. Insects 2022, 13, 281. [Google Scholar] [CrossRef] [PubMed]
- Menozzi, D.; Sogari, G.; Veneziani, M.; Simoni, E.; Mora, C. Eating Novel Foods: An application of the theory of planned behaviour to predict the consumption of an insect-based product. Food Qual. Prefer. 2017, 59, 27–34. [Google Scholar] [CrossRef]
- Verheyen, G.R.; Ooms, T.; Vogels, L.; Vreysen, S.; Bovy, A.; Van Miert, S.; Meersman, F. Insects as an Alternative Source for the Production of Fats for Cosmetics. J. Cosmet. Sci. 2018, 69, 187–202. [Google Scholar] [PubMed]
- de Carvalho, N.M.; Madureira, A.R.; Pintado, M.E. The potential of insects as food sources—A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3642–3652. [Google Scholar] [CrossRef] [PubMed]
- Yazici, G.N.; Ozer, M.S. Using Edible Insects in the Production of Cookies, Biscuits, and Crackers: A Review. Biol. Life Sci. Forum. 2021, 6, 80. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional Composition and Safety Aspects of Edible Insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Zhou, J.; Han, D. Proximate, amino acid and mineral composition of pupae of the silkworm, Antheraea pernyl in China. J. Food Compos. Anal. 2006, 19, 850–853. [Google Scholar] [CrossRef]
- Dossey, A.T.; Tatum, J.T.; McGill, W.L. Modern Insect-Based Food Industry: Current Status, Insect Processing Technology, and Recommendations Moving Forward. In Insects as Sustainable Food Ingredients; Elsevier: Amsterdam, The Netherlands, 2016; pp. 113–152. [Google Scholar]
- Barker, D.; Fitzpatrick, M.P.; Dierenfeld, E.S. Nutrient composition of selected whole invertebrates. Zoo Biol. 1998, 17, 123–134. [Google Scholar] [CrossRef]
- Finke, M.D. Complete nutrient composition of commercially raised invertebrates used as food for insectivores. Zoo Biol. 2002, 21, 269–285. [Google Scholar] [CrossRef]
- Woods, M.J.; Cullere, M.; Van Emmenes, L.; Vincenzi, S.; Pieterse, E.; Hoffman, L.C.; Zotte, A.D. Hermetia Illucens Larvae reared on different substrates in broiler quail diets: Effect on apparent digestibility, feed-choice and growth performance. J. Insects Food Feed 2019, 5, 89–98. [Google Scholar] [CrossRef]
- Hall, H.N.; Masey O’Neill, H.V.; Scholey, D.; Burton, E.; Dickinson, M.; Fitches, E.C. Amino acid digestibility of Larval Meal (Musca Domestica) for broiler chickens. Poult. Sci. 2018, 97, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Bosch, G.; Zhang, S.; Oonincx, D.G.A.B.; Hendriks, W.H. Protein quality of insects as potential ingredients for dog and cat foods. J. Nutr. Sci. 2014, 3, e29. [Google Scholar] [CrossRef]
- Nginya, E.S.; Ondiek, J.O.; King’ori, A.M.; Nduko, J.M. Evaluation of grasshoppers as a protein source for improved indigenous chicken growers. Livest. Res. Rural. Dev. 2019, 31. Available online: http://www.lrrd.org/lrrd31/1/shilo31002.html (accessed on 12 April 2023).
- Khan, S.; Khan, R.U.; Alam, W.; Sultan, A. Evaluating the nutritive profile of three insect meals and their effects to replace soya bean in broiler diet. J. Anim. Physiol. Anim. Nutr. 2018, 102, e662–e668. [Google Scholar] [CrossRef]
- Brah, N.; Salissou, I.; Houndonougbo, F. Effect of Grasshopper Meal on Laying Hens’ Performance and Egg Quality Characteristics. Indian J. Anim. Sci. 2017, 87, 1005–1010. [Google Scholar] [CrossRef]
- Lee, C.G.; Da Silva, C.A.; Lee, J.-Y.; Hartl, D.; Elias, J.A. Chitin Regulation of Immune Responses: An Old Molecule with New Roles. Curr. Opin. Immunol. 2008, 20, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Elieh Ali Komi, D.; Sharma, L.; Dela Cruz, C.S. Chitin and Its Effects on Inflammatory and Immune Responses. Clin. Rev. Allergy Immunol. 2018, 54, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Kipkoech, C.; Kinyuru, J.N.; Imathiu, S.; Meyer-Rochow, V.B.; Roos, N. In Vitro Study of Cricket Chitosan’s Potential as a Prebiotic and a Promoter of Probiotic Microorganisms to Control Pathogenic Bacteria in the Human Gut. Foods 2021, 10, 2310. [Google Scholar] [CrossRef]
- Mei, Y.-X.; Dai, X.-Y.; Yang, W.; Xu, X.-W.; Liang, Y.-X. Antifungal Activity of Chitooligosaccharides against the Dermatophyte Trichophyton Rubrum. Int. J. Biol. Macromol. 2015, 77, 330–335. [Google Scholar] [CrossRef]
- Vidanarachchi, J.K.; Kurukulasuriya, M.S.; Kim, S.K. Chitin, Chitosan, and their oligosaccharides in food industry. In Chitin, Chitosan, Oligosaccharides and Their Derivatives; CRC Press: Boca Raton, FL, USA, 2010; p. 560. [Google Scholar]
- Choi, Y.C.; Park, K.H.; Nam, S.H.; Jang, B.G.; Kim, J.H.; Kim, D.W.; Yu, D.J. The Effect on Growth Performance of Chicken Meat in Broiler Chicks by Dietary Supplementation of Black Soldier Fly Larvae, Hermetia Illucens (Diptera: Stratmyidae). J. Sericultural Entomol. Sci. 2013, 51, 30–35. [Google Scholar] [CrossRef]
- Mosaheb, M.U.W.F.Z.; Khan, N.A.; Siddiqui, R. Cockroaches, Locusts, and envenomating Arthropods: A promising source of antimicrobials. Iran. J. Basic Med. Sci. 2018, 21, 873–877. [Google Scholar] [PubMed]
- Chernysh, S.; Gordya, N.; Suborova, T. Insect Antimicrobial Peptide Complexes Prevent Resistance Development in Bacteria. PLoS ONE 2015, 10, e0130788. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Han, M.; Zhang, G.; Qiao, S.; Li, D.; Ma, X. The Signal Pathway of Antibiotic Alternatives on Intestinal Microbiota and Immune Function. Curr. Protein Pept. Sci. 2016, 17, 785–796. [Google Scholar] [CrossRef]
- Dierick, N.A.; Decuypere, J.A.; Molly, K.; Van Beek, E.; Vanderbeke, E. The Combined Use of Triacylglycerols (TAGs) Containing Medium Chain Fatty Acids (MCFAs) and Exogenous Lipolytic Enzymes as an Alternative to Nutritional Antibiotics in Piglet Nutrition: II. In Vivo Release of MCFAs in Gastric Cannulated and Slaughtered Piglets by Endogenous and Exogenous Lipases; Effects on the Luminal Gut Flora and Growth Performance. Livest. Prod. Sci. 2002, 76, 1–16. [Google Scholar]
- Zuidhof, M.J.; Molnar, C.L.; Morley, F.M.; Wray, T.L.; Robinson, F.E.; Khan, B.A.; Al-Ani, L.; Goonewardene, L.A. Nutritive Value of House Fly (Musca Domestica) Larvae as a Feed Supplement for Turkey Poults. Anim. Feed Sci. Technol. 2003, 105, 225–230. [Google Scholar] [CrossRef]
- Broeckx, L.; Frooninckx, L.; Slegers, L.; Berrens, S.; Noyens, I.; Goossens, S.; Verheyen, G.; Wuyts, A.; Van Miert, S. Growth of Black Soldier Fly Larvae Reared on Organic Side-Streams. Sustainability 2021, 13, 12953. [Google Scholar] [CrossRef]
- Mbhele, F.G.T.; Mnisi, C.M.; Mlambo, V. A Nutritional Evaluation of Insect Meal as a Sustainable Protein Source for Jumbo Quails: Physiological and Meat Quality Responses. Sustainability 2019, 11, 6592. [Google Scholar] [CrossRef]
- Kim, W.; Bae, S.; Park, K.; Lee, S.; Choi, Y.; Han, S.; Koh, Y. Biochemical Characterization of Digestive Enzymes in the Black Soldier Fly, Hermetia Illucens (Diptera: Stratiomyidae). J. Asia. Pac. Entomol. 2011, 14, 11–14. [Google Scholar] [CrossRef]
- Cullere, M.; Tasoniero, G.; Giaccone, V.; Miotti-Scapin, R.; Claeys, E.; De Smet, S.; Dalle Zotte, A. Black Soldier Fly as Dietary Protein Source for Broiler Quails: Apparent Digestibility, Excreta Microbial Load, Feed Choice, Performance, Carcass and Meat Traits. Animal 2016, 10, 1923–1930. [Google Scholar] [CrossRef]
- De Marco, D.; Martínez, M.; Hernandez, S.; Madrid, F.; Gai, J.; Rotolo, F.; Schiavone, L. Nutritional Value of Two Insect Larval Meals (Tenebrio Molitor and Hermetia Illucens) for Broiler Chickens: Apparent Nutrient Digestibility, Apparent Ileal Amino Acid Digestibility and Apparent Metabolizable Energy. Anim. Feed. Sci. Technol. 2015, 209, 211–218. [Google Scholar] [CrossRef]
- Jayanegara, A.; Goel, G.; Makkar, H.P.S.; Becker, K. Divergence between Purified Hydrolysable and Condensed Tannin Effects on Methane Emission, Rumen Fermentation and Microbial Population in Vitro. Anim. Feed Sci. Technol. 2015, 209, 60–68. [Google Scholar] [CrossRef]
- De Souza-Vilela, D.; Andrew, J.; Ruhnke, N.R. Insect Protein in Animal Nutrition. Anim. Prod. Sci. 2019, 59, 2029–2036. [Google Scholar] [CrossRef]
- Benzertiha, A.; Kierończyk, B.; Rawski, M.; Kołodziejski, P.; Bryszak, M.; Józefiak, D. Insect Oil as an Alternative to Palm Oil and Poultry Fat in Broiler Chicken Nutrition. Animals 2019, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Neumann, C.; Velten, S.; Liebert, F. Improving the Dietary Protein Quality by Amino Acid Fortification with a High Inclusion Level of Micro Algae (Spirulina Platensis) or Insect Meal (Hermetia Illucens) in Meat Type Chicken Diets. Open J. Anim. Sci. 2017, 8, 12–26. [Google Scholar] [CrossRef]
- Dahiru, S.J.; Azhar, B.; Anjas, A.B.; Asmara, B.S. Performance of Spring Chicken Fed Different Inclusion Levels of Black Soldier Fly Larvae Meal. Entomol. Ornithol. Herpetol. 2016, 5, 1000185. [Google Scholar]
- Vilela, D.S.; Alvarenga, J.; Andrew, T.I.; Mcphee, N.R.; Kolakshyapati, M.; Hopkins, M.; Ruhnke, D.L. Technological Quality, Amino Acid and Fatty Acid Profile of Broiler Meat Enhanced by Dietary Inclusion of Black Soldier Fly Larvae. Foods 2021, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Biasato, I.; Ferrocino, I.; Dabbou, S.; Evangelista, R.; Gai, F.; Gasco, L.; Cocolin, L.; Capucchio, M.T.; Schiavone, A. Black Soldier Fly and Gut Health in Broiler Chickens: Insights into the Relationship between Cecal Microbiota and Intestinal Mucin Composition. J. Anim. Sci. Biotechnol. 2020, 11, 11. [Google Scholar] [CrossRef]
- Schiavone, A.; Dabbou, S.; Petracci, M.; Zampiga, M.; Sirri, F.; Biasato, I.; Gai, F.; Gasco, L. Black Soldier Fly Defatted Meal as a Dietary Protein Source for Broiler Chickens: Effects on Carcass Traits, Breast Meat Quality and Safety. Animal 2019, 13, 2397–2405. [Google Scholar] [CrossRef]
- Dabbou, S.; Gai, F.; Biasato, I.; Capucchio, M.T.; Biasibetti, E.; Dezzutto, D.; Meneguz, M.; Plachà, I.; Gasco, L.; Schiavone, A. Black Soldier Fly Defatted Meal as a Dietary Protein Source for Broiler Chickens: Effects on Growth Performance, Blood Traits, Gut Morphology and Histological Features. J. Anim. Sci. Biotechnol. 2018, 9, 49. [Google Scholar] [CrossRef]
- Kareem, K.Y.; Abdulla, N.R.; Foo, H.L.; Zamri, A.N.M.; Shazali, N.; Loh, T.C.; Alshelmani, M.I. Effect of Feeding Larvae Meal in the Diets on Growth Performance, Nutrient Digestibility and Meat Quality in Broiler Chicken. Indian J. Anim. Sci. 2018, 88, 1180–1185. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.-M.; Park, Y.-K.; Yang, Y.-C.; Jung, B.-G.; Lee, B.-J. Black Soldier Fly (Hermetia Illucens) Larvae Enhances Immune Activities and Increases Survivability of Broiler Chicks against Experimental Infection of Salmonella Gallinarum. J. Vet. Med. Sci. 2018, 80, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, A.; Cullere, M.; De Marco, M.; Meneguz, M.; Biasato, I.; Bergagna, S.; Dezzutto, D.; Gai, F.; Dabbou, S.; Gasco, L.; et al. Partial or Total Replacement of Soybean Oil by Black Soldier Fly Larvae (Hermetia Illucens L.) Fat in Broiler Diets: Effect on Growth Performances, Feed-Choice, Blood Traits, Carcass Characteristics and Meat Quality. Ital. J. Anim. Sci. 2017, 16, 93–100. [Google Scholar] [CrossRef]
- Cullere, M.; Schiavone, A.; Dabbou, S.; Gasco, L.; Dalle Zotte, A. Meat Quality and Sensory Traits of Finisher Broiler Chickens Fed with Black Soldier Fly (Hermetia Illucens L.) Larvae Fat as Alternative Fat Source. Animals 2019, 9, 140. [Google Scholar] [CrossRef]
- Marono, S.; Loponte, R.; Lombardi, P.; Vassalotti, G.; Pero, M.E.; Russo, F.; Gasco, L.; Parisi, G.; Piccolo, G.; Nizza, S.; et al. Productive performance and blood profiles of laying hens fed Hermetia illucens larvae meal as total replacement of soybean meal from 24 to 45 weeks of age. Poult. Sci. 2017, 96, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Ruhnke, I.; Normant, C.; Campbell, D.L.M.; Iqbal, Z.; Lee, C.; Hinch, G.N.; Roberts, J. Impact of on-range choice feeding with black soldier fly larvae (Hermetia illucens) on flock performance, egg quality, and range use of free-range laying hens. Anim. Nutr. 2018, 4, 452–460. [Google Scholar] [CrossRef]
- Cutrignelli, M.I.; Messina, M.; Tulli, F.; Randazzo, B.; Olivotto, I.; Gasco, L.; Loponte, R.; Bovera, F. Evaluation of an insect meal of the Black Soldier Fly (Hermetia illucens) as soybean substitute: Intestinal morphometry, enzymatic and microbial activity in laying hens. Res. Vet. Sci. 2018, 117, 209–215. [Google Scholar] [CrossRef]
- Tahamtani, F.M.; Ivarsson, E.; Wiklicky, V.; Lalander, C.; Wall, H.; Rodenburg, T.B.; Tuyttens, F.A.M.; Hernandez, C.E. Feeding live Black Soldier Fly larvae (Hermetia illucens) to laying hens: Effects on feed consumption, hen health, hen behavior, and egg quality. Poult Sci. 2021, 100, 101400. [Google Scholar] [CrossRef]
- Chu, X.; Li, M.; Wang, G.; Wang, K.; Shang, R.; Wang, Z.; Li, L. Evaluation of the Low Inclusion of Full-Fatted Hermetia Illucens Larvae Meal for Layer Chickens: Growth Performance, Nutrient Digestibility, and Gut Health. Front. Vet. Sci. 2020, 7, 585843. [Google Scholar] [CrossRef]
- Heuel, M.; Sandrock, C.; Leiber, F.; Mathys, A.; Gold, M.; Zurbrügg, C.; Gangnat, I.D.M.; Kreuzer, M.; Terranova, M. Black Soldier Fly Larvae Meal and Fat Can Completely Replace Soybean Cake and Oil in Diets for Laying Hens. Poult. Sci. 2021, 100, 101034. [Google Scholar] [CrossRef]
- Mwaniki, Z.; Neijat, M.; Kiarie, E. Egg Production and Quality Responses of Adding up to 7.5% Defatted Black Soldier Fly Larvae Meal in a Corn–Soybean Meal Diet Fed to Shaver White Leghorns from Wk 19 to 27 of Age. Poult. Sci. 2018, 97, 2829–2835. [Google Scholar] [CrossRef]
- Elahi, U.; Xu, C.-C.; Wang, J.; Lin, J.; Wu, S.-G.; Zhang, H.-J.; Qi, G.-H. Insect Meal as a Feed Ingredient for Poultry. Anim. Biosci. 2022, 35, 332–346. [Google Scholar] [CrossRef]
- Zadeh, Z.S.; Kheiri, F.; Faghani, M. Use of Yellow Mealworm (Tenebrio Molitor) as a Protein Source on Growth Performance, Carcass Traits, Meat Quality and Intestinal Morphology of Japanese Quails (Coturnix Japonica). Vet. Anim. Sci. 2019, 8, 100066. [Google Scholar] [CrossRef]
- Bovera, F.; Piccolo, G.; Gasco, L.; Marono, S.; Loponte, R.; Vassalotti, G.; Mastellone, V.; Lombardi, P.; Attia, Y.A.; Nizza, A. Yellow Mealworm Larvae (Tenebrio Molitor L.) as a Possible Alternative to Soybean Meal in Broiler Diets. Br. Poult. Sci. 2015, 56, 569–575. [Google Scholar] [CrossRef]
- Sedgh-Gooya, S.; Torki, M.; Darbemamieh, M.; Khamisabadi, H.; Karimi Torshizi, M.A.; Abdolmohamadi, A. Yellow Mealworm, Tenebrio Molitor (Col: Tenebrionidae), Larvae Powder as Dietary Protein Sources for Broiler Chickens: Effects on Growth Performance, Carcass Traits, Selected Intestinal Microbiota and Blood Parameters. J. Anim. Physiol. Anim. Nutr. 2021, 105, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Churchward-Venne, T.A.; Pinckaers, P.J.M.; van Loon, J.J.A.; van Loon, L.J.C. Consideration of Insects as a Source of Dietary Protein for Human Consumption. Nutr. Rev. 2017, 75, 1035–1045. [Google Scholar] [CrossRef]
- Elahi, U.; Wang, J.; Ma, Y.-B.; Wu, S.-G.; Wu, J.; Qi, G.-H.; Zhang, H.-J. Evaluation of Yellow Mealworm Meal as a Protein Feedstuff in the Diet of Broiler Chicks. Animals 2020, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Shaviklo, A.R.; Alizadeh-Ghamsari, A.H.; Hosseini, S.A. Sensory Attributes and Meat Quality of Broiler Chickens Fed with Mealworm (Tenebrio Molitor). J. Food Sci. Technol. 2021, 58, 4587–4597. [Google Scholar] [CrossRef] [PubMed]
- Dabbou, S.; Gasco, L.; Lussiana, C.; Brugiapaglia, A.; Biasato, I.; Renna, M.; Schiavone, A. Yellow Mealworm (Tenebrio Molitor L.) Larvae Inclusion in Diets for Free-Range Chickens: Effects on Meat Quality and Fatty Acid Profile. Renew. Agric. Food Syst. 2020, 35, 571–578. [Google Scholar] [CrossRef]
- Biasato, I.; Gasco, L.; De Marco, M.; Renna, M.; Rotolo, L.; Dabbou, S.; Capucchio, M.T.; Biasibetti, E.; Tarantola, M.; Sterpone, L.; et al. Yellow Mealworm Larvae (Tenebrio Molitor) Inclusion in Diets for Male Broiler Chickens: Effects on Growth Performance, Gut Morphology, and Histological Findings. Poult. Sci. 2018, 97, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Biasato, I.; Gasco, L.; De Marco, M.; Renna, M.; Rotolo, L.; Dabbou, S.; Capucchio, M.T.; Biasibetti, E.; Tarantola, M.; Bianchi, C.; et al. Effects of Yellow Mealworm Larvae (Tenebrio Molitor) Inclusion in Diets for Female Broiler Chickens: Implications for Animal Health and Gut Histology. Anim. Feed Sci. Technol. 2017, 234, 253–263. [Google Scholar] [CrossRef]
- Kim, S.G.; Kim, J.E.; Oh, H.K.; Kang, S.J.; Koo, H.Y.; Kim, H.J.; Choi, H.C. Feed Supplementation of Yellow Mealworms (Tenebrio Molitor L.) Improves Blood Characteristics and Meat Quality in Broiler. J. Agric. Sci. Technol. 2014, 49, 9–18. [Google Scholar] [CrossRef]
- Benzertiha, A.; Kierończyk, B.; Kołodziejski, P.; Pruszyńska-Oszmałek, E.; Rawski, M.; Józefiak, D.; Józefiak, A. Tenebrio Molitor and Zophobas Morio Full-Fat Meals as Functional Feed Additives Affect Broiler Chickens’ Growth Performance and Immune System Traits. Poult. Sci. 2020, 99, 196–206. [Google Scholar] [CrossRef]
- Radulović, S.; Pavlović, M.; Šefer, D.; Katoch, S.; Hadži-Milić, M.; Jovanović, D.; Marković, R. Effects of Housefly Larvae (Musca Domestica) Dehydrated Meal on Production Performances and Sensory Properties of Broiler Meat. Thai J. Vet. Med. 2018, 48, 63–70. [Google Scholar]
- Awoniyi, T.A.; Aletor, V.A.; Aina, J.M. Performance of Broiler-Chickens Fed on Maggot Meal in Place of Fishmeal. Int. J. Poult. Sci. 2003, 2, 271–274. [Google Scholar]
- Khan, S.; Khan, R.U.; Sultan, A.; Khan, M.; Hayat, S.U.; Shahid, M.S. Evaluating the Suitability of Maggot Meal as a Partial Substitute of Soya Bean on the Productive Traits, Digestibility Indices and Organoleptic Properties of Broiler Meat. J. Anim. Physiol. Anim. Nutr. 2016, 100, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Aniebo, A.O.; Owen, O.J. Effects of Age and Method of Drying on the Proximate Composition of Housefly Larvae (Musca Domestica Linnaeus) Meal (HFLM). Pak. J. Nutr. 2010, 9, 485–487. [Google Scholar] [CrossRef]
- Pretorius, Q. The Evaluation of Larvae of Musca Domestica (Common House Fly) as Protein Source for Broiler Production. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2011. [Google Scholar]
- Okah, U.; Onwujiariri, E.B. Performance of finisher broiler chickens fed maggot meal as a replacement for fish meal. J. Agric. Technol 2012, 8, 471–477. [Google Scholar]
- Khan, M.; Chand, N.; Khan, S.; Khan, R.U.; Sultan, A. Utilizing the House Fly (Musca Domestica) Larva as an Alternative to Soybean Meal in Broiler Ration during the Starter Phase. Rev. Bras. Cienc. Avic. 2018, 20, 9–14. [Google Scholar] [CrossRef]
- Elahi, U.; Ma, Y.-B.; Wu, S.-G.; Wang, J.; Zhang, H.-J.; Qi, G.-H. Growth Performance, Carcass Characteristics, Meat Quality and Serum Profile of Broiler Chicks Fed on Housefly Maggot Meal as a Replacement of Soybean Meal. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1075–1084. [Google Scholar] [CrossRef]
- Pieterse, E.; Pretorius, Q.; Hoffman, L.C.; Drew, D.W. The Carcass Quality, Meat Quality and Sensory Characteristics of Broilers Raised on Diets Containing Either Musca Domestica Larvae Meal, Fish Meal or Soya Bean Meal as the Main Protein Source. Anim. Prod. Sci. 2014, 54, 622. [Google Scholar] [CrossRef]
- Agunbiade, J.A.; Adeyemi, O.A.; Ashiru, O.M.; Awojobi, H.A.; Taiwo, A.A.; Oke, D.B.; Adekunmisi, A.A. Replacement of Fish Meal with Maggot Meal in Cassava-Based Layers’ Diets. J. Poult. Sci. 2007, 44, 278–282. [Google Scholar] [CrossRef]
- Wang, D.; Zhai, S.-W.; Zhang, C.-X.; Zhang, Q.; Chen, H. Nutrition Value of the Chinese Grasshopper Acrida Cinerea (Thunberg) for Broilers. Anim. Feed Sci. Technol. 2007, 135, 66–74. [Google Scholar] [CrossRef]
- Ghosh, S.; Haldar, P.; Mandal, D.K. Evaluation of Nutrient Quality of a Short Horned Grasshopper, Oxya Hyla Hyla Serville (Orthoptera: Acrididae) in Search of New Protein Source. J. Entomol. Zool. Stud. 2016, 4, 193–197. [Google Scholar]
- Amobi, M.I.; Saleh, A.; Okpoko, V.O.; Abdullahi, A.M. Growth performance of broiler chickens based on grasshopper meal inclusions in feed formulation. Zoologist 2020, 18, 39–43. [Google Scholar] [CrossRef]
- Sanusi, M.; Garba, A.; Saidu, I.; Ali, Y.Z. Performance of Broiler Chickens Fed Graded Levels of Grasshopper Meals. Int. J. Appl. Res. Technol. 2013, 2, 235–240. [Google Scholar]
- Sun, T.; Long, R.J.; Liu, Z.Y. The Effect of a Diet Containing Grasshoppers and Access to Free-Range on Carcase and Meat Physicochemical and Sensory Characteristics in Broilers. Br. Poult. Sci. 2013, 54, 130–137. [Google Scholar] [CrossRef]
- Acay, R.P. Silkworm Pupa Meal as Feed Supplement for Growing-Finishing Broilers. Master’s Thesis, Benguet State University, La Trinidad, Philippines, 2011. [Google Scholar]
- Banday, M.T.; Adil, S.; Sheikh, I.U.; Hamadani, H.; Qadri, F.I.; Sahfi, M.E.; Sait, H.S.A.W.; Abd El-Mageed, T.A.; Salem, H.M.; Taha, A.E.; et al. The Use of Silkworm Pupae (Bombyx Mori) Meal as an Alternative Protein Source for Poultry. Worlds. Poult. Sci. J. 2023, 79, 1–16. [Google Scholar] [CrossRef]
- Jintasataporn, O. Production performance of broiler chickens fed with silkworm Pupa (Bombyx Mori). J. Agric. Sci. Technol. A 2012, 2, 505–510. [Google Scholar]
- Ullah, R.; Khan, S.; Khan, N.A.; Mobashar, M.; Sultan, A.; Ahmad, N.; Lohakare, J. Replacement of Soybean Meal with Silkworm Meal in the Diets of White Leghorn Layers and Effects on Performance, Apparent Total Tract Digestibility, Blood Profile and Egg Quality. Int. J. Vet. Health Sci. Res. 2017, 5, 200–207. [Google Scholar]
- Miah, M.Y.; Singh, Y.; Cullere, M.; Tenti, S.; Dalle Zotte, A. Effect of Dietary Supplementation with Full-Fat Silkworm (Bombyx Mori L.) Chrysalis Meal on Growth Performance and Meat Quality of Rhode Island Red× Fayoumi Crossbred Chickens. Ital. J. Anim. Sci. 2020, 19, 447–456. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, S.; Hafeez, A.; Sultan, A.; Khan, N.A.; Chand, N.; Ahmad, N. Silkworm (Bombyx Mori) Meal as Alternate Protein Ingredient in Broiler Finisher Ration. Pak. J. Zool. 2017, 49, 1463–1470. [Google Scholar] [CrossRef]
- Musa, U.; Yusuf, J.; Haruna, E.S.; Karsin, P.D.; Ali, U.D. Termites as possible animal protein supplement for Japanese Quail (Cotumix Cotumix Japonica) chicks feed. Niger. J. Biotechnol. 2004, 15, 48–51. [Google Scholar]
- Pousga, S.; Sankara, F.; Coulibaly, K.; Nacoulma, J.P.; Ouedraogo, S.; Kenis, M.; Ouedraogo, G.A. Effects of replacement of fishmeal by Termites (Macrotermes Sp.) on the weight evolution and carcass characteristics of local poultry in Burkina Faso. Afr. J. Food Agric. Nutr. Dev. 2019, 19, 14354–14371. [Google Scholar]
- Purwadaria, T.; Ketaren, P.P.; Sinurat, A.P.; Sutikno, I. Identification and evaluation of fiber hydrolytic enzymes in the extract of Termites (Glyptotermes Montanus) for poultry feed application. Indones. J. Agric. Sci. 2003, 4, 40–47. [Google Scholar] [CrossRef]
- Imathiu, S. Benefits and food safety concerns associated with consumption of edible insects. NFS J. 2020, 18, 1–11. [Google Scholar] [CrossRef]
- Lähteenmäki-Uutela, A.; Marimuthu, S.B.; Meijer, N. Regulations on Insects as Food and Feed: A Global Comparison. J. Insects Food Feed 2021, 7, 849–856. [Google Scholar] [CrossRef]
- Van der Fels-Klerx, H.J.; Camenzuli, L.; Belluco, S.; Meijer, N.; Ricci, A. Food safety issues related to uses of insects for feeds and foods: Food Safety of Insects for Feeds/Foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Vandeweyer, D.; De Smet, J.; Van Looveren, N.; Van Campenhout, L. Biological Contaminants in Insects as Food and Feed. J. Insects Food Feed 2021, 7, 807–822. [Google Scholar] [CrossRef]
- Govorushko, S. Global Status of Insects as Food and Feed Source: A Review. Trends Food Sci. Technol. 2019, 91, 436–445. [Google Scholar] [CrossRef]
- Müller, A.; Wiedmer, S.; Kurth, M. Risk Evaluation of Passive Transmission of Animal Parasites by Feeding of Black Soldier Fly (Hermetia Illucens) Larvae and Prepupae. J. Food Prot. 2019, 82, 948–954. [Google Scholar] [CrossRef]
- Diener, S.; Zurbrügg, C.; Tockner, K. Bioaccumulation of Heavy Metals in the Black Soldier Fly, Hermetia Illucens and Effects on Its Life Cycle. J. Insects Food Feed 2015, 1, 261–270. [Google Scholar] [CrossRef]
- Van der Fels-Klerx, H.J.; Camenzuli, L.; van der Lee, M.K.; Oonincx, D.G.A.B. Uptake of Cadmium, Lead and Arsenic by Tenebrio Molitor and Hermetia Illucens from Contaminated Substrates. PLoS ONE 2016, 11, e0166186. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, L.; Block, M. Excretion of Cadmium during Moulting and Metamorphosis in Tenebrio Molitor (Coleoptera; Tenebrionidae). Comp. Biochem. Physiol. C 1995, 111, 325–328. [Google Scholar] [CrossRef]
- Meyer, A.M.; Meijer, N.; Hoek-van den Hil, E.F.; van der Fels-Klerx, H.J. Chemical Food Safety Hazards of Insects Reared for Food and Feed. J. Insects Food Feed 2021, 7, 823–831. [Google Scholar] [CrossRef]
- DiGiacomo, K.; Leury, B.J. Review: Insect Meal: A Future Source of Protein Feed for Pigs? Animal 2019, 13, 3022–3030. [Google Scholar] [CrossRef]
- Premrov-Bajuk, B.; Zrimšek, P.; Kotnik, T.; Leonardi, A.; Križaj, I.; Strajn, B. Insect Protein-Based Diet as Potential Risk of Allergy in Dogs. Animals 2021, 11, 1942. [Google Scholar] [CrossRef]
- Ojha, S.; Bekhit, A.E.-D.; Grune, T.; Schlüter, O.K. Bioavailability of Nutrients from Edible Insects. Curr. Opin. Food Sci. 2021, 41, 240–248. [Google Scholar] [CrossRef]
- Sogari, G.; Amato, M.; Biasato, I.; Chiesa, S.; Gasco, L. The Potential Role of Insects as Feed: A Multi-Perspective Review. Animals 2019, 9, 119. [Google Scholar] [CrossRef]
- Lähteenmäki-Uutela, A.; Grmelová, N.; Hénault-Ethier, L.; Deschamps, M.H.; Vandenberg, G.W.; Zhao, A.; Zhang, Y.; Yang, B.; Nemane, V. Insects as Food and Feed: Laws of the European Union, United States, Canada, Mexico, Australia, and China. Eur. Food Feed. Law Rev. EFFL 2017, 12, 22. [Google Scholar]
- Kim, J.W. Insect Industry for Future Super Foods. Food Sci Anim Resour Ind 2019, 8, 74–77. [Google Scholar]
- Usman, H.S.; Yusuf, A.A. Legislation and legal framework for sustainable edible insects use in Nigeria. Int. J. Trop. Insect Sci. 2021, 41, 2201–2209. [Google Scholar] [CrossRef]
- IPIFF (International Platform Insects for Food and Feed). EU Authorisation of Insect Proteins in Poultry and Swine Feed: Opportunities for More Resilient and Sustainable Agri-Food Supply Chains. Available online: https://ipiff.org/insect-proteins-in-poultry-and-pig-feed-are-fully-authorised-in-the-european-union/ (accessed on 21 September 2022).
- Gasco, L.; Biasato, I.; Dabbou, S.; Schiavone, A.; Gai, F. Animals Fed Insect-Based Diets: State-of-the-Art on digestibility, performance and product quality. Animals 2019, 9, 170. [Google Scholar] [CrossRef]
- Koutsos, L.; McComb, A.; Finke, M. Insect Composition and Uses in Animal Feeding Applications: A Brief Review. Ann. Entomol. Soc. Am. 2019, 112, 544–551. [Google Scholar] [CrossRef]
- Danieli, P.P.; Lussiana, C.; Gasco, L.; Amici, A.; Ronchi, B. The Effects of Diet Formulation on the Yield, Proximate Composition, and Fatty Acid Profile of the Black Soldier Fly (Hermetia Illucens L) Prepupae Intended for Animal Feed. Animals 2019, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Finke, M.D. Complete nutrient content of four species of commercially available feeder insects fed enhanced diets during growth: Complete nutrient content of four species of feeder insects. Zoo Biol. 2015, 34, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Biasato, I.; Ferrocino, I.; Grego, E.; Dabbou, S.; Gai, F.; Gasco, L.; Cocolin, L.; Capucchio, M.T.; Schiavone, A. Gut Microbiota and Mucin Composition in Female Broiler Chickens Fed Diets Including Yellow Mealworm (Tenebrio Molitor). Animals 2019, 9, 213–221. [Google Scholar] [CrossRef]
- Henry, M.A.; Gasco, L.; Chatzifotis, S.; Piccolo, G. Does Dietary Insect Meal Affect the Fish Immune System? The Case of Mealworm, Tenebrio Molitor on European Sea Bass, Dicentrarchus Labrax. Dev. Comp. Immunol. 2018, 81, 204–209. [Google Scholar] [CrossRef]
- Antonopoulou, E.; Nikouli, E.; Piccolo, G.; Gasco, L.; Gai, F.; Chatzifotis, S.; Mente, E.; Kormas, K. Reshaping Gut Bacterial Communities after Dietary Tenebrio Molitor Larvae Meal Supplementation in Three Different Fish Species. Aquaculture 2019, 503, 628–635. [Google Scholar] [CrossRef]
- Wu, Q.; Patočka, J.; Kuča, K. Insect Antimicrobial Peptides, a Mini Review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef]
- Mancuso, T.; Poppinato, L.; Gasco, L. The European Insects Sector and Its Role in the Provision of Green Proteins in Feed Supply. Calitatea 2019, 20, 374–381. [Google Scholar]
- Amro, A.B.N. Insectenkweek: Kleine Sector, Grote Kansen. Available online: https://www.bom.nl/uploads/content/file/Insectenkweek-def_1565254395.pdf (accessed on 16 March 2023).
- Arru, B.; Furesi, R.; Gasco, L.; Madau, F.; Pulina, P. The Introduction of Insect Meal into Fish Diet: The first economic analysis on European Sea Bass farming. Sustainability 2019, 11, 1697. [Google Scholar] [CrossRef]
- Dalle Zotte, A.; Singh, Y.; Michiels, J.; Cullere, M. Black Soldier Fly (Hermetia Illucens) as dietary source for laying quails: Live performance, and egg physico-chemical quality, sensory profile and storage stability. Animals 2019, 9, 115. [Google Scholar] [CrossRef]
- Pinotti, L.; Giromini, C.; Ottoboni, M.; Tretola, M.; Marchis, D. Review: Insects and former foodstuffs for upgrading food waste biomasses/streams to feed ingredients for farm animals. Animal 2019, 13, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Pliner, P.; Hobden, K. Development of a scale to measure the trait of food neophobia in humans. Appetite 1992, 19, 105–120. [Google Scholar] [CrossRef]
- Verbeke, W. Profiling Consumers Who Are Ready to Adopt Insects as a Meat Substitute in a Western Society. Food Qual. Prefer. 2015, 39, 147–155. [Google Scholar] [CrossRef]
- IPIFF (International Platform Insects for Food and Feed). The Insect Sector Milestones towards Sustainable Food Supply Chains. Available online: https://ipiff.org/wp-content/uploads/2020/05/IPIFF-RegulatoryBrochure-update07-2020-1.pdf (accessed on 16 March 2023).
- Hua, K.; Cobcroft, J.M.; Cole, A.; Condon, K.; Jerry, D.R.; Mangott, A.; Praeger, C.; Vucko, M.J.; Zeng, C.; Zenger, K.; et al. The future of aquatic protein: Implications for protein sources in aquaculture diets. One Earth 2019, 1, 316–329. [Google Scholar] [CrossRef]
- Moula, N.; Detilleux, J. A Meta-Analysis of the effects of insects in feed on poultry growth performances. Animals 2019, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Belluco, S.; Bertola, M.; Montarsi, F.; Di Martino, G.; Granato, A.; Stella, R.; Martinello, M.; Bordin, F.; Mutinelli, F. Insects and Public Health: An Overview. Insects 2023, 14, 240. [Google Scholar] [CrossRef]


| Insect Name | Protein (%) | Fat (%) | Methionine (%) * | Lysine (%) * | Ca (%) | P (%) | References |
|---|---|---|---|---|---|---|---|
| Black soldier | 42.3 | 33.5 | 2.1 | 5.7 | 3.2 | 0.9 | [12,34] |
| House fly | 52 | 18 | 2.2 | 6.1 | 0.47 | 1.6 | [35] |
| Meal worm | 45 | 30 | 1.5 | 5.4 | 0.27 | 0.78 | [12,36] |
| Locusts | 57.3 | 8.5 | 2.3 | 5.8 | 0.13 | - | [37] |
| Silkworm | 54 | 12 | 3 | 7 | 0.38 | 0.60 | [38] |
| Grasshopper | 47.71 | 12.21 | - | - | - | - | [39] |
| Soybean meal | 44 | 0.9 | 0.65 | 2.95 | 0.32 | 0.65 | [4] |
| Bird Species | Replacements Rate (%) | Main Results | References |
|---|---|---|---|
| Growing quails | 10–15% |
| [56] |
| Growing quails | 10% |
| [34] |
| Growing quails | 10% |
| [54] |
| Broilers | 0, 5, 10, 15, and 20% |
| [57] |
| Broilers | 75 and 100% * |
| [58] |
| Cobb broiler | 5, 7.5, and 10% |
| [60] |
| Ross 308 broiler | 20% |
| [61] |
| Ross 708 broiler | 5% |
| [62] |
| Hy-line brown laying hens | 3% |
| [73] |
| White Leghorn laying hens | 5 and 7.5% |
| [75] |
| Bird Line | Replacements Rate (%) | Main Results | References |
|---|---|---|---|
| Arbor Acres broilers | 2.5 and 5% from total feed |
| [79] |
| Ross 308 male broilers | 4% from from total feed |
| [76] |
| Hubbard hybrid free-range broilers | 7.5% from from total feed |
| [83] |
| Ross 708 male broilers | 15% from from total feed |
| [84] |
| Broilers | 100% from soybean oil |
| [87] |
| Bird Line | Replacements Rate (%) | Main Results | References |
|---|---|---|---|
| Anak broilers | 20 and 40% of fish meal |
| [93] |
| Broilers | 60% from soybean meal |
| [94] |
| Ross 308 male broilers | 4% from from total feed |
| [95] |
| Ross 308 male broilers | 10% from from total feed |
| [96] |
| Country | Authority | Regulation and Content | References |
|---|---|---|---|
| European Union (EU) | European Food Safety Authority (EFSA) |
| [126] |
| United States | Federal Food and Drug Administration (FDA) and Association of American Feed Control Officials (AAFCO) |
| [113] |
| Canada | Canadian Food Inspection Agency (CFIA) |
| [126] |
| Republic of Korea | The Ministry of Agriculture, Food, and Rural Affairs (MAFRA) |
| [127] |
| China | The Ministry of Agriculture and Rural Affairs |
| [126] |
| Japan | The Ministry of Agriculture, Forestry and Fisheries |
| [113] |
| Australia | Australian Pesticides and Veterinary Medicine Authority (APVMA) |
| [122] |
| Nigeria | National Agency for Food and Drug Administration and Control (NAFDAC) |
| [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalifah, A.; Abdalla, S.; Rageb, M.; Maruccio, L.; Ciani, F.; El-Sabrout, K. Could Insect Products Provide a Safe and Sustainable Feed Alternative for the Poultry Industry? A Comprehensive Review. Animals 2023, 13, 1534. https://doi.org/10.3390/ani13091534
Khalifah A, Abdalla S, Rageb M, Maruccio L, Ciani F, El-Sabrout K. Could Insect Products Provide a Safe and Sustainable Feed Alternative for the Poultry Industry? A Comprehensive Review. Animals. 2023; 13(9):1534. https://doi.org/10.3390/ani13091534
Chicago/Turabian StyleKhalifah, Ayman, Sara Abdalla, Mai Rageb, Lucianna Maruccio, Francesca Ciani, and Karim El-Sabrout. 2023. "Could Insect Products Provide a Safe and Sustainable Feed Alternative for the Poultry Industry? A Comprehensive Review" Animals 13, no. 9: 1534. https://doi.org/10.3390/ani13091534








