Characterization of Virulence Factors and Antimicrobial Susceptibility of Streptococcus agalactiae Associated with Bovine Mastitis Cases in Thailand
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Ethical Approval Statement
2.2. Bacterial Isolates and Sample Collection
2.3. Reference Strains
2.4. Preparation of the DNA Model for Molecular Procedures
2.5. Characterization of Molecular Virulence Factors
2.6. Antimicrobial Susceptibility of S. agalactiae Strains
2.7. Statistical Analysis
3. Results
3.1. Molecular Characterization of Virulence Factors
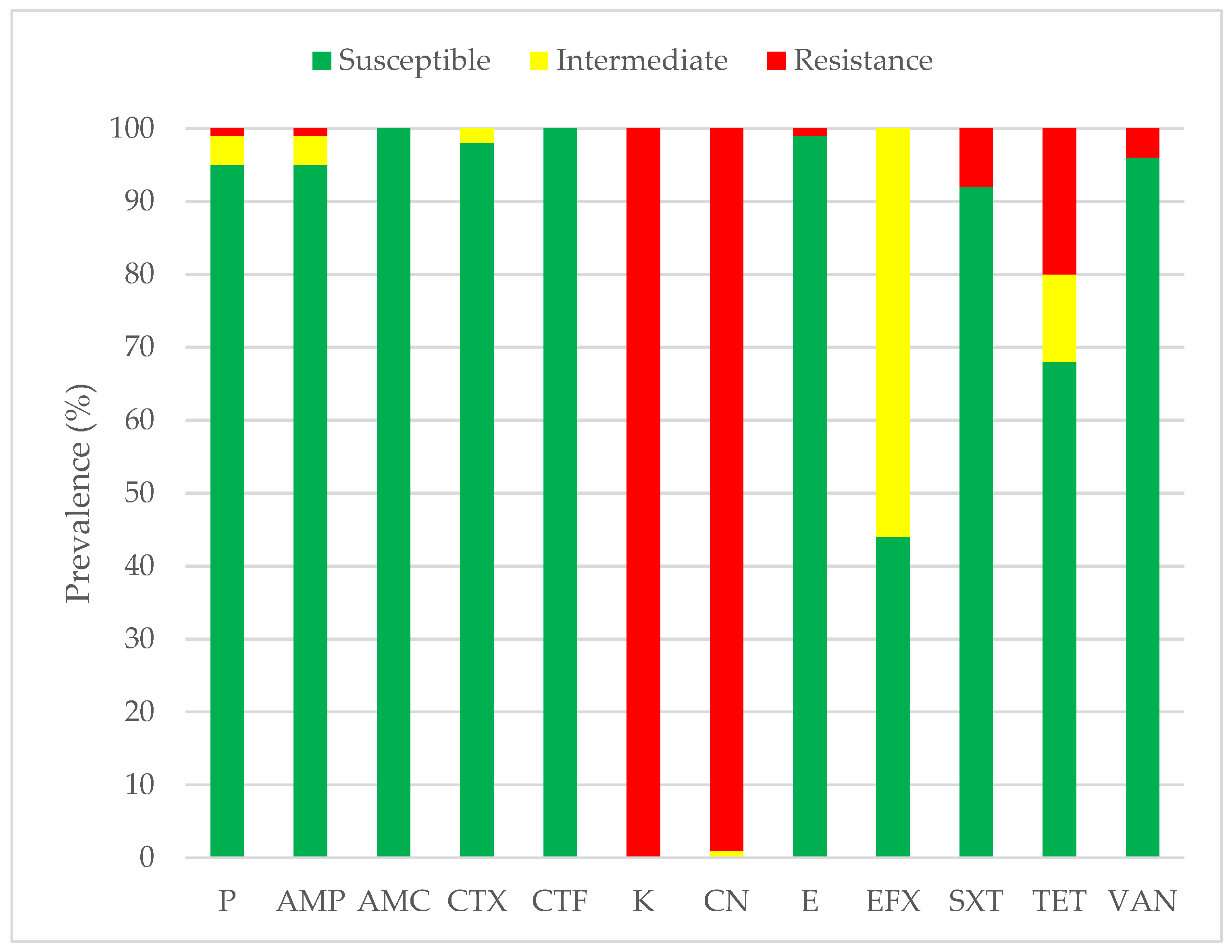
3.2. Antimicrobial Susceptibility Profiles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keefe, G. Update on control of Staphylococcus aureus and Streptococcus agalactiae for management of mastitis. Vet. Clin. N. Am. Food A Food Anim. Pract. 2012, 28, 203–216. [Google Scholar] [CrossRef]
- Reyes, J.; Chaffer, M.; Rodriguez-Lecompte, J.C.; Sánchez, J.; Zadoks, R.N.; Robinson, N.; Cardona, X.; Ramírez, N.; Keefe, G. Molecular epidemiology of Streptococcus agalactiae differs between countries. J. Dairy Sci. 2017, 100, 9294–9297. [Google Scholar] [CrossRef]
- Reyes, J.; Chaffer, M.; Sanchez, J.; Torres, G.; Macias, D.; Jaramillo, M.; Duque, P.; Ceballos, A.; Keefe, G. Evaluation of the efficacy of intramuscular versus intramammary treatment of subclinical Streptococcus agalactiae mastitis in dairy cows in Colombia. J. Dairy Sci. 2015, 98, 5294–5303. [Google Scholar] [CrossRef]
- Holmøy, I.H.; Toft, N.; Jørgensen, H.J.; Mørk, T.; Sølverød, L.; Nødtvedt, A. Latent class analysis of real time qPCR and bacteriological culturing for the diagnosis of Streptococcus agalactiae in cow composite milk samples. Prev. Vet. Med. 2018, 154, 119–123. [Google Scholar] [CrossRef]
- Boonyayatra, S.; Thaboonpeng, J.; Kreausukon, K.; Suriyasathaporn, W. Anitimicrobial resistance of mastitis associated bacteria in lactating dairy cows in chiang mai province. Chiang Mai Vet. J. 2007, 5, 135–145. [Google Scholar]
- Kampa, J.; Sukolapong, V.; Buttasri, A.; Charoenchai, A. Prevalence of Mycoplasma bovis and other contagious bovine mastitis pathogens in bulk tank milk of dairy cattle herds in Khon Kaen Province, Thailand. Thai J. Vet. Med. 2009, 39, 275–280. [Google Scholar] [CrossRef]
- Wataradee, S.; Samngamnim, S.; Boonserm, T.; Ajariyakhajorn, K. Genotypic and antimicrobial susceptibility of Streptococcus agalactiae causing bovine mastitis in the central region of Thailand. Front. Vet. Sci 2023, 10, 1250436. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, A.A.; Rocha, C.M.; Bruhn, F.R.; Custódio, D.A.; Braz, M.S.; Pinto, S.M.; Silva, D.B.; Costa, G.M. Staphylococcus aureus and Streptococcus agalactiae: Prevalence, resistance to antimicrobials, and their relationship with the milk quality of dairy cattle herds in Minas Gerais state, Brazil. Pesqui. Veterinária Bras. 2019, 39, 308–316. [Google Scholar] [CrossRef]
- Chaffin, D.O.; Beres, S.B.; Yim, H.H.; Rubens, C.E. The serotype of type Ia and III group B streptococci is determined by the polymerase gene within the polycistronic capsule operon. J. Bacteriol. 2000, 182, 4466–4477. [Google Scholar] [CrossRef]
- Heath, P.T. Status of vaccine research and development of vaccines for GBS. Vaccine 2016, 34, 2876–2879. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Castro, G.A.; Silva, J.R.; Paiva, L.V.; Custódio, D.A.C.; Moreira, R.O.; Mian, G.F.; Prado, I.A.; Chalfun-Junior, A.; Costa, G.M. Molecular epidemiology of Streptococcus agalactiae isolated from mastitis in Brazilian dairy herds. Braz. J. Microbiol. 2017, 48, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Shabayek, S.; Spellerberg, B. Group B streptococcal colonization, molecular characteristics, and epidemiology. Front. Microbiol. 2018, 9, 437. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, R.N.; Schukken, Y.H. Use of molecular epidemiology in veterinary practice. Vet. Clin. N. Am. Food A Food Anim. Pract. 2006, 22, 229–261. [Google Scholar] [CrossRef] [PubMed]
- Boonyayatra, S.; Wongsathein, D.; Tharavichitkul, P. Genetic relatedness among Streptococcus agalactiae isolated from Cattle, Fish, and Humans. Foodborne Pathog. Dis. 2020, 17, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Sun, L.; He, T.; Bao, H.; Zhang, L.; Zhou, Y.; Zhang, H.; Wei, R.; Liu, Y.; Wang, R. Molecular and virulence characterization of highly prevalent Streptococcus agalactiae circulated in bovine dairy herds. Vet. Res. 2017, 48, 65. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Ding, Y.; Yi, L.; Ma, Z.; Fan, H.; Lu, C. Molecular characterization of Streptococcus agalactiae isolated from bovine mastitis in Eastern China. PLoS ONE 2013, 8, 67755. [Google Scholar] [CrossRef]
- Lin, L.; Huang, X.; Yang, H.; He, Y.; He, X.; Huang, J.; Li, S.; Wang, X.; Tang, S.; Liu, G. Molecular epidemiology, antimicrobial activity, and virulence gene clustering of Streptococcus agalactiae isolated from dairy cattle with mastitis in China. J. Dairy Sci. 2021, 104, 4893–4903. [Google Scholar] [CrossRef]
- Kulkarni, A.G.; Kaliwal, B. Bovine mastitis: A review. Int. J Recent Sci. 2013, 4, 543–548. [Google Scholar]
- Rajagopal, L. Understanding the regulation of Group B Streptococcal virulence factors. Future Microbiol. 2009, 4, 201–221. [Google Scholar] [CrossRef]
- Hensler, M.E.; Quach, D.; Hsieh, C.-J.; Doran, K.S.; Nizet, V. CAMP factor is not essential for systemic virulence of Group B Streptococcus. Microb. Pathog. 2008, 44, 84–88. [Google Scholar] [CrossRef]
- Doran, K.S.; Liu, G.Y.; Nizet, V. Group B streptococcal β-hemolysin/cytolysin activates neutrophil signaling pathways in brain endothelium and contributes to development of meningitis. J. Clin. Investig. 2003, 112, 736–744. [Google Scholar] [CrossRef]
- Emaneini, M.; Jabalameli, F.; Abani, S.; Dabiri, H.; Beigverdi, R. Comparison of virulence factors and capsular types of Streptococcus agalactiae isolated from human and bovine infections. Microb. Pathog. 2016, 91, 1–4. [Google Scholar] [CrossRef]
- Zastempowska, E.; Twarużek, M.; Grajewski, J.; Lassa, H. Virulence Factor Genes and Cytotoxicity of Streptococcus agalactiae Isolated from Bovine Mastitis in Poland. Microbiol. Spectr. 2022, 10, e0222421. [Google Scholar] [CrossRef] [PubMed]
- Kaczorek, E.; Małaczewska, J.; Wójcik, R.; Rękawek, W.; Siwicki, A. Phenotypic and genotypic antimicrobial susceptibility pattern of Streptococcus spp. isolated from cases of clinical mastitis in dairy cattle in Poland. J. Dairy Sci. 2017, 100, 6442–6453. [Google Scholar] [CrossRef]
- Tomazi, T.; de Souza Filho, A.F.; Heinemann, M.B.; dos Santos, M.V. Molecular characterization and antimicrobial susceptibility pattern of Streptococcus agalactiae isolated from clinical mastitis in dairy cattle. PLoS ONE 2018, 13, e0199561. [Google Scholar] [CrossRef]
- Lechner, I.; Freivogel, C.; Stärk, K.D.; Visschers, V.H. Exposure pathways to antimicrobial resistance at the human-animal interface—A qualitative comparison of Swiss expert and consumer opinions. Front. Public Health 2020, 8, 345. [Google Scholar] [CrossRef]
- Serwecińska, L. Antimicrobials and antibiotic-resistant bacteria: A risk to the environment and to public health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Koonawootrittriron, S.; Elzo, M. Challenges and opportunities for improvement in dairy production and genetic progress in Thailand. In Proceedings of the 14th AAAP Animal Science Congress, Pingtung, Taiwan, China, 23–27 August 2010; pp. 389–394. [Google Scholar]
- Kayansamruaj, P.; Pirarat, N.; Katagiri, T.; Hirono, I.; Rodkhum, C. Molecular characterization and virulence gene profiling of pathogenic Streptococcus agalactiae populations from tilapia (Oreochromis sp.) farms in Thailand. J. Vet. Diagn. Investig. 2014, 26, 488–495. [Google Scholar] [CrossRef]
- Firon, A.; Tazi, A.; Da Cunha, V.; Brinster, S.; Sauvage, E.; Dramsi, S.; Golenbock, D.T.; Glaser, P.; Poyart, C.; Trieu-Cuot, P. The Abi-domain protein Abx1 interacts with the CovS histidine kinase to control virulence gene expression in group B Streptococcus. PLoS Pathog. 2013, 9, e1003179. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; CLSI: Wayne, PA, USA, 2019. [Google Scholar]
- Sweeney, M.T.; Lubbers, B.V.; Schwarz, S.; Watts, J.L. Applying definitions for multidrug resistance, extensive drug resistance and pandrug resistance to clinically significant livestock and companion animal bacterial pathogens. J. Antimicrob. Chemother. 2018, 73, 1460–1463. [Google Scholar] [CrossRef]
- Olde Riekerink, R.G.M.; Barkema, H.W.; Veenstra, S.; Poole, D.E.; Dingwell, R.T.; Keefe, G.P. Prevalence of contagious mastitis pathogens in bulk tank milk in Prince Edward Island. Can. Vet. J. 2006, 47, 567–572. [Google Scholar] [PubMed]
- Hernandez, L.; Bottini, E.; Cadona, J.; Cacciato, C.; Monteavaro, C.; Bustamante, A.; Sanso, A.M. Multidrug resistance and molecular characterization of Streptococcus agalactiae isolates from dairy cattle with mastitis. Front. Cell. Infect. Microbiol. 2021, 11, 647324. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, W.; Lu, C. Comparative genomics analysis of Streptococcus agalactiae reveals that isolates from cultured tilapia in China are closely related to the human strain A909. BMC Genom. 2013, 14, 775. [Google Scholar] [CrossRef]
- Rosa-Fraile, M.; Dramsi, S.; Spellerberg, B. Group B streptococcal haemolysin and pigment, a tale of twins. FEMS Microbiol. Rev. 2014, 38, 932–946. [Google Scholar] [CrossRef] [PubMed]
- Ke, D.; Ménard, C.; Picard, F.J.; Boissinot, M.; Ouellette, M.; Roy, P.H.; Bergeron, M.G. Development of Conventional and Real-Time PCR Assays for the Rapid Detection of Group B Streptococci. Clin. Chem. 2000, 46, 324–331. [Google Scholar] [CrossRef]
- Guo, D.; Xi, Y.; Wang, S.; Wang, Z. Is a positive Christie-Atkinson-Munch-Peterson (CAMP) test sensitive enough for the identification of Streptococcus agalactiae? BMC Infect. Dis. 2019, 19, 7. [Google Scholar] [CrossRef]
- Lasagno, M.C.; Reinoso, E.B.; Dieser, S.A.; Calvinho, L.F.; Buzzola, F.; Vissio, C.; Bogni, C.I.; Odierno, L.M. Phenotypic and genotypic characterization of Streptococcus uberis isolated from bovine subclinical mastitis in Argentinean dairy farms. Rev. Argent. Microbiol. 2011, 43, 212–217. [Google Scholar] [CrossRef]
- Duarte, R.S.; Miranda, O.P.; Bellei, B.C.; Brito, M.A.V.P.; Teixeira, L.M. Phenotypic and Molecular Characteristics of Streptococcus agalactiae Isolates Recovered from Milk of Dairy Cows in Brazil. J. Clin. Microbiol. 2004, 42, 4214. [Google Scholar] [CrossRef] [PubMed]
- Baron, M.J.; Filman, D.J.; Prophete, G.A.; Hogle, J.M.; Madoff, L.C. Identification of a glycosaminoglycan binding region of the alpha C protein that mediates entry of group B streptococci into host cells. J. Biol. Chem. 2007, 282, 10526–10536. [Google Scholar] [CrossRef]
- Kabelitz, T.; Aubry, E.; van Vorst, K.; Amon, T.; Fulde, M. The role of Streptococcus spp. in bovine mastitis. Microorganisms 2021, 9, 1497. [Google Scholar] [CrossRef]
- da Costa, G.M.; Ribeiro, N.A.; Gonçalves, M.S.; da Silva, J.R.; da Costa Custódio, D.A.; Mian, G.F. Antimicrobial susceptibility profile of Streptococcus agalactiae strains isolated from bovine mastitis. Braz. J. Vet. Res. Anim. Sci. 2021, 58, e178109. [Google Scholar] [CrossRef]
- Chehabi, C.N.; Nonnemann, B.; Astrup, L.B.; Farre, M.; Pedersen, K. In vitro antimicrobial resistance of causative agents to clinical mastitis in Danish dairy cows. Foodborne Pathog. Dis. 2019, 16, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Zhang, B.; Luo, Z.; Lu, B.; Luo, Z.; Zhang, J.; Wang, Y.; Luo, Y.; Yang, Z.; Shen, L. Molecular typing and prevalence of antibiotic resistance and virulence genes in Streptococcus agalactiae isolated from Chinese dairy cows with clinical mastitis. PLoS ONE 2022, 17, e0268262. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zheng, N.; Han, R.; Ho, H.; Wang, J.; Wang, Y.; Wang, S.; Li, H.; Liu, H.; Yu, Z. Antimicrobial resistance and virulence genes of Streptococcus isolated from dairy cows with mastitis in China. Microb. Pathog. 2019, 131, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Dogan, B.; Schukken, Y.; Santisteban, C.; Boor, K.J. Distribution of serotypes and antimicrobial resistance genes among Streptococcus agalactiae isolates from bovine and human hosts. J. Clin. Microbiol. 2005, 43, 5899–5906. [Google Scholar] [CrossRef] [PubMed]
- Meroni, G.; Sora, V.M.; Martino, P.A.; Sbernini, A.; Laterza, G.; Zaghen, F.; Soggiu, A.; Zecconi, A. Epidemiology of antimicrobial resistance genes in Streptococcus agalactiae sequences from a public database in a One Health perspective. Antibiotics 2022, 11, 1236. [Google Scholar] [CrossRef] [PubMed]
- Premashthira, S. Overview of Foot and Mouth Disease Control in Thailand and Southeast Asia. In Proceedings of the 20th FAVA & the 15th KIVNAS PDHI, Bali, Indonesia, 1–3 November 2018. [Google Scholar]
- Ajariyakhajorn, K.; Boonserm, T.; Samngamnim, S. Detection of Pathogens Associated with Bovine Respiratory Disease: Clinical Cases in Thai Dairy Herds. In Proceedings of the 20th FAVA & the 15th KIVNAS PDHI, Bali, Indonesia, 1–3 November 2018. [Google Scholar]
- Carra, E.; Russo, S.; Micheli, A.; Garbarino, C.; Ricchi, M.; Bergamini, F.; Bassi, P.; Prosperi, A.; Piva, S.; Cricca, M. Evidence of common isolates of Streptococcus agalactiae in bovines and humans in Emilia Romagna region (Northern Italy). Front. Microbiol. 2021, 12, 673126. [Google Scholar] [CrossRef] [PubMed]
- Rato, M.G.; Bexiga, R.; Florindo, C.; Cavaco, L.M.; Vilela, C.L.; Santos-Sanches, I. Antimicrobial resistance and molecular epidemiology of streptococci from bovine mastitis. Vet. Microbiol. 2013, 161, 286–294. [Google Scholar] [CrossRef]
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An overview. Cold Spring Harb. Perspect. 2016, 6, a027029. [Google Scholar] [CrossRef]
- Magnet, S.; Blanchard, J.S. Molecular insights into aminoglycoside action and resistance. Chem. Rev. 2005, 105, 477–498. [Google Scholar] [CrossRef]
- Tenover, F.C. Mechanisms of antimicrobial resistance in bacteria. Am. J. Med. 2006, 119, S3–S10. [Google Scholar] [CrossRef]
- Sendi, P.; Furitsch, M.; Mauerer, S.; Florindo, C.; Kahl, B.C.; Shabayek, S.; Berner, R.; Spellerberg, B. Chromosomally and extrachromosomally mediated high-level gentamicin resistance in Streptococcus agalactiae. Antimicrob. Agents Chemother. 2016, 60, 1702–1707. [Google Scholar] [CrossRef]
- Sukalić, T.; Đuričić, D.; Pavljak, I.; Končurat, A.; Cvetnić, Ž.; Grbavac, J.; Bačanek, B.; Jurmanović, J.; Samardžija, M. Antimicrobial susceptibility of bovine mastitis pathogens from northwestern Croatia in the period 2014 to 2018. Vet. Stanica 2021, 52, 149–158. [Google Scholar] [CrossRef]
- Holko, I.; Tančin, V.; Vršková, M.; Tvarožková, K. Prevalence and antimicrobial susceptibility of udder pathogens isolated from dairy cows in Slovakia. J. Dairy Res. 2019, 86, 436–439. [Google Scholar] [CrossRef]
- Hooper, D.C.; Jacoby, G.A. Mechanisms of drug resistance: Quinolone resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 12–31. [Google Scholar] [CrossRef]
- Leghari, A.; Lakho, S.A.; Khand, F.M.; Lone, S.Q.; Aleem, M.T.; Iqra, B.; Chandio, M.A.; Shah, J.M.; Lin, H.-X.; Fan, H.-J. Molecular epidemiology, characterization of virulence factors and antibiotic resistance profile of Streptococcus agalactiae isolated from dairy farms in China and Pakistan. J. Integr. Agric. 2023, 22, 1514–1528. [Google Scholar] [CrossRef]
- Gao, J.; Barkema, H.W.; Zhang, L.; Liu, G.; Deng, Z.; Cai, L.; Shan, R.; Zhang, S.; Zou, J.; Kastelic, J.P. Incidence of clinical mastitis and distribution of pathogens on large Chinese dairy farms. J. Dairy Sci. 2017, 100, 4797–4806. [Google Scholar] [CrossRef]
- Ruegg, P.; Oliveira, L.; Jin, W.; Okwumabua, O. Phenotypic antimicrobial susceptibility and occurrence of selected resistance genes in gram-positive mastitis pathogens isolated from Wisconsin dairy cows. J. Dairy Sci. 2015, 98, 4521–4534. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Metcalf, B.J.; Knipe, K.M.; Ouattara, M.; McGee, L.; Shewmaker, P.L.; Glennen, A.; Nichols, M.; Harris, C.; Brimmage, M. vanG element insertions within a conserved chromosomal site conferring vancomycin resistance to Streptococcus agalactiae and Streptococcus anginosus. MBio 2014, 5, e01386-14. [Google Scholar] [CrossRef] [PubMed]
- Ikiz, S.; Başaran, B.; Bingöl, E.B.; Çetin, Ö.; Kaşikçi, G.; Özgür, N.Y.; Ucmak, M.; Yilmaz, Ö.; Gündüz, M.C.; Sabuncu, A. Presence and antibiotic susceptibility patterns of contagious mastitis agents (Staphylococcus aureus and Streptococcus agalactiae) isolated from milks of dairy cows with subclinical mastitis. Turk. J. Vet. Anim. Sci. 2013, 37, 569–574. [Google Scholar] [CrossRef]
- Naranjo-Lucena, A.; Slowey, R. Invited review: Antimicrobial resistance in bovine mastitis pathogens: A review of genetic determinants and prevalence of resistance in European countries. J. Dairy Sci. 2023, 106, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Chohan, L.; Hollier, L.M.; Bishop, K.; Kilpatrick, C.C. Patterns of antibiotic resistance among group B streptococcus isolates: 2001–2004. Infect. Dis. Obstet. Gynecol. 2006, 2006, 57492. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, A.; Koops, W.; Wemmenhove, H. Antibiotic use in dairy herds in the Netherlands from 2005 to 2012. J. Dairy Sci. 2016, 99, 1632–1648. [Google Scholar] [CrossRef] [PubMed]
- Almansour, A.M.; Alhadlaq, M.A.; Alzahrani, K.O.; Mukhtar, L.E.; Alharbi, A.L.; Alajel, S.M. The Silent Threat: Antimicrobial-Resistant Pathogens in Food-Producing Animals and Their Impact on Public Health. Microorganisms 2023, 11, 2127. [Google Scholar] [CrossRef] [PubMed]
- Botelho, A.C.; Ferreira, A.F.; Fracalanzza, S.E.; Teixeira, L.M.; Pinto, T.C. A perspective on the potential zoonotic role of Streptococcus agalactiae: Searching for a missing link in alternative transmission routes. Front. Microbiol. 2018, 9, 608. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, U.B.S.; Klaas, I.C.; Boes, J.; Farre, M. The distribution of clones of Streptococcus agalactiae (group B streptococci) among herdspersons and dairy cows demonstrates lack of host specificity for some lineages. Vet. Microbiol. 2019, 235, 71–79. [Google Scholar] [CrossRef]
- Crestani, C.; Forde, T.L.; Lycett, S.J.; Holmes, M.A.; Fasth, C.; Persson-Waller, K.; Zadoks, R.N. The fall and rise of group B Streptococcus in dairy cattle: Reintroduction due to human-to-cattle host jumps? Microb. Genom. 2021, 7, 000648. [Google Scholar] [CrossRef]

| Herds | Province | Year | Herd Size 1 | Number of Strains | Number of Cases | Number of Clinical Cases | Number of Subclinical Cases |
|---|---|---|---|---|---|---|---|
| Total strains/Cases | 100 | 58 | 21 | 37 | |||
| A | Saraburi | 2018–2019 | L | 31 | 22 | 12 | 10 |
| B | Nakorn-Ratsima | 2016 | M | 1 | 1 | 0 | 1 |
| C | Saraburi | 2017 | S | 1 | 1 | 0 | 1 |
| D | Nakorn-Ratsima | 2016–2017 | M | 9 | 4 | 0 | 4 |
| E | Saraburi | 2018 | S | 1 | 1 | 1 | 0 |
| F | Nakorn-Ratsima | 2016–2017 | M | 18 | 6 | 2 | 4 |
| G | Nakorn-Ratsima | 2017 | S | 3 | 3 | 0 | 3 |
| H | Saraburi | 2017 | M | 3 | 2 | 0 | 2 |
| I | Saraburi | 2017 | S | 8 | 3 | 0 | 3 |
| J | Saraburi | 2016 | M | 15 | 8 | 2 | 6 |
| K | Saraburi | 2017 | M | 4 | 2 | 0 | 2 |
| L | Ratburi | 2019 | L | 5 | 4 | 4 | 0 |
| M | Nakorn-Ratsima | 2019 | M | 1 | 1 | 0 | 1 |
| Function | Gene | Sequence Forward (5′ to 3′) | Tm (°C) | Sequence Reverse (5′ to 3′) | Tm (°C) |
|---|---|---|---|---|---|
| Adhesion | bibA | AATCGAAAACAACGTTGGAAAG | 52.3 | AAACCAGGCTTCATCAGTCATT | 54.7 |
| bca | CTACAATTCCAGGGAGTGCA | 54.5 | ACTTTCTTCCGTCCACTTAG | 51.8 | |
| bac | AAGCAACTAGAAGAGGAAGC | 52.1 | TTCTGCTCTGGTGTTTTAGG | 52.3 | |
| Invasion | fbsA | GTCACCTTGACTAGAGTGATTAT | 51.6 | CCAAGTAGGTCAACTTATAGGGA | 53.4 |
| fbsB | TCTGTCCAACAGCCGGCTCC | 62.3 | TTCCGCAGTTGTTACACCGGC | 60.5 | |
| PI-1 | AACAATAGTGGCGGGGTCAACTG | 59.6 | TTTCGCTGGGCGTTCTTGTGAC | 60.4 | |
| PI-2a | CACGTGTCGCATCTTTTTGGTTGC | 59.6 | AACACTTGCTCCAGCAGGATTTGC | 60.4 | |
| PI-2b | AGGAGATGGAGCCACTGATACGAC | 59.9 | ACGACGACGAGCAACAAGCAC | 60.4 | |
| Infection and Tissue damage | cfb | AAGCGTGTATTCCAGATTTCC | 52.9 | AGACTTCATTGCGTGCCAAC | 56.2 |
| cyl | ACGGCTTGAACGACGTGACTAT | 58.6 | CACCAATTGGCAGAGCCT | 55.6 |
| Profiles | Virulence Factors | Number of Strains | Herds | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adherence | Invasion and Colonize | Infection | ||||||||||
| bibA | bca | bac | fbsA | fbsB | PI-1 | PI-2a | PI-2b | cfb | cyl | |||
| ATCC 12400CPS type Ia | + | − | − | + | + | − | − | + | + | + | Reference | |
| ATCC 13813CPS type II | + | − | − | + | + | − | + | + | + | + | Reference | |
| ATCC 31475CPS type III | + | + | + | + | + | + | + | − | + | + | Reference | |
| A | + | − | − | + | + | − | − | + | + | + | 51 | A–M |
| B | + | − | − | + | + | − | − | − | + | + | 45 | A–M |
| C | + | − | − | + | + | − | − | + | + | − | 2 | A and F |
| D | + | − | − | − | + | − | − | − | + | + | 1 | J |
| E | + | − | − | − | + | − | − | − | + | − | 1 | A |
| Total | 100 | 0 | 0 | 98 | 100 | 0 | 0 | 53 | 100 | 97 | 100 | |
| Multidrug Resistance Profile | Virulence Profiles | Number of Strains | Herds (n) |
|---|---|---|---|
| Penicillin, Ampicillin, Kanamycin, Gentamicin, Sulfamethoxazole-Trimethoprim | A | 1 | F (1) |
| Kanamycin, Gentamicin, Sulfamethoxazole-Trimethoprim, Tetracycline | A (3) and B (1) | 4 | C (1), D (1), K (2) |
| Kanamycin, gentamicin, tetracycline, vancomycin | B | 1 | D (1) |
| Kanamycin, gentamicin, erythromycin, vancomycin | B | 1 | D (1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wataradee, S.; Boonserm, T.; Samngamnim, S.; Ajariyakhajorn, K. Characterization of Virulence Factors and Antimicrobial Susceptibility of Streptococcus agalactiae Associated with Bovine Mastitis Cases in Thailand. Animals 2024, 14, 447. https://doi.org/10.3390/ani14030447
Wataradee S, Boonserm T, Samngamnim S, Ajariyakhajorn K. Characterization of Virulence Factors and Antimicrobial Susceptibility of Streptococcus agalactiae Associated with Bovine Mastitis Cases in Thailand. Animals. 2024; 14(3):447. https://doi.org/10.3390/ani14030447
Chicago/Turabian StyleWataradee, Sirirat, Thanasak Boonserm, Sukuma Samngamnim, and Kittisak Ajariyakhajorn. 2024. "Characterization of Virulence Factors and Antimicrobial Susceptibility of Streptococcus agalactiae Associated with Bovine Mastitis Cases in Thailand" Animals 14, no. 3: 447. https://doi.org/10.3390/ani14030447





