Effects of Garlic Oil and Cinnamaldehyde on Sheep Rumen Fermentation and Microbial Populations in Rusitec Fermenters in Two Different Sampling Periods
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Donor Animals and Diet
2.2. Additives
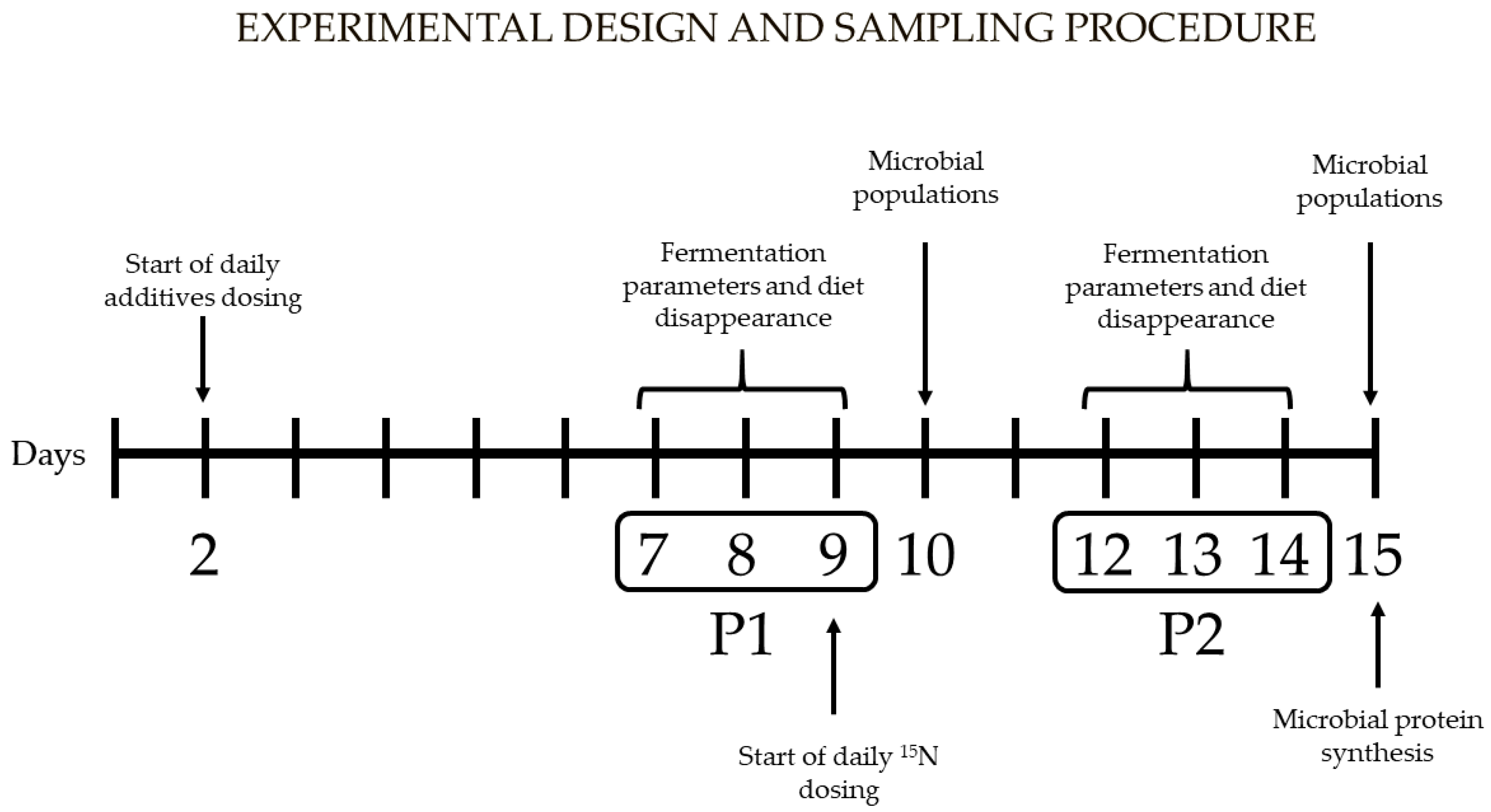
2.3. Experimental Procedures
2.4. Analysis of Bacterial Diversity and Characterization of Microbial Populations
2.5. Analytical Procedures
2.6. Calculations and Statistical Analyses
3. Results
3.1. Diet Disappearance, Rumen Fermentation Parameters, and Enzymatic Activity
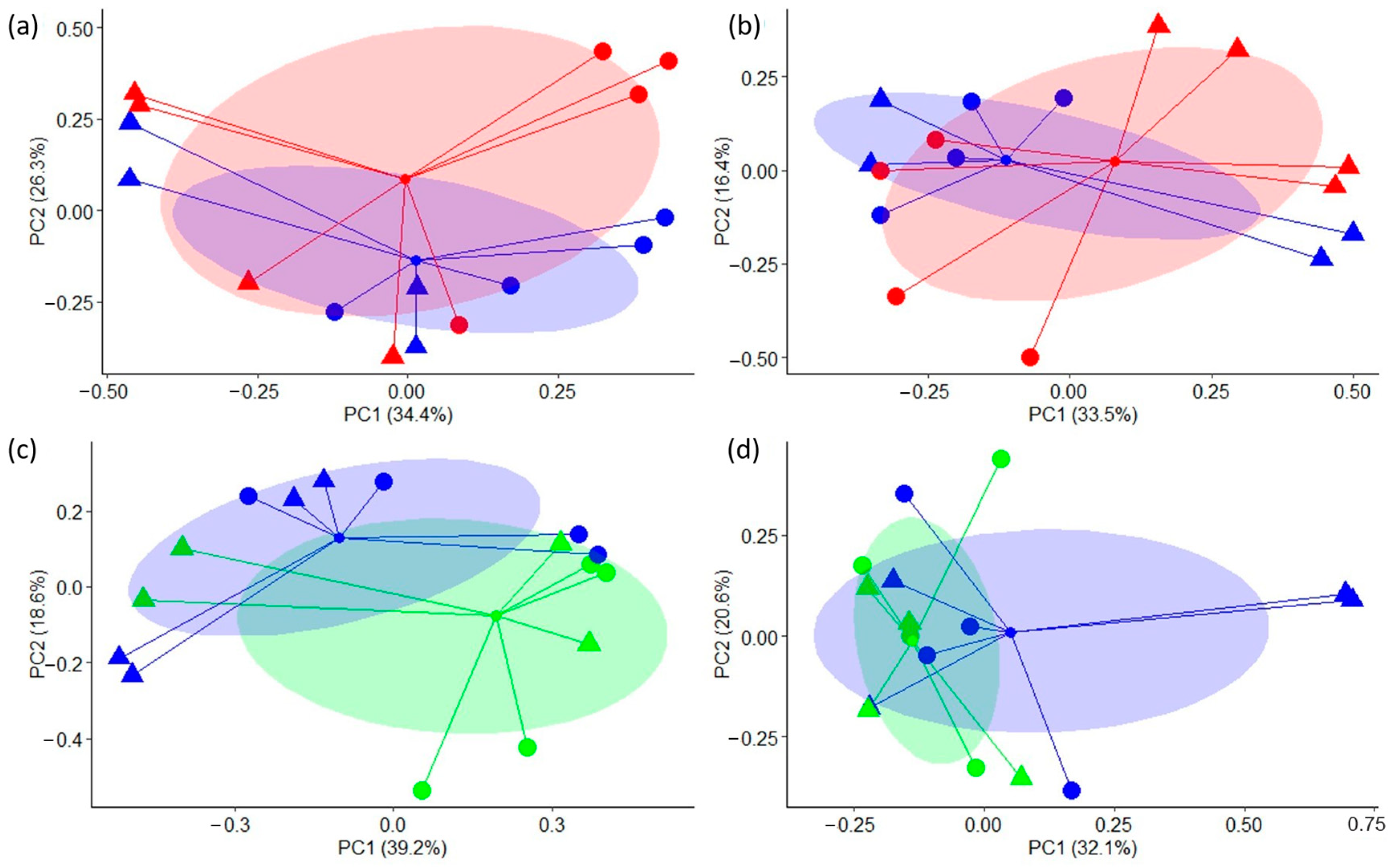
3.2. Microbial Protein Synthesis (MPS), Bacterial Diversity, and Microbial Populations
4. Discussion
4.1. Garlic Oil
4.2. Cinnamaldehyde
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sari, N.F.; Ray, P.; Rymer, C.; Kliem, K.E.; Stergiadis, S. Garlic and Its Bioactive Compounds: Implications for Methane Emissions and Ruminant Nutrition. Animals 2022, 12, 2998. [Google Scholar] [CrossRef] [PubMed]
- Cieslak, A.; Szumacher-Strabel, M.; Stochmal, A.; Oleszek, W. Plant components with specific activities against rumen methanogens. Animal 2013, 7, 253–265. [Google Scholar] [CrossRef]
- Calsamiglia, S.; Busquet, M.; Cardozo, P.W.; Castillejos, L.; Ferret, A. Invited review: Essential oils as modifiers of rumen microbial fermentation. J. Dairy Sci. 2007, 90, 2580–2595. [Google Scholar] [CrossRef] [PubMed]
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H.B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Ao, C.; Zhang, X. Potential use of garlic products in ruminant feeding: A review. Anim. Nutr. 2023, 14, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Meng, X.; Li, Y.; Zhao, C.N.; Tang, G.Y.; Li, H.B. Antibacterial and Antifungal Activities of Spices. Int. J. Mol. Sci. 2017, 18, 1283. [Google Scholar] [CrossRef] [PubMed]
- Yasin, G.; Jasin, S.A.; Mahmudiono, T.; Al-shawi, S.G.; Shichiyakh, R.A.; Shoukat, S.; Kadhim, A.J.; Iswanto, A.H.; Saleh, M.M.; Fenjan, M. Investigating the effect of garlic (Allium sativum) essential oil on foodborne pathogenic microorganisms. Food Sci. Technol. 2022, 42, e03822. [Google Scholar] [CrossRef]
- Soliva, C.R.; Amelchanka, S.L.; Duval, S.M.; Kreuzer, M. Ruminal methane inhibition potential of various pure compounds in comparison with garlic oil as determined with a rumen simulation technique (Rusitec). Br. J. Nutr. 2011, 106, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Busquet, M.; Calsamiglia, S.; Ferret, A.; Carro, M.D.; Kamel, C. Effect of garlic oil and four of its compounds on rumen microbial fermentation. J. Dairy Sci. 2005, 88, 4393–4404. [Google Scholar] [CrossRef]
- Busquet, M.; Calsamiglia, S.; Ferret, A.; Cardozo, P.W.; Kamel, C. Effects of cinnamaldehyde and garlic oil on rumen microbial fermentation in a dual flow continuous culture. J. Dairy Sci. 2005, 88, 2508–2516. [Google Scholar] [CrossRef]
- Mateos, I.; Ranilla, M.J.; Tejido, M.L.; Saro, C.; Kamel, C.; Carro, M.D. The influence of diet type (dairy versus intensive fattening) on the effectiveness of garlic oil and cinnamaldehyde to manipulate in vitro ruminal fermentation and methane production. Anim. Prod. Sci. 2013, 53, 299–307. [Google Scholar] [CrossRef]
- Singh, R.K.; Dey, A.; Thakur, S.; Singh, M.; Lailer, P.C. Modulation of Murrah Buffalo (Bubalus bubalis) Rumen Functions for In Vitro Fatty Acid Bio-Hydrogenation, Methane Production and Fermentation Pattern of Total Mixed Ration Supplemented with Allium sativum (Garlic) Essential Oils. Fermentation 2023, 9, 615. [Google Scholar] [CrossRef]
- Patra, A.K.; Yu, Z. Effects of essential oils on methane production and fermentation by, and abundance and diversity of, rumen microbial populations. Appl. Environ. Microbiol. 2012, 78, 4271–4280. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Yu, Z. Effects of garlic oil, nitrate, saponin and their combinations supplemented to different substrates on in vitro fermentation, ruminal methanogenesis, and abundance and diversity of microbial populations. J. Appl. Microbiol. 2015, 119, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Yu, Z. Effects of adaptation of in vitro rumen culture to garlic oil, nitrate, and saponin and their combinations on methanogenesis, fermentation, and abundances and diversity of microbial populations. Front. Microbiol. 2015, 6, 1434. [Google Scholar] [CrossRef] [PubMed]
- Anassori, E.; Dalir-Naghadeh, B.; Pirmohammadi, R.; Taghizadeh, A.; Asri-Rezaei, S.; Maham, M.; Farahmand-Azar, S.; Farhoomand, P. Garlic: A potential alternative for monensin as a rumen modifier. Livest. Sci. 2011, 142, 276–287. [Google Scholar] [CrossRef]
- Chapman, C.E.; Ort, S.B.; Aragona, K.M.; Cabral, R.G.; Erickson, P.S. Effect of cinnamaldehyde on feed intake, rumen fermentation, and nutrient digestibility, in lactating dairy cows. J. Anim. Sci. 2019, 97, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Ishlak, A.; Günal, M.; AbuGhazaleh, A.A. The effects of cinnamaldehyde, monensin and quebracho condensed tannin on rumen fermentation, biohydrogenation and bacteria in continuous culture system. Anim. Feed Sci. Technol. 2015, 207, 31–40. [Google Scholar] [CrossRef]
- Cantet, J.M.; Zhantao, Y.; Tucker, H.A.; Ríus, A.G. A cinnamaldehyde feed additive improved feed use-efficiency in lactating dairy cows. Livest. Sci. 2023, 272, 105236. [Google Scholar] [CrossRef]
- Cattani, M.; Maccarana, L.; Rossi, G.; Tagliapietra, F.; Schiavon, S.; Bailoni, L. Dose-response and inclusion effects of pure natural extracts and synthetic compounds on in vitro methane production. Anim. Feed Sci. Technol. 2016, 218, 100–109. [Google Scholar] [CrossRef]
- Cardozo, P.; Calsamiglia, S.; Ferret, A.; Kamel, C. Effects of natural plant extracts on protein degradation and fermentation profiles in continuous culture. J. Anim. Sci. 2004, 82, 3230–3236. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.E.; Ranilla, M.J.; Ramos, S.; Tejido, M.L.; Carro, M.D. Effects of dilution rate and retention time of concentrate on efficiency of microbial growth, methane production, and ruminal fermentation in Rusitec fermenters. J. Dairy Sci. 2009, 92, 3930–3938. [Google Scholar] [CrossRef]
- McDougall, E.I. Studies on ruminant saliva I. The composition and output of sheep’s saliva. Biochem. J. 1948, 43, 99–109. [Google Scholar] [CrossRef]
- Ranilla, M.J.; López, S.; Giráldez, F.J.; Valdés, C.; Carro, M.D. Comparative digestibility and digesta flow kinetics in two breeds of sheep. Anim. Sci. 1998, 66, 389–396. [Google Scholar] [CrossRef]
- Carro, M.D.; Miller, E.L. Effect of supplementing a fibre basal diet with different nitrogen forms on ruminal fermentation and microbial growth in an in vitro semi-continuous culture system (RUSITEC). Br. J. Nutr. 1999, 82, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.; Tejido, M.L.; Ranilla, M.J.; Martínez, M.E.; Saro, C.; Carro, M.D. Influence of detachment procedure and diet on recovery of solid-associated bacteria from sheep ruminal digesta and representativeness of bacterial isolates as assessed by automated ribosomal intergenic spacer analysis-polymerase chain reaction. J. Dairy Sci. 2009, 92, 5659–5668. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Morrison, M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. BioTechniques 2004, 36, 808–812. [Google Scholar] [CrossRef]
- Danovaro, R.; Luna, G.M.; Dell’Anno, A.; Pietrangeli, B. Comparison of two fingerprinting techniques, terminal restriction fragment length polymorphism and automated ribosomal intergenic spacer analysis, for determination of bacterial diversity in aquatic environments. Appl. Environ. Microbiol. 2006, 72, 5982–5989. [Google Scholar] [CrossRef]
- Saro, C.; Ranilla, M.J.; Cifuentes, A.; Rosselló-Mora, R.; Carro, M.D. Technical note: Comparison of automated ribosomal intergenic spacer analysis (ARISA) and denaturing gradient gel electrophoresis (DGGE) to assess bacterial diversity in the rumen of sheep. J. Anim. Sci. 2014, 92, 1083–1088. [Google Scholar] [CrossRef][Green Version]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; R Package Version 2.5-5; 2019. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Denman, S.E.; McSweeney, C.S. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol. Ecol. 2006, 58, 572–582. [Google Scholar] [CrossRef]
- Sylvester, J.T.; Karnati, S.K.R.; Yu, Z.; Morrison, M.; Firkins, J.L. Development of an assay to quantify rumen ciliate protozoal biomass in cows using real-time PCR. J. Nutr. 2004, 134, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- Denman, S.E.; Tomkins, N.W.; McSweeney, C.S. Quantitation and diversity analysis of ruminal methanogenic populations in response to the antimethanogenic compound bromochloromethane. FEMS Microbiol. Ecol. 2007, 62, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Koike, S.; Kobayashi, Y. Development and use of competitive PCR assays for the rumen cellulolytic bacteria: Fibrobacter succinogenes, Ruminococcus albus and Ruminococcus flavefaciens. FEMS Microbiol. Lett. 2001, 204, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Saro, C.; Ranilla, M.J.; Carro, M.D. Postprandial changes of fiber-degrading microbes in the rumen of sheep fed diets varying in type of forage as monitored by real-time PCR and automated ribosomal intergenic spacer analysis. J. Anim. Sci. 2012, 90, 4487–4494. [Google Scholar] [CrossRef] [PubMed][Green Version]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.E.; Ranilla, M.J.; Tejido, M.L.; Ramos, S.; Carro, M.D. Comparison of fermentation of diets of variable composition and microbial populations in the rumen of sheep and Rusitec fermenters. I. Digestibility, fermentation parameters, and microbial growth. J. Dairy Sci. 2010, 93, 3684–3698. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.-E.; Ranilla, M.J.; Tejido, M.L.; Saro, C.; Carro, M.D. The effect of the diet fed to donor sheep on in vitro methane production and ruminal fermentation of diets of variable composition. Anim. Feed Sci. Technol. 2010, 158, 126–135. [Google Scholar] [CrossRef]
- Giraldo, L.A.; Ranilla, M.J.; Tejido, M.L.; Carro, M.D. Influence of exogenous fibrolytic enzymes and fumarate on methane production, microbial growth and fermentation in Rusitec fermenters. Br. J. Nutr. 2007, 98, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Demeyer, D.I. Quantitative aspects of microbial metabolism in the rumen and hindgut. In Rumen Microbial Metabolism and Ruminant Digestion, 1st ed.; INRA Editions: Paris, France, 1991; pp. 217–237. [Google Scholar]
- Dey, A.; Paul, S.S.; Lailer, P.C.; Dahiya, S.S. Reducing enteric methane production from buffalo (Bubalus bubalis) by garlic oil supplementation in in vitro rumen fermentation system. SN Appl. Sci. 2021, 3, 187. [Google Scholar] [CrossRef]
- Patra, A.K.; Kamra, D.N.; Agarwal, N. Effects of extracts of spices on rumen methanogenesis, enzyme activities and fermentation of feeds in vitro. J. Sci. Food Agric. 2010, 90, 511–520. [Google Scholar] [CrossRef]
- Belanche, A.; de la Fuente, G.; Newbold, J.C. Study of methanogen communities associated with different rumen protozoal populations. FEMS Microbiol. Ecol. 2014, 90, 663–677. [Google Scholar] [CrossRef]
- Tan, C.; Ramírez-Restrepo, C.A.; Shah, A.M.; Hu, R.; Bell, M.; Wang, Z.; McSweeney, C. The community structure and microbial linkage of rumen protozoa and methanogens in response to the addition of tea seed saponins in the diet of beef cattle. J. Anim. Sci. Biotechnol. 2020, 11, 80. [Google Scholar] [CrossRef]
- Ungerfeld, E.M. Limits to dihydrogen incorporation into electron sinks alternative to methanogenesis in ruminal fermentation. Front. Microbiol. 2015, 6, 1272. [Google Scholar] [CrossRef]
- Tager, L.R.; Krause, K.M. Effects of cinnamaldehyde, eugenol, and capsicum on fermentation of a corn-based dairy ration in continuous culture. Can. J. Anim. Sci. 2010, 90, 413–420. [Google Scholar] [CrossRef]
- Şahan, Z. Bacteriolytic activity of ruminal protozoa is affected by rate and type of common essential oils: Effect of thyme oil. S. Afr. J. Anim. Sci. 2023, 53, 589–597. [Google Scholar] [CrossRef]
- Khorrami, B.; Vakili, A.R.; Mesgaran, M.D.; Klevenhusen, F. Thyme and cinnamon essential oils: Potential alternatives for monensin as a rumen modifier in beef production systems. Anim. Feed Sci. Technol. 2015, 200, 8–16. [Google Scholar] [CrossRef]
- Cobellis, G.; Trabalza-Marinucci, M.; Marcotullio, M.C.; Yu, Z. Evaluation of different essential oils in modulating methane and ammonia production, rumen fermentation, and rumen bacteria in vitro. Anim. Feed Sci. Technol. 2016, 215, 25–36. [Google Scholar] [CrossRef]
| Item | g/kg DM 1 |
|---|---|
| Ingredients | |
| Alfalfa hay | 500 |
| Barley | 199 |
| Maize Soybean meal | 96.0 |
| 71.0 | |
| Lupins | 60.0 |
| Oat | 31.5 |
| Full-fat soybean | 15.0 |
| Calcium carbonate (CaCO3) | 6.9 |
| Sugarcane molasses | 5.0 |
| NaCl | 3.5 |
| Dicalcium phosphate (CaHPO4) | 2.1 |
| Mineral/vitamin premix 2 | 10.0 |
| Chemical composition | |
| Organic matter | 935 |
| Crude protein | 176 |
| Neutral detergent fibre 3 | 368 |
| Acid detergent fibre 3 | 162 |
| Ether extract | 26 |
| Non-structural carbohydrates | 355 |
| Item | Treatment | SEM 4 | p-Value | ||
|---|---|---|---|---|---|
| CON 1 | GO 2 | CIN 3 | |||
| Diet disappearance (g/kg DM 5) | |||||
| Dry matter | 640 | 622 | 640 | 10.9 | 0.08 |
| Organic matter | 633 | 614 | 632 | 11.2 | 0.09 |
| Neutral detergent fibre | 457 | 439 | 465 | 16.2 | 0.17 |
| Acid detergent fibre | 302 | 249 * | 302 | 16.9 | <0.001 |
| pH | 6.40 | 6.51 | 6.45 | 0.078 | 0.24 |
| NH3-N 6 (mg/day) | 234 | 221 | 215 | 9.9 | 0.07 |
| Total VFA 7 (mmol/day) | 113 | 106 | 109 | 3.8 | 0.10 |
| Molar proportions (mol/100 mol) | |||||
| Acetate | 55.2 | 50.8 * | 55.7 | 0.84 | <0.001 |
| Propionate | 16.0 | 19.1 * | 15.3 | 0.61 | <0.001 |
| Butyrate | 16.4 | 15.0 * | 16.2 | 0.38 | 0.002 |
| Isobutyrate | 1.19 | 1.32 | 1.16 | 0.090 | 0.08 |
| Valerate | 4.90 | 6.61 | 4.61 | 0.315 | <0.001 |
| Isovalerate | 3.27 | 2.37 * | 3.67 | 0.271 | <0.001 |
| Caproate | 3.06 | 4.80 * | 3.40 | 0.261 | <0.001 |
| Acetate/propionate | 3.46 | 2.70 * | 3.64 | 0.111 | <0.001 |
| Methane (mmol/day) | 23.1 | 16.6 * | 26.3 | 1.59 | <0.001 |
| Methane/VFA (mol/mol) | 0.20 | 0.17 * | 0.24 * | 0.017 | <0.001 |
| Enzymatic activity 8 | |||||
| Amylase | 285 | 305 | 315 | 30.3 | 0.55 |
| Xylanase | 696 | 673 | 642 | 42.4 | 0.67 |
| Endoglucanase | 84.0 | 98.9 | 96.9 | 8.76 | 0.49 |
| Item | Treatment | SEM 4 | p-Value | ||
|---|---|---|---|---|---|
| CON 1 | GO 2 | CIN 3 | |||
| Diet disappearance (g/kg DM 5) | |||||
| Dry matter | 619 | 623 | 628 | 13.1 | 0.73 |
| Organic matter | 615 | 617 | 621 | 13.1 | 0.82 |
| Neutral detergent fibre | 459 | 446 | 473 | 16.7 | 0.15 |
| Acid detergent fibre | 277 | 274 | 327 * | 22.5 | 0.01 |
| pH | 6.51 | 6.51 | 6.49 | 0.082 | 0.93 |
| NH3-N 6 (mg/day) | 210 | 221 | 195 | 9.8 | 0.01 |
| Total VFA 7 (mmol/day) | 103 | 100 | 98 | 3.7 | 0.31 |
| Molar proportions (mol/100 mol) | |||||
| Acetate | 54.5 | 53.6 * | 53.7 | 0.45 | 0.01 |
| Propionate | 14.9 | 17.1 * | 13.4 * | 0.37 | <0.001 |
| Butyrate | 17.2 | 14.6 * | 17.6 | 0.48 | <0.001 |
| Isobutyrate | 1.22 | 1.32 * | 1.23 | 0.035 | <0.01 |
| Valerate | 4.85 | 5.86 * | 4.82 | 0.170 | <0.001 |
| Isovalerate | 4.30 | 2.62 * | 5.99 * | 0.418 | <0.001 |
| Caproate | 3.06 | 4.90 * | 3.30 | 0.271 | <0.001 |
| Acetate/propionate | 3.67 | 3.14 * | 4.01 * | 0.096 | <0.001 |
| Methane (mmol/day) | 27.5 | 24.5 * | 27.4 | 0.98 | <0.001 |
| Methane/VFA (mol/mol) | 0.27 | 0.24 * | 0.28 | 0.007 | <0.001 |
| Enzymatic activity 8 | |||||
| Amylase | 259 | 242 | 232 | 25.5 | 0.75 |
| Xylanase | 491 | 511 | 443 | 25.0 | 0.17 |
| Endoglucanase | 67.6 | 63.7 | 61.9 | 3.17 | 0.22 |
| Item | Treatment | SEM 4 | p-Value | ||
|---|---|---|---|---|---|
| CON 1 | GO 2 | CIN 3 | |||
| Microbial protein synthesis (mg N/day) | |||||
| Solid | 128 | 137 | 160 * | 6.0 | 0.02 |
| Liquid | 110 | 110 | 128 | 5.0 | 0.05 |
| Total | 238 | 247 | 288 * | 5.6 | <0.001 |
| Efficiency of microbial growth 5 | 27.5 | 30.5 | 35.5 * | 0.99 | <0.01 |
| Phase | Item | Treatment | SEM 4 | p-Value | ||
|---|---|---|---|---|---|---|
| CON 1 | GO 2 | CIN 3 | ||||
| Solid | Number of peaks | 35.5 | 37.8 | 39.0 | 2.00 | 0.91 |
| Shannon index | 3.57 | 3.63 | 3.64 | 0.053 | 0.98 | |
| Total bacteria (μg DNA/g DM 5) | 1900 | 1349 | 3138 | 911.8 | 0.41 | |
| Total protozoa (μg DNA/g DM 5) | 28.95 | 13.65 * | 7.55 * | 3.333 | <0.01 | |
| Fibrobacter succinogenes 6 | 10.41 | 11.31 | 9.50 | 3.888 | 0.95 | |
| Ruminococcus flavefaciens 6 | 0.14 | 0.14 | 0.19 | 0.041 | 0.67 | |
| Ruminococcus albus 6 | 10.29 | 18.64 | 13.72 | 4.520 | 0.46 | |
| Fungi 6 | 3.09 | 0.675 * | 1.94 | 0.442 | 0.02 | |
| Archaea 6 | 0.37 | 0.20 | 0.52 | 0.090 | 0.10 | |
| Liquid | Number of peaks | 41.5 | 36.0 | 51.3 * | 1.75 | 0.001 |
| Shannon index | 3.72 | 3.58 | 3.93 * | 0.043 | 0.002 | |
| Total bacteria (μg DNA/mL) | 115 | 1160.02 | 136 | 10.0 | 0.33 | |
| Total protozoa (μg DNA/mL) | 30.47 | 3.16 * | 13.93 | 5.390 | 0.03 | |
| Fungi 6 | 0.88 | 0.02 | 0.25 | 0.217 | 0.06 | |
| Archaea 6 | 0.24 | 0.07 * | 0.24 | 0.034 | 0.01 | |
| Phase | Item | Treatment | SEM 4 | p-Value | ||
|---|---|---|---|---|---|---|
| CON 1 | GO 2 | CIN 3 | ||||
| Solid | Number of peaks | 37.8 | 35.5 | 41.3 | 1.77 | 0.14 |
| Shannon index | 3.63 | 3.57 | 3.71 | 0.045 | 0.14 | |
| Total bacteria (μg DNA/g DM 5) | 2243 | 1564 | 1111 * | 258.0 | 0.047 | |
| Total protozoa (μg DNA/g DM 5) | 27.54 | 0.93 | 2.82 | 8.069 | 0.09 | |
| Fibrobacter succinogenes 6 | 4.60 | 0.44 * | 2.68 | 0.527 | 0.01 | |
| Ruminococcus flavefaciens 6 | 0.17 | 0.23 | 0.17 | 0.032 | 0.37 | |
| Ruminococcus albus 6 | 14.37 | 21.89 | 8.28 | 2.188 | 0.01 | |
| Fungi 6 | 5.45 | 0.03 | 29.77 | 10.54 | 0.18 | |
| Archaea 6 | 0.32 | 0.10 | 0.65 | 0.103 | 0.02 | |
| Liquid | Number of peaks | 41.0 | 45.5 | 51.5 | 3.05 | 0.21 |
| Shannon index | 3.69 | 3.81 | 3.94 | 0.074 | 0.20 | |
| Total bacteria (μg DNA/mL) | 101 | 138 | 85 | 13.5 | 0.07 | |
| Total protozoa (μg DNA/mL) | 18.82 | 1.04 | 3.53 * | 3.645 | 0.049 | |
| Fungi 6 | 0.31 | 0.00 | 0.07 | 0.112 | 0.19 | |
| Archaea 6 | 0.17 | 0.08 | 0.25 | 0.040 | 0.06 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Rodríguez, J.; Saro, C.; Mateos, I.; Carro, M.D.; Ranilla, M.J. Effects of Garlic Oil and Cinnamaldehyde on Sheep Rumen Fermentation and Microbial Populations in Rusitec Fermenters in Two Different Sampling Periods. Animals 2024, 14, 1067. https://doi.org/10.3390/ani14071067
García-Rodríguez J, Saro C, Mateos I, Carro MD, Ranilla MJ. Effects of Garlic Oil and Cinnamaldehyde on Sheep Rumen Fermentation and Microbial Populations in Rusitec Fermenters in Two Different Sampling Periods. Animals. 2024; 14(7):1067. https://doi.org/10.3390/ani14071067
Chicago/Turabian StyleGarcía-Rodríguez, Jairo, Cristina Saro, Iván Mateos, María Dolores Carro, and María José Ranilla. 2024. "Effects of Garlic Oil and Cinnamaldehyde on Sheep Rumen Fermentation and Microbial Populations in Rusitec Fermenters in Two Different Sampling Periods" Animals 14, no. 7: 1067. https://doi.org/10.3390/ani14071067
APA StyleGarcía-Rodríguez, J., Saro, C., Mateos, I., Carro, M. D., & Ranilla, M. J. (2024). Effects of Garlic Oil and Cinnamaldehyde on Sheep Rumen Fermentation and Microbial Populations in Rusitec Fermenters in Two Different Sampling Periods. Animals, 14(7), 1067. https://doi.org/10.3390/ani14071067







