A 3D-Printed Dummy for Training Distal Phalanx Amputation in Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Micro-Computed Tomography
2.3. Dummy Creation
2.4. Course and Survey
2.5. Survey and General Content of the Experiment
2.6. Data Analysis
3. Results
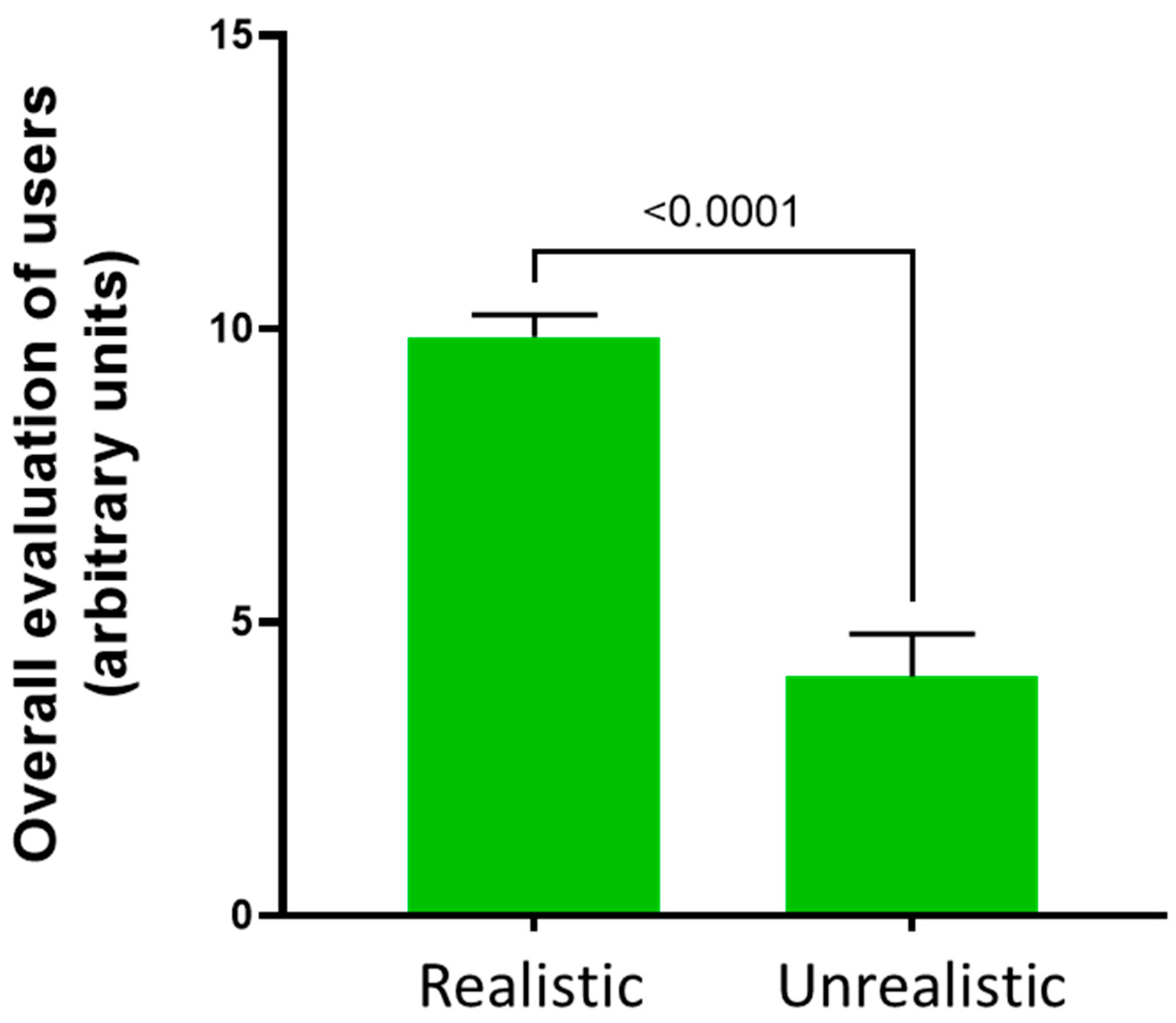
3.1. Users’ Perceptions about the Distal Phalangeal Amputation
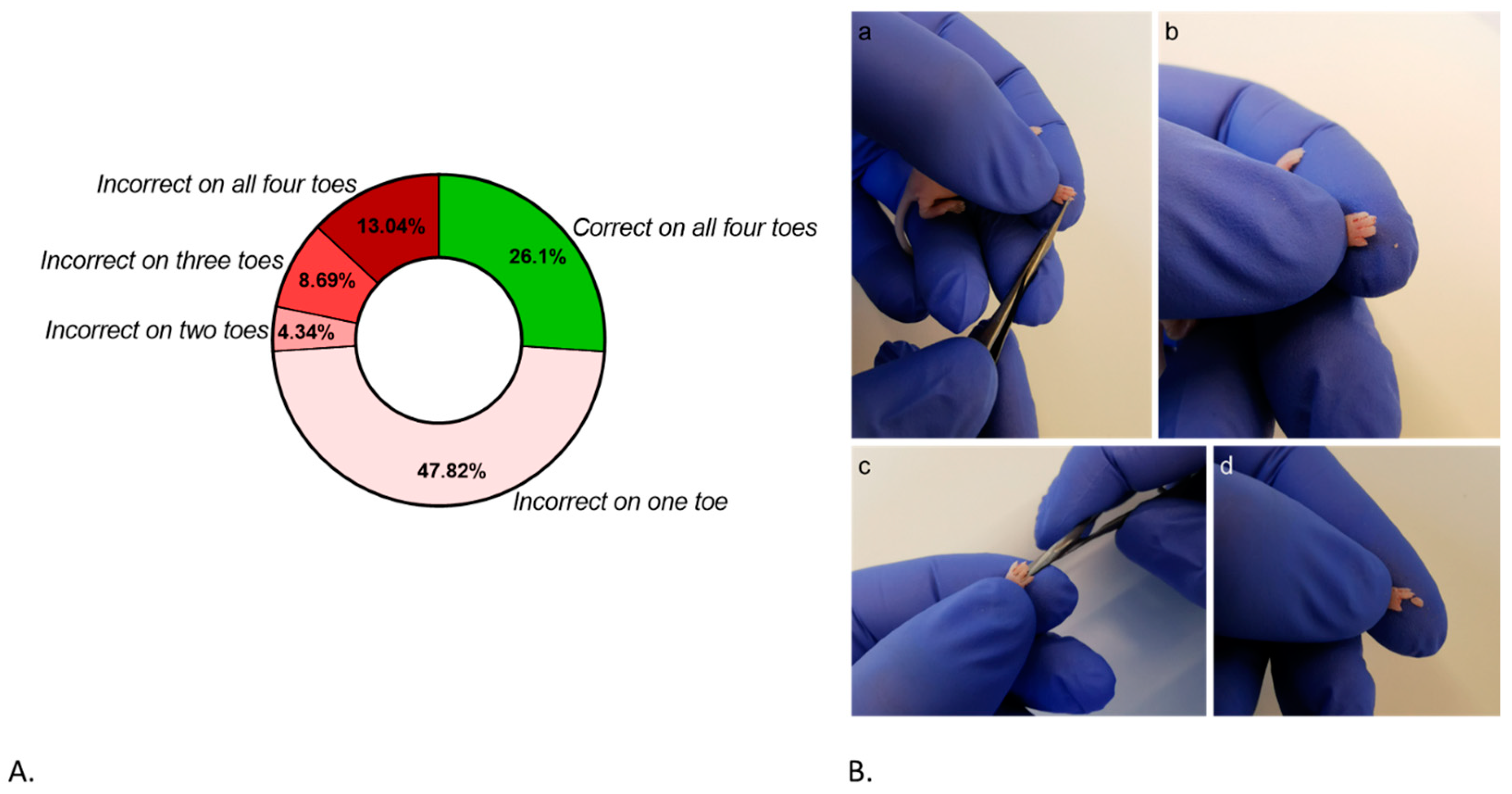
3.2. Users’ Performance on the Distal Phalangeal Amputation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matthews, G.A.; Nieh, E.H.; Vander Weele, C.M.; Halbert, S.A.; Pradhan, R.V.; Yosafat, A.S.; Glober, G.F.; Izadmehr, E.M.; Thomas, R.E.; Lacy, G.D.; et al. Dorsal Raphe Dopamine Neurons Represent the Experience of Social Isolation. Cell 2016, 164, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Schipper, L.; Harvey, L.; van der Beek, E.M.; van Dijk, G. Home alone: A systematic review and meta-analysis on the effects of individual housing on body weight, food intake and visceral fat mass in rodents. Obes. Rev. 2018, 19, 614–637. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Choi, E.Y.; Magee, D.J.; Stets, C.W.; During, M.J.; Lin, E.-J.D. Metabolic Effects of Social Isolation in Adult C57BL/6 Mice. Int. Sch. Res. Not. 2014, 2014, 690950. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Kan, L.; Ledford, B.T.; He, J.-Q. Tattooing Various Combinations of Ears, Tail, and Toes to Identify Mice Reliably and Permanently. J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 189–198. [Google Scholar] [PubMed]
- Paluch, L.-R.; Lieggi, C.C.; Dumont, M.; Monette, S.; Riedel, E.R.; Lipman, N.S. Developmental and behavioral effects of toe clipping on neonatal and preweanling mice with and without vapocoolant anesthesia. J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 132–140. [Google Scholar] [PubMed]
- Schaefer, D.C.; Asner, I.N.; Seifert, B.; Bürki, K.; Cinelli, P. Analysis of physiological and behavioural parameters in mice after toe clipping as newborns. Lab. Anim. 2010, 44, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Vachon, P. Anatomical and histological observations of fore- and hind limb toes in adult mice after amputations performed at the age of two weeks. Can. J. Vet. Res. 1998, 62, 311–313. [Google Scholar]
- Castelhano-Carlos, M.J.; Sousa, N.; Ohl, F.; Baumans, V. Identification methods in newborn C57BL/6 mice: A developmental and behavioural evaluation. Lab. Anim. 2010, 44, 88–103. [Google Scholar] [CrossRef]
- Dahlborn, K.; Bugnon, P.; Nevalainen, T.; Raspa, M.; Verbost, P.; Spangenberg, E. Report of the Federation of European Laboratory Animal Science Associations Working Group on animal identification. Lab. Anim. 2013, 47, 2–11. [Google Scholar] [CrossRef]
- Walshaw, S. Incorporating animal alternatives in a training programme in laboratory animal care and use. Altern. Lab. Anim. 2004, 32 (Suppl. S1B), 549–551. [Google Scholar] [CrossRef]
- Conarello, S.L.; Shepherd, M.J. Training strategies for research investigators and technicians. ILAR J. 2007, 48, 120–130. [Google Scholar] [CrossRef]
- EC. Summary Report on the Statistics on the Use of Animals for Scientific Purposes in the Member States of the European Union and Norway in 2020. 2023. Available online: https://circabc.europa.eu/ui/group/8ee3c69a-bccb-4f22-89ca-277e35de7c63/library/10ad28d6-e17e-4367-b459-20883402cfcc/details?download=true (accessed on 30 November 2023).
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959; pp. 238p. [Google Scholar]
- EU. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 50, 33–79. [Google Scholar]
- Akaike, M.; Fukutomi, M.; Nagamune, M.; Fujimoto, A.; Tsuji, A.; Ishida, K.; Iwata, T. Simulation-based medical education in clinical skills laboratory. J. Med. Investig. 2012, 59, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Duff, J.; Grant, E.; Kissoon, N.; Grant, V.J. Simulation in paediatrics: An educational revolution. Paediatr. Child. Health 2007, 12, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Humpenöder, M.; Corte, G.M.; Pfützner, M.; Wiegard, M.; Merle, R.; Hohlbaum, K.; Erickson, N.A.; Plendl, J.; Thöne-Reineke, C. Alternatives in Education-Rat and Mouse Simulators Evaluated from Course Trainers’ and Supervisors’ Perspective. Animals 2021, 11, 1848. [Google Scholar] [CrossRef] [PubMed]
- Micallef, J.; Broekhuyse, A.; Vuyyuru, S.; Wax, R.; Sridhar, S.K.; Heath, J.; Clarke, S.; Dubrowski, A. Application of 3D Printing in Training Health Care Providers; the Development of Diverse Facial Overlays for Simulation-Based Medical Training. Cureus 2022, 14, e26637. [Google Scholar] [CrossRef] [PubMed]
- Noyes, J.A.; Carbonneau, K.J.; Matthew, S.M. Comparative Effectiveness of Training with Simulators Versus Traditional Instruction in Veterinary Education: Meta-Analysis and Systematic Review. J. Vet. Med. Educ. 2022, 49, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.G.; Bordim, N.B.C.; Fantoni, D.T.; Villamizar-Martinez, L.A. Evaluation of 3D-Printed Dog Teeth for Pre-clinical Training of Endodontic Therapy in Veterinary Dentistry. J. Vet. Dent. 2023, 7, 08987564231210409. [Google Scholar] [CrossRef] [PubMed]
- McMenamin, P.G.; Quayle, M.R.; McHenry, C.R.; Adams, J.W. The production of anatomical teaching resources using three-dimensional (3D) printing technology. Anat. Sci. Educ. 2014, 7, 479–486. [Google Scholar] [CrossRef]
- Conigliaro, R.L.; Peterson, K.D.; Stratton, T.D. Lack of Diversity in Simulation Technology: An Educational Limitation? Simul. Healthc. 2020, 15, 112–114. [Google Scholar] [CrossRef]
- Nair, L.; Adetayo, O.A. Cultural Competence and Ethnic Diversity in Healthcare. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2219. [Google Scholar] [CrossRef] [PubMed]
- Corte, G.M.; Humpenöder, M.; Pfützner, M.; Merle, R.; Wiegard, M.; Hohlbaum, K.; Richardson, K.; Thöne-Reineke, C.; Plendl, J. Anatomical Evaluation of Rat and Mouse Simulators for Laboratory Animal Science Courses. Animals 2021, 11, 3432. [Google Scholar] [CrossRef]
- Meyer-Szary, J.; Luis, M.S.; Mikulski, S.; Patel, A.; Schulz, F.; Tretiakow, D.; Fercho, J.; Jaguszewska, K.; Frankiewicz, M.; Pawłowska, E.; et al. The Role of 3D Printing in Planning Complex Medical Procedures and Training of Medical Professionals-Cross-Sectional Multispecialty Review. Int. J. Environ. Res. Public. Health 2022, 19, 3331. [Google Scholar] [CrossRef]
- Goudie, C.; Shanahan, J.; Gill, A.; Murphy, D.; Dubrowski, A. Investigating the Efficacy of Anatomical Silicone Models Developed from a 3D Printed Mold for Perineal Repair Suturing Simulation. Cureus 2018, 10, e3181. [Google Scholar] [CrossRef]
- Hoang, D.; Perrault, D.; Stevanovic, M.; Ghiassi, A. Surgical applications of three-dimensional printing: A review of the current literature & how to get started. Ann. Transl. Med. 2016, 4, 456. [Google Scholar]
- Jin, Z.; Li, Y.; Yu, K.; Liu, L.; Fu, J.; Yao, X.; Zhang, A.; He, Y. 3D Printing of Physical Organ Models: Recent Developments and Challenges. Adv. Sci. 2021, 8, e2101394. [Google Scholar] [CrossRef]
- Oberoi, G.; Eberspächer-Schweda, M.C.; Hatamikia, S.; Königshofer, M.; Baumgartner, D.; Kramer, A.-M.; Schaffarich, P.; Agis, H.; Moscato, F.; Unger, E. 3D Printed Biomimetic Rabbit Airway Simulation Model for Nasotracheal Intubation Training. Front. Vet. Sci. 2020, 7, 587524. [Google Scholar] [CrossRef] [PubMed]
- Gyles, C. 3D printing comes to veterinary medicine. Can. Vet. J. 2019, 60, 1033–1034. [Google Scholar] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. BMJ Open Sci. 2020, 4, e100115. [Google Scholar]
- Bonaparte, D.; Cinelli, P.; Douni, E.; Hérault, Y.; Maas, A.; Pakarinen, P.; Poutanen, M.; Lafuente, M.S.; Scavizzi, F. FELASA guidelines for the refinement of methods for genotyping genetically-modified rodents: A report of the Federation of European Laboratory Animal Science Associations Working Group. Lab. Anim. 2013, 47, 134–145. [Google Scholar] [CrossRef]
- Randall, M.S.; Moody, C.M.; Turner, P.V. Mental Wellbeing in Laboratory Animal Professionals: A Cross-Sectional Study of Compassion Fatigue, Contributing Factors, and Coping Mechanisms. J. Am. Assoc. Lab. Anim. Sci. 2021, 60, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, C.M.; Kneebone, R.L.; Woloshynowych, M.; Nestel, D.; Moorthy, K.; Kidd, J.; Darzi, A. The effects of stress on surgical performance. Am. J. Surg. 2006, 191, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, C.M.; Black, S.A.; Hanna, G.B.; Athanasiou, T.F.; Kneebone, R.L.; Nestel, D.; Wolfe, J.H.N.; Woloshynowych, M. The effects of stress and coping on surgical performance during simulations. Ann. Surg. 2010, 251, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Braid, H.R. The Use of Simulators for Teaching Practical Clinical Skills to Veterinary Students—A Review. Altern. Lab. Anim. 2022, 50, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Bergmeister, K.D.; Aman, M.; Kramer, A.; Schenck, T.L.; Riedl, O.; Daeschler, S.C.; Aszmann, O.C.; Bergmeister, H.; Golriz, M.; Mehrabi, A.; et al. Simulating Surgical Skills in Animals: Systematic Review, Costs & Acceptance Analyses. Front. Vet. Sci. 2020, 7, 570852. [Google Scholar] [PubMed]
- Du, W.-Q.; Zhong, X.; Jiang, R.-Q.; Zong, Z.-W.; Jia, Y.-J.; Ye, Z.; Zhou, X.-L. Animal model-based simulation training for three emergent and urgent operations of penetrating thoracic injuries. Chin. J. Traumatol. 2023, 26, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Nuber, M.; Gonzalez-Uarquin, F.; Neufurth, M.; Brockmann, M.A.; Baumgart, J.; Baumgart, N. Development of a 3D simulator for training the mouse in utero electroporation. PLoS ONE 2022, 17, e0279004. [Google Scholar] [CrossRef] [PubMed]
- Bainier, M.; Su, A.; Redondo, R.L. 3D printed rodent skin-skull-brain model: A novel animal-free approach for neurosurgical training. PLoS ONE 2021, 16, e0253477. [Google Scholar] [CrossRef] [PubMed]
- Tevlin, R.M.; Shah, H.N.; Salhotra, A.B.; Di Iorio, S.E.S.; Griffin, M.M.; Januszyk, M.; Wan, D.C.; Longaker, M.T.M. An Inexpensive 3D Printed Mouse Model of Successful, Complication-free Long Bone Distraction Osteogenesis. Plast. Reconstr. Surg. Glob. Open 2023, 11, e4674. [Google Scholar] [CrossRef]
- Moore, L.K.; Lee, C.S.; Agha, O.; Liu, M.; Zhang, H.; Dang, A.B.C.; Dang, A.; Liu, X.; Feeley, B.T. A novel mouse model of hindlimb joint contracture with 3D-printed casts. J. Orthop. Res. 2022, 40, 2865–2872. [Google Scholar] [CrossRef]
- Ballard, D.H.; Trace, A.P.; Ali, S.; Hodgdon, T.; Zygmont, M.E.; DeBenedectis, C.M.; Smith, S.E.; Richardson, M.L.; Patel, M.J.; Decker, S.J.; et al. Clinical Applications of 3D Printing: Primer for Radiologists. Acad. Radiol. 2018, 25, 52–65. [Google Scholar] [CrossRef] [PubMed]


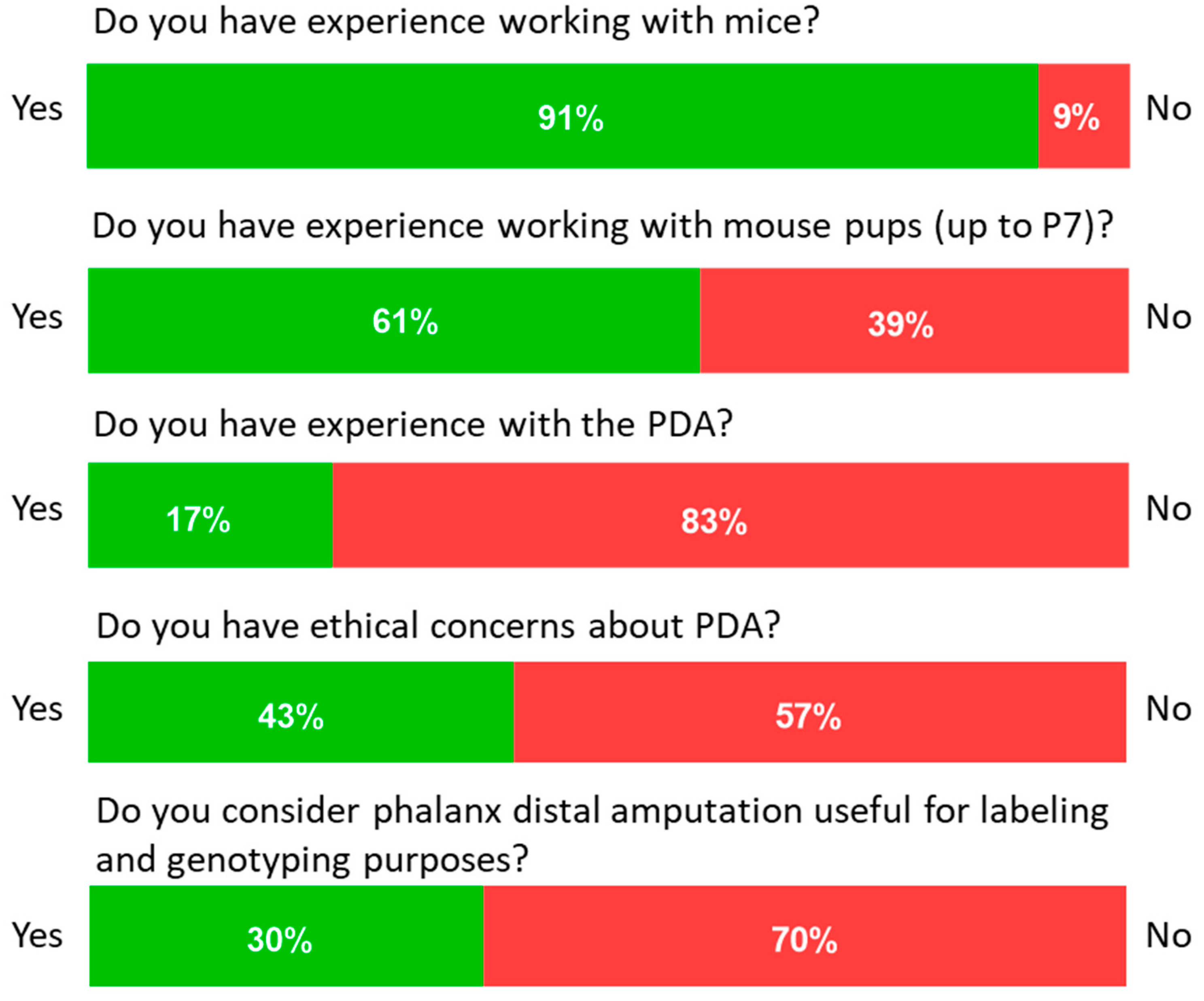


| Question | Answer | |
|---|---|---|
| Have you ever worked with mice? | Yes (1) | No (0) |
| Have you ever worked with young animals (Up to p7 = 7 days post-partum)? | Yes (1) | No (0) |
| Do you have previous experience in amputation of the distal phalanx amputation for marking purposes? | Yes (1) | No (0) |
| Do you have ethical concerns about performing distal phalanx amputation on young animals? | Yes (1) | No (0) |
| Do you feel that phalanx distalis amputation is useful for marking and genotyping purposes | Yes (1) | No (0) |
| On which paw/feet did you find marking easiest (Front paw (FP) or hind paw (HP))? | FP | HP |
| Based on your experience, was the model realistic? Please comment on this regard. | Realistic | Unrealistic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heuser, M.; Gonzalez-Uarquin, F.; Nuber, M.; Brockmann, M.A.; Baumgart, J.; Baumgart, N. A 3D-Printed Dummy for Training Distal Phalanx Amputation in Mice. Animals 2024, 14, 1253. https://doi.org/10.3390/ani14081253
Heuser M, Gonzalez-Uarquin F, Nuber M, Brockmann MA, Baumgart J, Baumgart N. A 3D-Printed Dummy for Training Distal Phalanx Amputation in Mice. Animals. 2024; 14(8):1253. https://doi.org/10.3390/ani14081253
Chicago/Turabian StyleHeuser, Miriam, Fernando Gonzalez-Uarquin, Maximilian Nuber, Marc A. Brockmann, Jan Baumgart, and Nadine Baumgart. 2024. "A 3D-Printed Dummy for Training Distal Phalanx Amputation in Mice" Animals 14, no. 8: 1253. https://doi.org/10.3390/ani14081253





