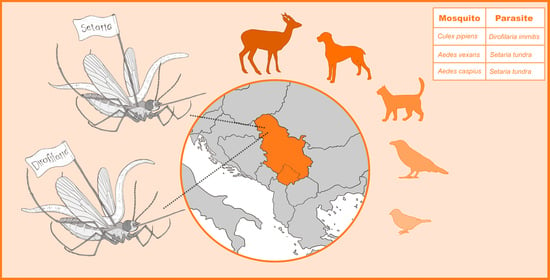
Dirofilaria sp. and Blood Meal Analysis in Mosquitoes Collected in Vojvodina and Mačva, and the First Report of Setaria tundra (Issaitshikoff & Rajewskaya, 1928) in Serbia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquito Sampling and Vector Identification
2.2. DNA Extraction
2.3. Identification of Dirofilaria sp.
2.4. Identification of Blood-Meal Host
2.5. Phylogenetic Analysis of Setaria tundra
3. Results
3.1. Presence of Dirofilaria immitis and Setaria tundra in Mosquitoes
3.2. Blood-Meal Host Detection
3.3. Phylogenetic Analysis of Setaria tundra
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, M.J.; Bandi, C.; Hoerauf, A. Wolbachia. Bacterial Endosymbionts of Filarial Nematodes. Adv. Parasitol. 2005, 60, 245–284. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C. Nematode Parasites of Vertebrates, Their Development and Transmission, 2nd ed.; CABI Publishing: New York, NY, USA, 2000. [Google Scholar]
- Morchón, R.; Montoya-Alonso, J.A.; Rodríguez-Escolar, I.; Carretón, E. What Has Happened to Heartworm Disease in Europe in the Last 10 Years? Pathogens 2022, 11, 1042. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Alonso, J.A.; Carretón, E.; García-Guasch, L.; Expósito, J.; Armario, B.; Morchón, R.; Simón, F. First Epidemiological Report of Feline Heartworm Infection in the Barcelona Metropolitan Area (Spain). Parasit. Vectors 2014, 7, 506. [Google Scholar] [CrossRef] [PubMed]
- Tarello, W. Clinical Aspects of Dermatitis Associated with Dirofilaria repens in Pets: A Review of 100 Canine and 31 Feline Cases (1990–2010) and a Report of a New Clinic Case Imported from Italy to Dubai. J. Parasitol. Res. 2011, 2011, 578385. [Google Scholar] [CrossRef] [PubMed]
- Traversa, D.; Di Cesare, A.; Conboy, G. Canine and Feline Cardiopulmonary Parasitic Nematodes in Europe: Emerging and Underestimated. Parasit. Vectors 2010, 3, 62. [Google Scholar] [CrossRef] [PubMed]
- Genchi, C.; Kramer, L.H. The Prevalence of Dirofilaria immitis and D. Repens in the Old World. Vet. Parasitol. 2020, 280, 108995. [Google Scholar] [CrossRef] [PubMed]
- Sadighian, A. Helminth Parasites of Stray Dogs and Jackals in Shahsavar Area, Caspian Region, Iran. J. Parasitol. 1969, 55, 372. [Google Scholar] [CrossRef] [PubMed]
- McCall, J.W.; Genchi, C.; Kramer, L.H.; Guerrero, J.; Venco, L. Chapter 4 Heartworm Disease in Animals and Humans. Adv. Parasitol. 2008, 66, 193–285. [Google Scholar] [CrossRef] [PubMed]
- Ćirović, D.; Penezić, A.; Pavlović, I.; Kulišić, Z.; Ćosić, N.; Burazerović, J.; Maletić, V. First Records of Dirofilaria repens in Wild Canids from the Region of Central Balkan. Acta Vet. Hung. 2014, 62, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Ionică, A.M.; Matei, I.A.; D’Amico, G.; Daskalaki, A.A.; Juránková, J.; Ionescu, D.T.; Mihalca, A.D.; Modrý, D.; Gherman, C.M. Role of Golden Jackals (Canis aureus) as Natural Reservoirs of Dirofilaria Spp. in Romania. Parasit. Vectors 2016, 9, 240. [Google Scholar] [CrossRef]
- Morchón, R.; Carretón, E.; González-Miguel, J.; Mellado-Hernández, I. Heartworm Disease (Dirofilaria immitis) and Their Vectors in Europe—New Distribution Trends. Front. Physiol. 2012, 3, 23265. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Dantas-Torres, F.; Brianti, E.; Traversa, D.; Petrić, D.; Genchi, C.; Capelli, G. Vector-Borne Helminths of Dogs and Humans in Europe. Parasit. Vectors 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Rivasi, F.; Boldorini, R.; Criante, P.; Leutner, M.; Pampiglione, S. Detection of Dirofilaria (Nochtiella) Repens DNA by Polymerase Chain Reaction in Embedded Paraffin Tissues from Two Human Pulmonary Locations. APMIS 2006, 114, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Simón, F.; Siles-Lucas, M.; Morchón, R.; González-Miguel, J.; Mellado, I.; Carretón, E.; Montoya-Alonso, J.A. Human and Animal Dirofilariasis: The Emergence of a Zoonotic Mosaic. Clin. Microbiol. Rev. 2012, 25, 507–544. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.K.; Bonnier, A.; Chong, W.H.; Chieng, H.; Austin, A.; Hu, K.; Shkolnik, B. Human Pulmonary Dirofilariasis: A Review for the Clinicians. Am. J. Med. Sci. 2022, 363, 11–17. [Google Scholar] [CrossRef]
- Sako, T.; Burioka, N.; Suyama, H.; Kinugasa, Y.; Watanabe, M.; Hirai, K.; Shimizu, E. Human Pulmonary Dirofilariasis Presenting as a Small Nodule with a Cavity. J. Med. Investig. 2000, 47, 161–163. [Google Scholar]
- Muro, A.; Genchi, C.; Cordero, M.; Simón, F. Human Dirofilariasis in the European Union. Parasitol. Today 1999, 15, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Savić, S.; Stosic, M.Z.; Marcic, D.; Hernández, I.; Potkonjak, A.; Otasevic, S.; Ruzic, M.; Morchón, R. Seroepidemiological Study of Canine and Human Dirofilariasis in the Endemic Region of Northern Serbia. Front. Vet. Sci. 2020, 7, 571. [Google Scholar] [CrossRef] [PubMed]
- Zumaquero, L.; Simón, F.; Carretón, E.; Hernández, I.; Sandoval, C.; Morchón, R. Prevalence of Canine and Human Dirofilariosis in Puebla, Mexico. Vet. Parasitol. 2020, 282, 109098. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, P.A.; Grisolia, A.; Reale, S.; Liotta, R.; Mularoni, A.; Bertani, A. A Rare Case of Human Pulmonary Dirofilariasis with Nodules Mimicking Malignancy: Approach to Diagnosis and Treatment. J. Cardiothorac. Surg. 2018, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Czajka, C.; Becker, N.; Poppert, S.; Jöst, H.; Schmidt-Chanasit, J.; Krüger, A. Molecular Detection of Setaria tundra (Nematoda: Filarioidea) and an Unidentified Filarial Species in Mosquitoes in Germany. Parasit. Vectors 2012, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Oloś, G.; Nowakowska, J.; Rojewska, S.; Welc-Falęciak, R. New Findings of Setaria tundra and Setaria cervi in the Red Deer (Cervus elaphus) in Poland. Parasitology 2019, 146, 1333–1337. [Google Scholar] [CrossRef] [PubMed]
- Čurlík, J.; Konjević, D.; Bujanić, M.; Sabol, Ž.; Martinković, F.; Sindičić, M. The First Description of Setaria tundra (Issaitshikoff & Rajewskaya, 1928) in Roe Deer from Croatia. Helminthologia 2019, 56, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Čurlík, J.; Šmigová, J.; Šmiga, Ľ.; Lazár, J.; Lazár, P.; Konjević, D.; Papajová, I. The First Report of Setaria tundra (Issaitshikoff & Rajewskaya, 1928) in Slovakia by Using of Molecular Methods. Vet. Res. Commun. 2023, 47, 2247–2251. [Google Scholar] [CrossRef] [PubMed]
- Kronefeld, M.; Kampen, H.; Sassnau, R.; Werner, D. Molecular Detection of Dirofilaria immitis, Dirofilaria repens and Setaria tundra in Mosquitoes from Germany. Parasit. Vectors 2014, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Zittra, C.; Kocziha, Z.; Pinnyei, S.; Harl, J.; Kieser, K.; Laciny, A.; Eigner, B.; Silbermayr, K.; Duscher, G.G.; Fok, É.; et al. Screening Blood-Fed Mosquitoes for the Diagnosis of Filarioid Helminths and Avian Malaria. Parasit. Vectors 2015, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Medlock, J.M.; Barrass, I.; Kerrod, E.; Taylor, M.A.; Leach, S. Analysis of Climatic Predictions for Extrinsic Incubation of Dirofilaria in The United Kingdom. Vector-Borne Zoonotic Dis. 2007, 7, 4–14. [Google Scholar] [CrossRef]
- Fortin, J.; Slocombe, J. Temperature Requirements for the Development of Dirofilaria immitis in Aedes triseriatus and Ae. vexans. Mosq. News 1981, 41, 625–633. [Google Scholar]
- Christensen, B.M.; Hollander, A. Effect of Temperature on Vector-Parasite Relationships of Aedes Trivittatus and Dirofilaria immitis. Proc. Helminthol. Soc. 1978, 45, 115–119. [Google Scholar]
- Jankowski, T.J.; Bickley, W.E. The Mosquitoes, Aedes Canadensis and A. Vexans as Potential Vectors of Dirofilaria immitis in Maryland 1. Ann. Entomol. Soc. Am. 1976, 69, 781–783. [Google Scholar] [CrossRef]
- Kutz, F.W.; Dobson, R.C. Effects of Temperature on the Development of Dirofilaria immitis (Leidy) in Anopheles Quadrimaculatus Say1 and on Vector Mortality Resulting from This Development2,3. Ann. Entomol. Soc. Am. 1974, 67, 325–331. [Google Scholar] [CrossRef]
- Cancrini, G.; Magi, M.; Gabrielli, S.; Arispici, M.; Tolari, F.; Dell’Omodarme, M.; Prati, M.C. Natural Vectors of Dirofilariasis in Rural and Urban Areas of the Tuscan Region, Central Italy. J. Med. Entomol. 2006, 43, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijević, S.; Tasić, A.; Tasić, S.; Adamović, V.; Ilić, T.; Miladinović-Tasić, N. Filariosis in Dogs in Serbia. In Dirofilaria immitis and D. repens in Dog and Cat and Human Infections, Mappe Parassitologiche; Genchi, C., Rinaldi, L., Cringoli, G., Eds.; Litografia Vigilante srl, Rolando Editore: Naples, Italy, 2007; Volume 8, p. 201. ISBN 88-89132-14-0. [Google Scholar]
- Tasić, A.; Rossi, L.; Tasić, S.; Miladinović-Tasić, N.; Ilić, T.; Dimitrijević, S. Survey of Canine Dirofilariasis in Vojvodina, Serbia. Parasitol. Res. 2008, 103, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Tasić, A.; Tasić-Otašević, S.; Gabrielli, S.; Miladinović-Tasić, N.; Ignjatović, A.; Đorđević, J.; Dimitrijević, S.; Cancrini, G. Canine Dirofilaria Infections in Two Uninvestigated Areas of Serbia: Epidemiological and Genetic Aspects. Vector-Borne Zoonotic Dis. 2012, 12, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Tasić, A.; Tasić, S.; Miladinović-Tasić, N.; Zdravković, D.; Đorđević, J. Prevalence of Dirofilaria repens-Cause of Zoonosis in Dogs. Acta Fac. Med. Naiss. 2007, 24, 71–74. [Google Scholar]
- Penezić, A.; Selaković, S.; Pavlović, I.; Ćirović, D. First Findings and Prevalence of Adult Heartworms (Dirofilaria immitis) in Wild Carnivores from Serbia. Parasitol. Res. 2014, 113, 3281–3285. [Google Scholar] [CrossRef] [PubMed]
- Gavrilović, P.; Blitva-Robertson, G.; Özvegy, J.; Kiskároly, F.; Becskei, Z. Case Report of Dirofilariasis in Grey Wolf in Serbia. Acta Parasitol. 2014, 60, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Potkonjak, A.; Rojas, A.; Gutiérrez, R.; Nachum-Biala, Y.; Kleinerman, G.; Savić, S.; Polaček, V.; Pušić, I.; Harrus, S.; Baneth, G. Molecular Survey of Dirofilaria Species in Stray Dogs, Red Foxes and Golden Jackals from Vojvodina, Serbia. Comp. Immunol. Microbiol. Infect. Dis. 2020, 68, 101409. [Google Scholar] [CrossRef] [PubMed]
- Kurucz, K.; Kepner, A.; Krtinic, B.; Zana, B.; Földes, F.; Bányai, K.; Oldal, M.; Jakab, F.; Kemenesi, G. First Molecular Identification of Dirofilaria Spp. (Onchocercidae) in Mosquitoes from Serbia. Parasitol. Res. 2016, 115, 3257–3260. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.; Petrić, D.; Zgomba, M.; Boase, C.; Madon, M.B.; Dahl, C.; Kaiser, A. Mosquitoes, 3rd ed.; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Casiraghi, M.; Anderson, T.J.C.; Bandi, C.; Bazzocchi, C.; Genchi, C. A Phylogenetic Analysis of Filarial Nematodes: Comparison with the Phylogeny of Wolbachia Endosymbionts. Parasitology 2001, 122, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, M.; Bain, O.; Guerrero, R.; Martin, C.; Pocacqua, V.; Gardner, S.L.; Franceschi, A.; Bandi, C. Mapping the Presence of Wolbachia Pipientis on the Phylogeny of Filarial Nematodes: Evidence for Symbiont Loss during Evolution. Int. J. Parasitol. 2004, 34, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, S.; Mangano, V.; Furzi, F.; Oliva, A.; Vita, S.; Poscia, R.; Fazii, P.; Di Paolo, J.; Marocco, R.; Mastroianni, C.M.; et al. Molecular Identification of New Cases of Human Dirofilariosis (Dirofilaria repens) in Italy. Pathogens 2021, 10, 251. [Google Scholar] [CrossRef] [PubMed]
- Tasić-Otašević, S.; Savić, S.; Jurhar-Pavlova, M.; Stefanovska, J.; Stalević, M.; Ignjatović, A.; Ranđelović, M.; Gajić, B.; Cvetkovikj, A.; Gabrielli, S. Molecular Survey of Dirofilaria and Leishmania Species in Dogs from Central Balkan. Animals 2022, 12, 911. [Google Scholar] [CrossRef] [PubMed]
- Boessenkool, S.; Epp, L.S.; Haile, J.; Bellemain, E.; Edwards, M.; Coissac, E.; Willerslev, E.; Brochmann, C. Blocking Human Contaminant DNA during PCR Allows Amplification of Rare Mammal Species from Sedimentary Ancient DNA. Mol. Ecol. 2012, 21, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Morchón, R.; Bargues, M.D.; Latorre, J.M.; Melero-Alcíbar, R.; Pou-Barreto, C.; Mas-Coma, S.; Simón, F. Haplotype H1 of Culex pipiens Implicated as Natural Vector of Dirofilaria immitis in an Endemic Area of Western Spain. Vector-Borne Zoonotic Dis. 2007, 7, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Inci, A.; Duzlu, O.; Biskin, Z.; Ica, A.; Sahin, I. Aedes Vexans and Culex Pipiens as the Potential Vectors of Dirofilaria immitis in Central Turkey. Vet. Parasitol. 2011, 178, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Santa-Ana, M.; Khadem, M.; Capela, R. Natural Infection of Culex Theileri (Diptera: Culicidae) with Dirofilaria immitis (Nematoda: Filarioidea) on Madeira Island, Portugal. J. Med. Entomol. 2006, 43, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Morchón, R.; Bargues, M.D.; Latorre-Estivalis, J.M.; Pou-Barreto, C.; Melero-Alcibar, R.; Moreno, M.; Valladares, B.; Molina, R.; Montoya-Alonso, J.A.; Mas-Coma, S.; et al. Molecular Characterization of Culex Theileri from Canary Islands, Spain, a Potential Vector of Dirofilaria immitis. J. Clin. Exp. Pathol. 2011, 1, 1–6. [Google Scholar] [CrossRef]
- Bışkın, Z.; Düzlü, O.; Yildirim, A.; Incı, A. The Molecular Diagnosis of Dirofilaria immitis in Vector Mosquitoes in Felahiye District of Kayseri. Turkiye Parazitol. Derg. 2010, 34, 200–205. [Google Scholar] [PubMed]
- Cancrini, G.; Pietrobelli, M.; Frangipane di Regalbono, A.F.; Tampieri, M.P.; della Torre, A. Development of Dirofilaria and Setaria Nematodes in Aedes Albopictus. Parassitologia 1995, 37, 141–145. [Google Scholar] [PubMed]
- Cancrini, G.; Frangipane di Regalbono, A.; Ricci, I.; Tessarin, C.; Gabrielli, S.; Pietrobelli, M. Aedes Albopictus Is a Natural Vector of Dirofilaria immitis in Italy. Vet. Parasitol. 2003, 118, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Petrić, D.; Petrović, T.; Hrnjaković Cvjetković, I.; Zgomba, M.; Milošević, V.; Lazić, G.; Ignjatović Ćupina, A.; Lupulović, D.; Lazić, S.; Dondur, D.; et al. West Nile Virus “circulation” in Vojvodina, Serbia: Mosquito, Bird, Horse and Human Surveillance. Mol. Cell Probes 2017, 31, 28–36. [Google Scholar] [CrossRef]
- Petrić, M.; Lalić, B.; Ducheyne, E.; Djurdjević, V.; Petrić, D. Modelling the regional impact of climate change on the suitability of the establishment of the Asian tiger mosquito (Aedes albopictus) in Serbia. Clim. Chang. 2017, 142, 361–374. [Google Scholar] [CrossRef]
- Capelli, G.; Genchi, C.; Baneth, G.; Bourdeau, P.; Brianti, E.; Cardoso, L.; Danesi, P.; Fuehrer, H.-P.; Giannelli, A.; Ionică, A.M.; et al. Recent Advances on Dirofilaria repens in Dogs and Humans in Europe. Parasit. Vectors 2018, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- Krstić, M.; Gabrielli, S.; Ignjatović, M.; Savić, S.; Cancrini, G.; Ranđelović, G.; Momčilović, S.; Stojnev, S.; Otašević, S. An Appraisal of Canine and Human Cases Reveals an Endemic Status of Dirofilariosis in Parts of Serbia. Mol. Cell Probes 2017, 31, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Spasojević-Kosić, L.; Lalošević, V.; Lalošević, D.; Simin, S.; Vasić, I.; Kuruca, L. Prevalence of Dirofilariosis in Pet Dogs in Novi Sad. Contemp. Agric. 2012, 61, 247–254. [Google Scholar]
- Spasojević Kosić, L.; Lalošević, V.; Simin, S.; Kuruca, L. Dirofilariosis and Angiostrongilosis in Pet and Hunting Dogs in Novi Sad, Vojvodina, Serbia. Arch. Vet. Med. 2017, 9, 53–62. [Google Scholar] [CrossRef]
- Gawor, J.J. The Prevalence and Abundance of Internal Parasites in Working Horses Autopsied in Poland. Vet. Parasitol. 1995, 58, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, M.T.; Piccolo, G.; Fraquelli, C.; Perco, F. Elmintofauna Del Cervo Nel Parco Nazionale Dello Stelvio. J. Mt. Ecol. 2003, 7, 245–249. [Google Scholar]
- Demiaszkiewicz, A.W.; Lachowicz, J.; Karbowiak, G. Wzrost Zarażenia Żubrów w Puszczy Białowieskiej Nicieniami Setaria Labiatopapillosa. Wiadomoœci Parazytol. 2007, 53, 335–338. [Google Scholar]
- Demiaszkiewicz, A.W.; Kuligowska, I.; Pyziel, A.M.; Lachowicz, J. First Cases of Nematodes Setaria tundra Invasion in Elk (Alces alces) in Poland. Med. Weter. 2015, 71, 510–512. [Google Scholar]
- Raevskaya, Z.A. Setaria and Their Pathogenic Significance. Tr. Gos. Instituta Eksperimental’noi Vet. 1928, 5, 1–58. [Google Scholar]
- Oloś, G.; Nowakowska, J.; Welc-Falęciak, R. Setaria tundra, What Do We Know, What Is Still to Be Discovered? Ann. Parasitol. 2021, 67, 1–10. [Google Scholar] [CrossRef]
- Kutzer, E.; Hinaidy, H.K. Die Parasiten Der Wildlebenden Wiederkäuer Österreichs. Z. Für Parasitenkd. 1969, 32, 354–368. [Google Scholar]
- Savonen, E. The Implementation of Domestic Reindeer Meat Inspection. Suom. Eläinlääkäril. 1978, 84, 651–653. [Google Scholar]
- Rehbinder, C.; Christensson, D.; Glatthard, V. Parasitic Granulomas in Swedish Forest Reindeer (Rangifer tarandus). In Wildlife Diseases; Springer US: Boston, MA, USA, 1976; pp. 597–607. [Google Scholar] [CrossRef]
- Poppe, T.T. Parasitic Diseases Recorded in Reindeer during Meat Inspection in Kautokeino (Norway) in Autumn and Winter. Nor. Veterinaertidsskrift 1977, 89, 791–795. [Google Scholar]
- Yanchev, Y. The Helminth Fauna of Roe Deer (Capreolus capreolus) in Bulgaria. 3. Material on Helminth Fauna in Roe Deer (Capreolus capreolus L.) in the Mountains of Southern Bulgaria. Izv. Natsentralnata Khelmintologichna Lab. 1973, 16, 205–220. [Google Scholar]
- Andrews, J.R.; Hörning, B.; Wandeler, A. Endoparasites of Roe Deer (Capreolus capreolus L.) from Switzerland with Special Reference to Hosts from the Emmental Region of Canton Berne. Rev. Suisse Zool. Ann. Soc. Zool. Suisse Mus. D’hist. Nat. Geneve 1974, 81, 13. [Google Scholar] [CrossRef]
- Büttner, K. Untersuchungen Zur Parasitierung Des Rehwildes Bei Steigendem Jagddruck. Z. Jagdwiss. 1978, 24, 139–155. [Google Scholar] [CrossRef]
- Favia, G. Molecular Assay for the Identification of Setaria tundra. Vet. Parasitol. 2003, 117, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Ferri, E.; Barbuto, M.; Bain, O.; Galimberti, A.; Uni, S.; Guerrero, R.; Ferté, H.; Bandi, C.; Martin, C.; Casiraghi, M. Integrated Taxonomy: Traditional Approach and DNA Barcoding for the Identification of Filarioid Worms and Related Parasites (Nematoda). Front. Zool. 2009, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Enemark, H.L.; Oksanen, A.; Chriél, M.; le Fèvre Harslund, J.; Woolsey, I.D.; Al-Sabi, M.N.S. Detection and Molecular Characterization of the Mosquito-Borne Filarial Nematode Setaria tundra in Danish Roe Deer (Capreolus capreolus). Int. J. Parasitol. Parasit. Wildl. 2017, 6, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Bednarski, M.; Piasecki, T.; Bednarska, M.; Soltysiak, Z. Invasion of Setaria tundra in Roedeer (Capreolus capreolus)—Case Report. Acta Sci. Polonorum. Med. Vet. 2010, 3, 21–25. [Google Scholar]
- Angelone-Alasaad, S.; Jowers, M.J.; Panadero, R.; Pérez-Creo, A.; Pajares, G.; Díez-Baños, P.; Soriguer, R.C.; Morrondo, P. First Report of Setaria tundra in Roe Deer (Capreolus capreolus) from the Iberian Peninsula Inferred from Molecular Data: Epidemiological Implications. Parasit. Vectors 2016, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, S.; Kuusela, J.; Nikander, S.; Nylund, M.; Oksanen, A. Outbreak of Parasitic Peritonitis in Reindeer in Finland. Vet. Rec. 2007, 160, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, S.; Solismaa, M.; Kortet, R.; Kuusela, J.; Oksanen, A. Vectors and Transmission Dynamics for Setaria tundra (Filarioidea; Onchocercidae), a Parasite of Reindeer in Finland. Parasit. Vectors 2009, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Kornaś, S.; Nosal, P.; Basiaga, M.; Lesiak, M. Setaria tundra in Roe Deer (Capreolus capreolus)–New Findings in Poland. Ann. Parasitol. 2013, 59, 179–182. [Google Scholar] [PubMed]
- Tomczuk, K.; Szczepaniak, K.; Grzybek, M.; Studzińska, M.; Demkowska-Kutrzepa, M.; Roczeń-Karczmarz, M.; Łopuszyński, W.; Junkuszew, A.; Gruszecki, T.; Dudko, P.; et al. Internal Parasites in Roe Deer of the Lubartów Forest Division in Postmortem Studies. Med. Weter. 2017, 73, 726–730. [Google Scholar] [CrossRef]
- Laaksonen, S.; Oksanen, A. Status and Review of the Vector-Borne Nematode Setaria tundra in Finnish Cervids. A J. Devoted Biol. Manag. Moose 2009, 45, 81–84. [Google Scholar]
- Bazargani, T.; Eslami, A.; Gholami, G.R.; Molai, A.; Ghafari-Charati, J.; Dawoodi, J.; Ashrafi, J. Cerebrospinal Nematodiasis of Cattle, Sheep and Goats in Iran. Iran. J. Parasitol. 2008, 3, 16–20. [Google Scholar]
- Sharhuu, G.; Sharkhuu, T. The Helminth Fauna of Wild and Domestic Ruminants in Mongolia-a Review. Eur. J. Wildl. Res. 2004, 50, 150–156. [Google Scholar] [CrossRef]
- Sundar, S.T.B.; D’Souza, P.E. Morphological Characterization of Setaria Worms Collected from Cattle. J. Parasit. Dis. 2015, 39, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Kuchboev, A.; Amirov, O.; Karimova, R.R.; Asakawa, M. Nematodes in the Digestive Tracts of Domestic Ruminants in Uzbekistan. Jpn. J. Vet. Parasitol. 2016, 15, 124–128. [Google Scholar]
- Lavadinovic, V.; Popovic, Z.; Beukovic, D.; Cokoski, K. Wild Boar Management (Sus scrofa L.) in the Republic of Serbia. Glas. Sumar. Fakulteta 2020, 2020, 47–60. [Google Scholar] [CrossRef]
- Milićević, V.; Sapundžić, Z.Z.; Glišić, D.; Kureljušić, B.; Vasković, N.; Đorđević, M.; Mirčeta, J. Cross-Sectional Serosurvey of Selected Infectious Diseases in Wild Ruminants in Serbia. Res. Vet. Sci. 2024, 170, 105183. [Google Scholar] [CrossRef] [PubMed]
- Lanková, S.; Vejl, P.; Melounová, M.; Čílová, D.; Vadlejch, J.; Miklisová, D.; Jankovská, I.; Langrová, I. Setaria cervi (Filarioidea, Onchocercidae) Undressing in Ungulates: Altered Morphology of Developmental Stages, Their Molecular Detection and Complete Sequence Cox 1 Gene. Parasitology 2021, 148, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Nechybová, S.; Vejl, P.; Hart, V.; Melounová, M.; Čílová, D.; Vašek, J.; Jankovská, I.; Vadlejch, J.; Langrová, I. Long-Term Occurrence of Trichuris Species in Wild Ruminants in the Czech Republic. Parasitol. Res. 2018, 117, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Genchi, C.; Rinaldi, L.; Mortarino, M.; Genchi, M.; Cringoli, G. Climate and Dirofilaria Infection in Europe. Vet. Parasitol. 2009, 163, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, S.; Pusenius, J.; Kumpula, J.; Venäläinen, A.; Kortet, R.; Oksanen, A.; Hoberg, E. Climate Change Promotes the Emergence of Serious Disease Outbreaks of Filarioid Nematodes. Ecohealth 2010, 7, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Rydzanicz, K.; Lonc, E.; Masny, A.; Golab, E. Detection of Setaria tundra Microfilariae in Mosquito Populations from Irrigated Fields in Wroclaw (Poland). Ann. Parasitol. 2016, 62 (Suppl. 132). Available online: https://annals-parasitology.eu/archive_2001_2022/2016-62-Suppl-S4_132.pdf (accessed on 18 April 2024).
- Masny, A.; Rożej-Bielicka, W.; Gołąb, E. Setaria tundra Invasive Larvae in a Mosquito Vector in Poland. Ann. Parasitol. 2013, 59, 178. [Google Scholar]
- Kemenesi, G.; Kurucz, K.; Kepner, A.; Dallos, B.; Oldal, M.; Herczeg, R.; Vajdovics, P.; Bányai, K.; Jakab, F. Circulation of Dirofilaria repens, Setaria tundra, and Onchocercidae Species in Hungary during the Period 2011–2013. Vet. Parasitol. 2015, 214, 108–113. [Google Scholar] [CrossRef] [PubMed]




| Mosquito Species | Number of | Total No. of Analyzed Mosquitoes | No. of Positive Samples for | ||
|---|---|---|---|---|---|
| Non-Blood-Fed | Blood-Fed | D. immitis | S. tundra | ||
| Anopheles maculipennis | 383 | 15 | 398 | – | – |
| Aedes vexans | 1253 | 87 | 1340 | – | 1 |
| Aedes caspius | 305 | 11 | 316 | – | 1 |
| Aedes sticticus | 0 | 8 | 8 | – | – |
| Aedes albopictus | 580 | 0 | 580 | – | – |
| Culex pipiens | 0 | 225 | 225 | 3 | – |
| Culiseta annulata | 0 | 7 | 7 | – | – |
| Coquillettidia richiardii | 0 | 28 | 28 | – | – |
| Total | 2521 | 381 | 2902 | 3 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šiljegović, S.; Mouillaud, T.; Jiolle, D.; Petrić, D.; Ignjatović-Ćupina, A.; Vasić, A.; Paupy, C.; Kavran, M. Dirofilaria sp. and Blood Meal Analysis in Mosquitoes Collected in Vojvodina and Mačva, and the First Report of Setaria tundra (Issaitshikoff & Rajewskaya, 1928) in Serbia. Animals 2024, 14, 1255. https://doi.org/10.3390/ani14091255
Šiljegović S, Mouillaud T, Jiolle D, Petrić D, Ignjatović-Ćupina A, Vasić A, Paupy C, Kavran M. Dirofilaria sp. and Blood Meal Analysis in Mosquitoes Collected in Vojvodina and Mačva, and the First Report of Setaria tundra (Issaitshikoff & Rajewskaya, 1928) in Serbia. Animals. 2024; 14(9):1255. https://doi.org/10.3390/ani14091255
Chicago/Turabian StyleŠiljegović, Sara, Théo Mouillaud, Davy Jiolle, Dušan Petrić, Aleksandra Ignjatović-Ćupina, Ana Vasić, Christophe Paupy, and Mihaela Kavran. 2024. "Dirofilaria sp. and Blood Meal Analysis in Mosquitoes Collected in Vojvodina and Mačva, and the First Report of Setaria tundra (Issaitshikoff & Rajewskaya, 1928) in Serbia" Animals 14, no. 9: 1255. https://doi.org/10.3390/ani14091255