Physical and Chemical Characteristics of Droppings as Sensitive Markers of Chicken Health Status
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Collection
2.2. Evaluation of pH, Dry Matter, Texture, and Color Coordinates of Droppings
2.3. The Evaluation of Short-Chain Fatty Acids in Chicken Droppings
2.4. Analysis of Volatile Compounds by Gas Chromatography–Mass Spectrometry
2.5. Fatty Acid Profile Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. pH, Dry Matter, Texture, and Color Coordinates of Chicken Droppings
3.2. Short-Chain Fatty Acid Profile of Chicken Droppings
3.3. Fatty Acid Profile of the Broiler Chicken Droppings
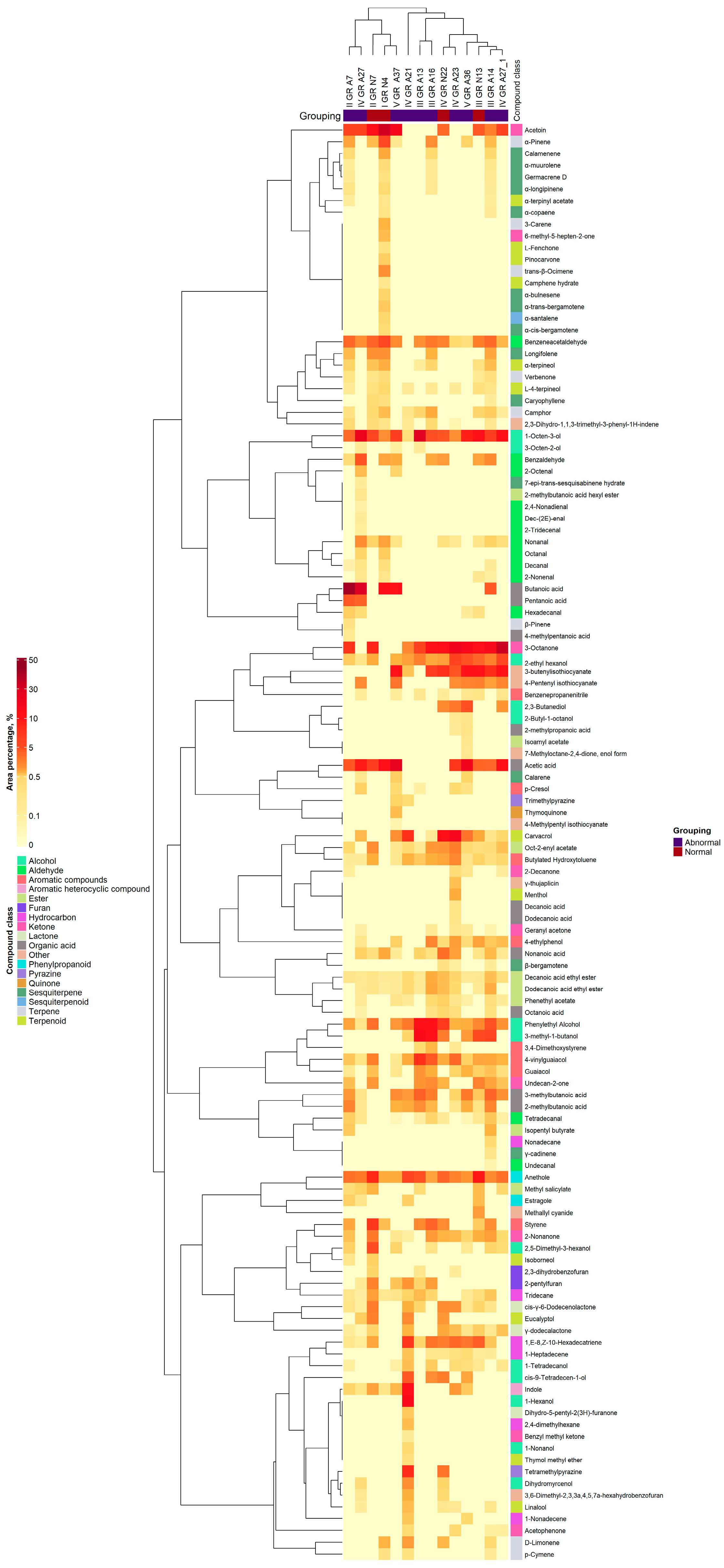
3.4. Volatile Compound Profile of the Broiler Chicken Droppings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maharjan, P.; Martinez, D.A.; Weil, J.; Suesuttajit, N.; Umberson, C.; Mullenix, G.; Hilton, K.M.; Beitia, A.; Coon, C.N. Review: Physiological Growth Trend of Current Meat Broilers and Dietary Protein and Energy Management Approaches for Sustainable Broiler Production. Animal 2021, 15, 100284. [Google Scholar] [CrossRef] [PubMed]
- Thi Huong-Anh, N.; Van Chinh, D.; Thi Tuyet-Hanh, T. Antibiotic Residues in Chickens and Farmers’ Knowledge of Their Use in Tay Ninh Province, Vietnam, in 2017. Asia Pac. J. Public Health 2020, 32, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Salsabil, U.S.; Syeed, M.M.; Rahman, M.M.; Fatema, K.; Uddin, M.F. SmartPoultry: Early Detection of Poultry Disease from Smartphone Captured Fecal Image. In Proceedings of the 2023 20th International Joint Conference on Computer Science and Software Engineering (JCSSE), Phitsanulok, Thailand, 28 June–1 July 2023; pp. 345–350. [Google Scholar]
- Duangnumsawang, Y.; Zentek, J.; Goodarzi Boroojeni, F. Development and Functional Properties of Intestinal Mucus Layer in Poultry. Front. Immunol. 2021, 12, 745849. [Google Scholar] [CrossRef] [PubMed]
- Wickramasuriya, S.S.; Park, I.; Lee, K.; Lee, Y.; Kim, W.H.; Nam, H.; Lillehoj, H.S. Role of Physiology, Immunity, Microbiota, and Infectious Diseases in the Gut Health of Poultry. Vaccines 2022, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Waite, D.W.; Taylor, M. Exploring the Avian Gut Microbiota: Current Trends and Future Directions. Front. Microbiol. 2015, 6, 673. [Google Scholar] [CrossRef]
- Borgonovo, F.; Ferrante, V.; Grilli, G.; Guarino, M. An Innovative Approach for Analysing and Evaluating Enteric Diseases in Poultry Farm. Acta IMEKO 2024, 13, 1–5. [Google Scholar] [CrossRef]
- Corrigan, A.; de Leeuw, M.; Penaud-Frézet, S.; Dimova, D.; Murphy, R.A. Phylogenetic and Functional Alterations in Bacterial Community Compositions in Broiler Ceca as a Result of Mannan Oligosaccharide Supplementation. Appl. Environ. Microbiol. 2015, 81, 3460–3470. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Singh, A.K.; Yadav, S.; Berrocoso, J.F.D.; Mishra, B. Early Nutrition Programming (in Ovo and Post-Hatch Feeding) as a Strategy to Modulate Gut Health of Poultry. Front. Vet. Sci. 2019, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- van Veen, L.A.; van den Oever, A.C.M.; Kemp, B.; van den Brand, H. Perception of Laying Hen Farmers, Poultry Veterinarians, and Poultry Experts Regarding Sensor-Based Continuous Monitoring of Laying Hen Health and Welfare. Poult. Sci. 2023, 102, 102581. [Google Scholar] [CrossRef]
- Vidic, J.; Manzano, M.; Chang, C.-M.; Jaffrezic-Renault, N. Advanced Biosensors for Detection of Pathogens Related to Livestock and Poultry. Vet. Res. 2017, 48, 11. [Google Scholar] [CrossRef]
- Cho, S.; Hwang, O.; Park, S. Effect of Dietary Protein Levels on Composition of Odorous Compounds and Bacterial Ecology in Pig Manure. Asian-Australas. J. Anim. Sci. 2015, 28, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Wu, R.; Liu, D.; Dou, J.; Hayat, K.; Shang, D.; Pan, J.; Lin, H. An Efficient Segmentation Model for Abnormal Chicken Droppings Recognition Based on Improved Deep Dual-Resolution Network. J. Anim. Sci. 2024, 102, skae098. [Google Scholar] [CrossRef] [PubMed]
- Nakrosis, A.; Paulauskaite-Taraseviciene, A.; Raudonis, V.; Narusis, I.; Gruzauskas, V.; Gruzauskas, R.; Lagzdinyte-Budnike, I. Towards Early Poultry Health Prediction through Non-Invasive and Computer Vision-Based Dropping Classification. Animals 2023, 13, 3041. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Gates, R.S.; Ramirez, B.C. An On-Site Feces Image Classifier System for Chicken Health Assessment: A Proof of Concept. Appl. Eng. Agric. 2023, 39, 417–426. [Google Scholar] [CrossRef]
- Pérez-Calvo, E.; Wicaksono, A.N.; Canet, E.; Daulton, E.; Ens, W.; Hoeller, U.; Verlhac, V.; Celi, P.; Covington, J.A. The Measurement of Volatile Organic Compounds in Faeces of Piglets as a Tool to Assess Gastrointestinal Functionality. Biosyst. Eng. 2019, 184, 122–129. [Google Scholar] [CrossRef]
- Bos, L.D.J.; Sterk, P.J.; Schultz, M.J. Volatile Metabolites of Pathogens: A Systematic Review. PLoS Pathog. 2013, 9, e1003311. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef] [PubMed]
- EUR-Lex. Official Journal of the European Union, L:2007:182:TOC—EN. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ%3AL%3A2007%3A182%3ATOC (accessed on 14 February 2024).
- EUR-Lex. Official Journal of the European Union Regulation—EU-2017/625-of the European Parliament and of the Council—EN. Available online: https://eur-lex.europa.eu/eli/reg/2017/625/oj (accessed on 11 February 2024).
- Managment Book Ross Broiler, Managment Handbook. 2018. Available online: https://aviagen.com/assets/Tech_Center/Ross_Broiler/Ross-BroilerHandbook2018-EN.pdf (accessed on 22 March 2024).
- Ross 300 PS, Managment Handbook. Available online: http://www.rosspoultrybreeders.co.za/downloads/breeder/2018RossPSHandbook.pdf (accessed on 15 March 2024).
- Oakley, B.B.; Lillehoj, H.S.; Kogut, M.H.; Kim, W.K.; Maurer, J.J.; Pedroso, A.; Lee, M.D.; Collett, S.R.; Johnson, T.J.; Cox, N.A. The Chicken Gastrointestinal Microbiome. FEMS Microbiol. Lett. 2014, 360, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Martínez, Y.; Altamirano, E.; Ortega, V.; Paz, P.; Valdivié, M. Effect of Age on the Immune and Visceral Organ Weights and Cecal Traits in Modern Broilers. Animals 2021, 11, 845. [Google Scholar] [CrossRef]
- Jaramillo, Á. Evaluation of a Prebiotic and an Organic Acid-Supplemented Diets on the Performance and Allometric Pa-Rameters of Broiler Chickens with Controlled Feeding. Rev. Colomb. Cienc. Pecu. 2012, 5, 52–66. [Google Scholar] [CrossRef]
- Ndelekwute, E.K.; Unah, U.L.; Udoh, U.H. Effect of Dietary Organic Acids on Nutrient Digestibility, Faecal Moisture, Digesta pH and Viscosity of Broiler Chickens. MOJ Anat. Physiol. 2019, 6, 40–43. [Google Scholar] [CrossRef]
- Damerow, G. The Chicken Health Handbook: A Complete Guide to Maximizing Flock Health and Dealing with Disease; Storey Publishing, LLC: North Adams, MA, USA, 2016; ISBN 1-60342-858-5. [Google Scholar]
- Machuve, D.; Nwankwo, E.; Mduma, N.; Mbelwa, J. Poultry Diseases Diagnostics Models Using Deep Learning. Front. Artif. Intell. 2022, 5, 733345. [Google Scholar] [CrossRef]
- Zhu, X.; Tao, L.; Liu, H.; Yang, G. Effects of Fermented Feed on Growth Performance, Immune Organ Indices, Serum Biochemical Parameters, Cecal Odorous Compound Production, and the Microbiota Community in Broilers. Poult. Sci. 2023, 102, 102629. [Google Scholar] [CrossRef] [PubMed]
- Wainaina, S.; Lukitawesa; Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of Anaerobic Digestion for Volatile Fatty Acids, Hydrogen or Methane Production: A Critical Review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef]
- Mahato, P.; Rajagopal, R.; Goyette, B.; Adhikary, S. Low-Temperature Anaerobic Digestion of Chicken Manure at High Organic and Nitrogen Loads—Strategies for Controlling Short Chain Fatty Acids. Bioresour. Technol. 2022, 351, 127049. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Corona, L.R.; Parra-Saavedra, K.J.; Mora-Alonzo, R.S.; Macías-Rodríguez, M.E.; Martínez-Preciado, A.H.; Guevara-Martínez, S.J.; Zamudio-Ojeda, A.; Macias-Lamas, A.M. HPLC-DAD Development and Validation Method for Short-Chain Fatty Acids Quantification from Chicken Feces by Solid-Phase Extraction. Separations 2023, 10, 308. [Google Scholar] [CrossRef]
- Ali, Q.; Ma, S.; La, S.; Guo, Z.; Liu, B.; Gao, Z.; Farooq, U.; Wang, Z.; Zhu, X.; Cui, Y.; et al. Microbial Short-Chain Fatty Acids: A Bridge between Dietary Fibers and Poultry Gut Health—A Review. Anim. Biosci. 2022, 35, 1461–1478. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Tampio, E.A.; Blasco, L.; Vainio, M.M.; Kahala, M.M.; Rasi, S.E. Volatile Fatty Acids (VFAs) and Methane from Food Waste and Cow Slurry: Comparison of Biogas and VFA Fermentation Processes. GCB Bioenergy 2019, 11, 72–84. [Google Scholar] [CrossRef]
- Yin, J.; Yu, X.; Wang, K.; Shen, D. Acidogenic Fermentation of the Main Substrates of Food Waste to Produce Volatile Fatty Acids. Int. J. Hydrogen Energy 2016, 41, 21713–21720. [Google Scholar] [CrossRef]
- Liu, H.Y.; Li, X.; Zhu, X.; Dong, W.G.; Yang, G.Q. Soybean Oligosaccharides Attenuate Odour Compounds in Excreta by Modulating the Caecal Microbiota in Broilers. Animal 2021, 15, 100159. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, Y.; Liu, H.; Yang, G.; Li, L. Microbiome-Metabolomics Analysis Reveals Abatement Effects of Itaconic Acid on Odorous Compound Production in Arbor Acre Broilers. BMC Microbiol. 2023, 23, 183. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Zeng, X.F.; Zhu, J.L.; Wang, S.; Liu, X.T.; Hou, C.L.; Thacker, P.A.; Qiao, S.Y. Effects of Dietary Lactobacillus Plantarum B1 on Growth Performance, Intestinal Microbiota, and Short Chain Fatty Acid Profiles in Broiler Chickens. Poult. Sci. 2016, 95, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Qaisrani, S.N.; Moquet, P.C.A.; van Krimpen, M.M.; Kwakkel, R.P.; Verstegen, M.W.A.; Hendriks, W.H. Protein Source and Dietary Structure Influence Growth Performance, Gut Morphology, and Hindgut Fermentation Characteristics in Broilers. Poult. Sci. 2014, 93, 3053–3064. [Google Scholar] [CrossRef] [PubMed]
- Palander, S. Volatile Fatty Acid Profile in Caecal Digesta of Growing Turkey Poults; SeAMK Julkaisut, Publications of Seinäjoki University of Applied Sciences: Seinäjoki, Finland; ISBN 978-952-7317-21-1.
- Palander, S.; Näsi, M.; Järvinen, S. Effect of Age of Growing Turkeys on Digesta Viscosity and Nutrient Digestibility of Maize, Wheat, Barley and Oats Fed as Such or with Enzyme Supplementation. Arch. Anim. Nutr. 2005, 59, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Xu, Y.-J.; Liu, Y. Influence of Different Dietary Oil Consumption on Nutrient Malabsorption: An Animal Trial Using Sprague Dawley Rats. J. Food Biochem. 2021, 45, e13695. [Google Scholar] [CrossRef]
- Loughrin, J.H.; Szogi, A.A. Free Fatty Acids and Sterols in Swine Manure. J. Environ. Sci. Health B 2006, 41, 31–42. [Google Scholar] [CrossRef]
- Yasuhara, A. Identification of Volatile Compounds in Poultry Manure by Gas Chromatography—Mass Spectrometry. J. Chromatogr. A 1987, 387, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Bicudo, J.R.; Schmidt, D.R.; Powers, W.; Zahn, J.A.; Tengman, C.L.; Clanton, C.J.; Jacobson, L.D. Odor and VOC Emissions from Swine Manure Storages. In Proceedings of the Odors and Air Pollutants Conference 2002, Diamond Bar, CA, USA, 5 April 2002; Water Environment Federation: Alexandria, VA, USA, 2002; pp. 123–135. [Google Scholar]
- Kimball, B.A.; Yamazaki, K.; Kohler, D.; Bowen, R.A.; Muth, J.P.; Opiekun, M.; Beauchamp, G.K. Avian Influenza Infection Alters Fecal Odor in Mallards. PLoS ONE 2013, 8, e75411. [Google Scholar] [CrossRef]
- Melaku, M.; Zhong, R.; Han, H.; Wan, F.; Yi, B.; Zhang, H. Butyric and Citric Acids and Their Salts in Poultry Nutrition: Effects on Gut Health and Intestinal Microbiota. Int. J. Mol. Sci. 2021, 22, 10392. [Google Scholar] [CrossRef]
- Tangtrakulwanich, K.; Albuquerque, T.A.; Brewer, G.J.; Baxendale, F.P.; Zurek, L.; Miller, D.N.; Taylor, D.B.; Friesen, K.A.; Zhu, J.J. Behavioural Responses of Stable Flies to Cattle Manure Slurry Associated Odourants. Med. Vet. Entomol. 2015, 29, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Ernstgård, L.; Norbäck, D.; Nordquist, T.; Wieslander, G.; Wålinder, R.; Johanson, G. Acute Effects of Exposure to Vapors of 3-Methyl-1-Butanol in Humans. Indoor Air 2013, 23, 227–235. [Google Scholar] [CrossRef]
- Joguet, N.; Jing, L.; Jamois, F.; Dumargue, P. Characterization of Volatile Organic Compounds (VOCs) from Farms Effluents: Interest of HS-SPME-GC-MS Technique for Laboratory and Field Test. Atmosphere 2023, 14, 928. [Google Scholar] [CrossRef]
- Al-Dalali, S.; Li, C.; Xu, B. Effect of Frozen Storage on the Lipid Oxidation, Protein Oxidation, and Flavor Profile of Marinated Raw Beef Meat. Food Chem. 2021, 376, 131881. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.; Maurer, S.; Herbert, U.; Kreyenschmidt, J.; Kaul, P. Detection of Volatile Organic Compounds Arising from Chicken Breast Filets Under Modified Atmosphere Packaging Using TD-GC/MS. Food Anal. Methods 2018, 11, 88–98. [Google Scholar] [CrossRef]
- Alagawany, M. Biological Effects and Modes of Action of Carvacrol in Animal and Poultry Production and Health—A Review. Adv. Anim. Vet. Sci. 2015, 3, 73–84. [Google Scholar] [CrossRef]
- Sirilun, S.; Chaiyasut, C.; Sivamaruthi, B.S.; Peerajan, S.; Kumar, N.; Kesika, P. Phenethyl Alcohol Is an Effective Non-Traditional Preservative Agent for Cosmetic Preparations. Asian J. Pharm. Clin. Res. 2017, 10, 129–133. [Google Scholar] [CrossRef]
- Kyoui, D.; Saito, Y.; Takahashi, A.; Tanaka, G.; Yoshida, R.; Maegaki, Y.; Kawarai, T.; Ogihara, H.; Suzuki, C. Antibacterial Activity of Hexanol Vapor In Vitro and on the Surface of Vegetables. Foods 2023, 12, 3097. [Google Scholar] [CrossRef]
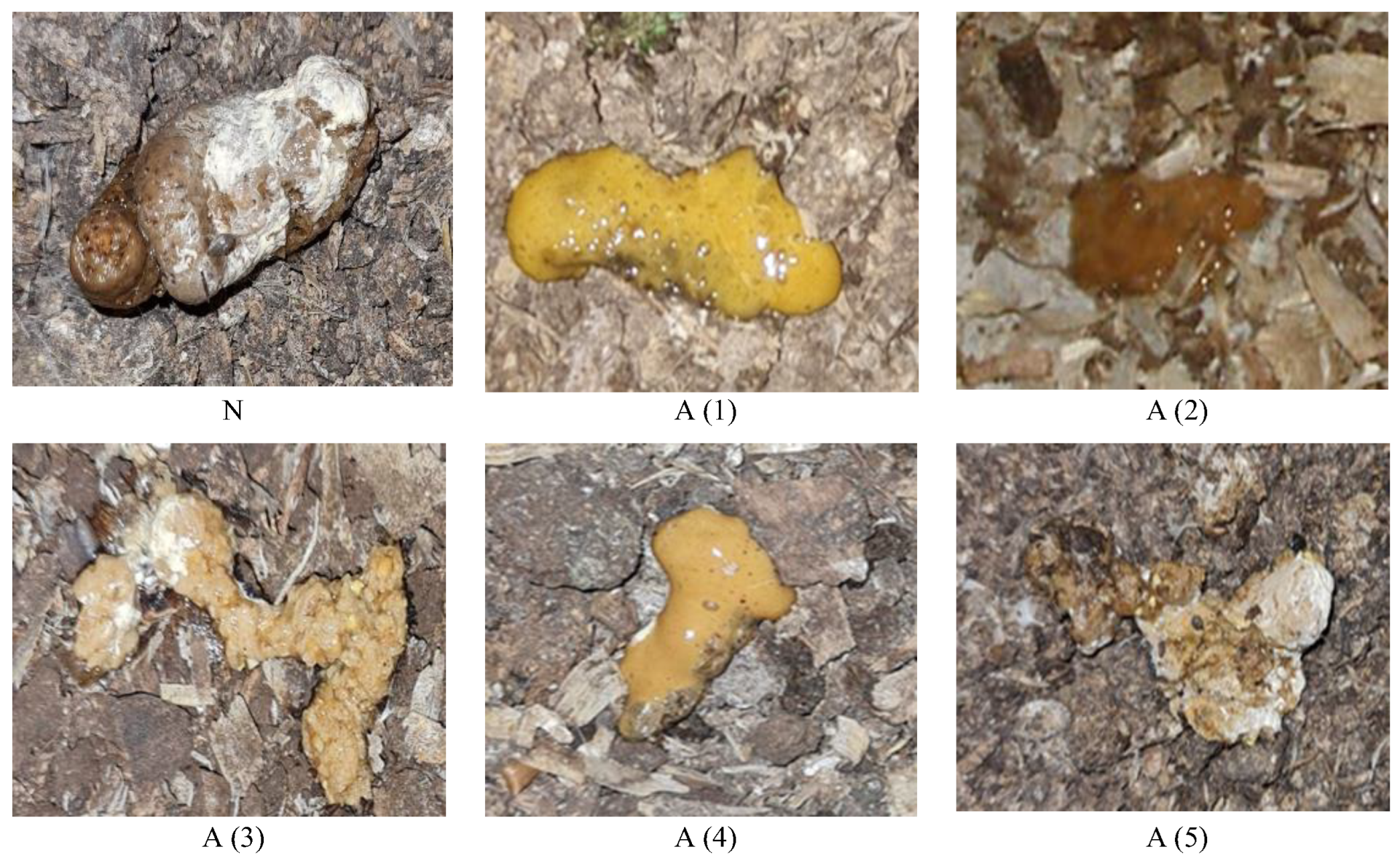
| Periods of Sample Collection | Group Name | Visual Droppings Evaluation | Explanation | Average Chicken Age |
|---|---|---|---|---|
| I GR (0–5 days) | I GR N4 | Normal | Brown or greyish-brown, solid, with a tiny bit of covered white on top | 4 days |
| II GR (6–10 days) | II GR N7 | Normal | Brown or greyish-brown, solid, with a tiny bit of covered white on top | 7 days |
| II GR A7 | Abnormal | Liquid | 7 days | |
| III GR (11–20 days) | III GR N13 | Normal | Brown or greyish-brown, solid, with a tiny bit of covered white on top | 13 days |
| III GR A13 | Abnormal | Mixed with feed residues | 13 days | |
| III GR A14 | Abnormal | Liquid | 14 days | |
| III GR A16 | Abnormal | Liquid/foamy | 16 days | |
| IV GR (21–30 days) | IV GR N22 | Normal | Brown or greyish-brown, solid, with a tiny bit of covered white on top | 22 days |
| IV GR A23 | Abnormal | Feed residues, possible pathology, coccidiosis, Eimeria averculina | 23 days | |
| IV GR A27 | Abnormal | Possible pathology, coccidiosis, Eimeria acervulina, Eimeria maxima | 27 days | |
| IV GR A27_1 | Abnormal | Feed residues, possible pathology, coccidiosis, Eimeria maxima | 27 days | |
| IV GR A21 | Abnormal | Mixed with feed residues | 21 days | |
| V GR (31–40 days) | V GR A37 | Abnormal | Possible pathology, mixed with intestinal mucosa | 37 days |
| V GR A36 | Abnormal | Possible pathology, mixed with feed residues | 36 days |
| Sample Group Name | Texture Hardness, mJ | pH | DM, % |
|---|---|---|---|
| I GR N4 | 0.20 ± 0.01 a | 5.10 ± 0.21 ab | 35.36 ± 1.38 jk |
| II GR N7 | 0.70 ± 0.03 e | 5.83 ± 0.23 adef | 37.30 ± 1.46 k |
| II GR A7 | 0.20 ± 0.01 a | 5.25 ± 0.21 adg | 21.03 ± 0.82 a |
| III GR N13 | 0.50 ± 0.02 d | 5.96 ± 0.24 eh | 33.38 ± 1.30 ghij |
| III GR A13 | 0.20 ± 0.01 a | 6.63 ± 0.27 hi | 38.68 k ± 1.51 |
| III GR A14 | 0.20 ± 0.01 a | 5.83 ± 0.23 cdef | 30.84 ± 1.20 fg |
| III GR A16 | 0.30 ± 0.01 b | 6.90 ± 0.28 i | 31.34 ± 1.22 fh |
| IV GR N22 | 0.40 ± 0.02 c | 5.27 ± 0.21 ae | 31.89 ± 1.24 fi |
| IV GR A23 | 0.30 ± 0.01 b | 5.42 ± 0.22 afgh | 26.14 ± 1.02 bd |
| IV GR A27 | 0.20 ± 0.01 a | 4.84 ± 0.19 a | 19.80 ± 0.77 a |
| IV GR A27_1 | 0.30 ± 0.01 b | 5.70 ± 0.23 bceg | 22.93 ± 0.89 ab |
| IV GR A21 | 0.40 ± 0.02 c | 6.99 ± 0.28 i | 26.79 ± 1.05 de |
| V GR A37 | 0.40 ± 0.02 c | 5.74 ± 0.23 bcdef | 28.88 ± 1.13 cdf |
| V GR A36 | 0.50 ± 0.02 d | 5.21 ± 0.21 acg | 25.64 ± 1.00 bce |
| Sample Group Name | Color Coordinates, NBS | ||
|---|---|---|---|
| L* | a* | b* | |
| I GR N4 | 53.13 ± 2.26 ef | 0.40 ± 0.02 a | 5.75 ± 0.26 a |
| II GR N7 | 48.58 ± 2.06 cde | 0.63 ± 0.02 ab | 10.06 ± 0.45 cdef |
| II GR A7 | 41.89 ± 1.78 b | 2.24 ± 0.09 d | 9.00 ± 0.40 bc |
| III GR N13 | 42.46 ± 1.80 b | 1.96 ± 0.08 d | 11.92 ± 0.53 fg |
| III GR A13 | 41.10 ± 1.75 b | 2.06 ± 0.08 d | 11.61 ± 0.52 fg |
| III GR A14 | 33.77 ± 1.43 ab | 3.18 ± 0.13 f | 11.43 ± 0.51 fg |
| III GR A16 | 34.64 ± 1.47 ab | 2.01 ± 0.08 d | 9.40 ± 0.42 be |
| IV GR N22 | 52.65 ± 2.24 ef | 0.75 ± 0.03 b | 9.28 ± 0.41 bd |
| IV GR A23 | 39.66 ± 1.68 b | 2.56 ± 0.10 e | 11.90 ± 0.53 fg |
| IV GR A27 | 54.60 ± 2.32 f | 1.46 ± 0.06 c | 11.59 ± 0.52 fg |
| IV GR A27_1 | 45.46 ± 1.93 bd | 3.14 ± 0.12 f | 15.03 ± 0.67 h |
| IV GR A21 | 68.00 ± 2.89 g | 1.20 ± 0.05 c | 8.11 ± 0.36 b |
| V GR A37 | 43.07 ± 1.83 bc | 4.68 ± 0.18 g | 18.75 ± 0.84 i |
| V GR A36 | 49.56 ± 2.10 df | 3.17 ± 0.13 f | 20.36 ± 0.91 i |
| Sample Group Name | Acetic Acid | Propanoic Acid | Isobutyric Acid | Butyric Acid | Isovaleric Acid | Valeric Acid | Caproic Acid |
|---|---|---|---|---|---|---|---|
| I GR N4 | 26.07 ± 1.05 bc | nd | nd | 1.71 ± 0.07 b | nd | nd | 0.040 ± 0.002 a |
| II GR N7 | 22.73 ± 0.92 b | nd | nd | nd | nd | nd | nd |
| II GR A7 | 93.39 ± 3.78 h | 5.41 ± 0.24 d | 0.44 ± 0.02 | 16.50 ± 0.64 e | 0.56 ± 0.02 d | 0.75 ± 0.03 b | nd |
| III GR N13 | 10.11 ± 0.41 a | nd | nd | nd | nd | nd | nd |
| III GR A13 | 5.96 ± 0.24 a | nd | nd | nd | 0.14 ± 0.01 b | nd | nd |
| III GR A14 | 62.70 ± 2.54 g | 1.74 ± 0.08 b | nd | 16.24 ± 0.63 e | nd | 0.28 ± 0.01 a | nd |
| III GR A16 | 10.32 ± 0.42 a | 0.83 ± 0.04 a | nd | 2.09 ± 0.08 bc | 0.28 ± 0.01 c | nd | nd |
| IV GR N22 | 5.99 ± 0.24 a | nd | nd | nd | nd | nd | nd |
| IV GR A23 | 37.64 ± 1.52 e | nd | nd | nd | nd | nd | nd |
| IV GR A27 | 45.10 ± 1.82 f | 2.27 ± 0.10 c | nd | 3.58 ± 0.14 d | nd | nd | nd |
| IV GR A27_1 | 32.65 ± 1.32 de | nd | nd | nd | nd | nd | nd |
| IV GR A21 | 5.46 ± 0.22 a | nd | nd | nd | 0.066 ± 0.03 a | nd | nd |
| V GR A37 | 93.22 ± 3.77 h | nd | nd | 2.55 ± 0.10 c | nd | nd | nd |
| V GR A36 | 28.45 ± 1.15 cd | nd | nd | 0.59 ± 0.02 a | nd | nd | nd |
| Sample Group | C16:0 | C18:0 | C20:0 | C18:1 cis | C20:1 | C22:1 | C18:2 cis | C20:2 | C18:3 γ | C18:3 α |
|---|---|---|---|---|---|---|---|---|---|---|
| I GR N4 | 13.39 ± 0.53 g | 5.03 ± 0.23 bd | nd | 19.93 ± 0.86 ac | 4.97 ± 0.23 b | 1.10 ± 0.04 | 31.94 ± 1.11 c | 0.69 ± 0.03 | nd | 22.96 ± 0.93 e |
| II GR N7 | 13.96 ± 0.56 gh | 5.43 ± 0.25 cde | nd | 17.59 ± 0.75 a | nd | nd | 48.01 ± 1.67 g | nd | nd | 15.00 ± 0.61 c |
| II GR A7 | 7.72 ± 0.31 ab | 7.05 ± 0.32 hij | nd | 24.35 ± 1.04 d | 1.03 ± 0.05 a | nd | 21.62 ± 0.75 a | nd | nd | 38.23 ± 1.55 h |
| III GR N13 | 14.96 ± 0.60 hi | 5.94 ± 0.27 ef | nd | 17.25 ± 0.74 a | nd | nd | 47.45 ± 1.65 g | nd | 0.35 ± 0.01 | 14.05 ± 0.57 c |
| III GR A13 | 14.81 ± 0.59 gi | 4.85 ± 0.22 bc | nd | 23.84 ± 1.02 d | nd | nd | 38.00 ± 1.32 def | nd | nd | 18.50 ± 0.75 d |
| III GR A14 | 8.92 ± 0.36 bcd | 4.64 ± 0.21 b | nd | 17.14 ± 0.74 a | nd | nd | 38.12 ± 1.32 def | nd | nd | 31.18 ± 1.26 f |
| III GR A16 | 9.97 ± 0.40 de | 6.07 ± 0.28 eg | nd | 28.43 ± 1.22 e | nd | nd | 20.66 ± 0.72 a | nd | nd | 34.86 ± 1.41 g |
| IV GR N22 | 9.05 ± 0.36 bce | 2.95 ± 0.14 a | nd | 27.61 ± 1.18 e | nd | nd | 55.60 ± 1.93 h | nd | nd | 4.78 ± 0.19 a |
| IV GR A23 | 19.05 ± 0.76 j | 7.15 ± 0.33 hik | nd | 24.46 ± 1.05 d | nd | nd | 27.76 ± 0.96 b | nd | nd | 21.57 ± 0.87 e |
| IV GR A27 | 15.61 ± 0.62 i | 6.66 ± 0.30 fgi | nd | 28.41 ± 1.22 e | nd | nd | 34.72 ± 1.20 cd | nd | nd | 14.59 ± 0.59 c |
| IV GR A27_1 | 8.11 ± 0.32 ac | 2.66 ± 0.12 a | nd | 31.49 ± 1.35 f | nd | nd | 49.57 ± 1.72 g | nd | nd | 8.17 ± 0.33 b |
| IV GR A21 | 6.86 ± 0.27 a | 2.27 ± 0.10 a | 0.14 ± 0.01 | 19.67 ± 0.84 ab | 5.43 ± 0.25 c | nd | 35.04 ± 1.22 cf | nd | nd | 30.59 ± 1.24 f |
| V GR A37 | 11.64 ± 0.47 f | 6.63 ± 0.30 fgh | nd | 20.58 ± 0.88 bc | nd | nd | 25.80 ± 0.90 b | nd | nd | 35.35 ± 1.43 gh |
| V GR A36 | 20.56 ± 0.82 j | 7.51 ± 0.34 jk | nd | 26.24 ± 1.13 bc | nd | nd | 34.76 ± 1.21 ce | nd | nd | 10.92 ± 0.44 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mozuriene, E.; Mockus, E.; Klupsaite, D.; Starkute, V.; Tolpeznikaite, E.; Gruzauskas, V.; Gruzauskas, R.; Paulauskaite-Taraseviciene, A.; Raudonis, V.; Bartkiene, E. Physical and Chemical Characteristics of Droppings as Sensitive Markers of Chicken Health Status. Animals 2024, 14, 1389. https://doi.org/10.3390/ani14091389
Mozuriene E, Mockus E, Klupsaite D, Starkute V, Tolpeznikaite E, Gruzauskas V, Gruzauskas R, Paulauskaite-Taraseviciene A, Raudonis V, Bartkiene E. Physical and Chemical Characteristics of Droppings as Sensitive Markers of Chicken Health Status. Animals. 2024; 14(9):1389. https://doi.org/10.3390/ani14091389
Chicago/Turabian StyleMozuriene, Erika, Ernestas Mockus, Dovile Klupsaite, Vytaute Starkute, Ernesta Tolpeznikaite, Valentas Gruzauskas, Romas Gruzauskas, Agne Paulauskaite-Taraseviciene, Vidas Raudonis, and Elena Bartkiene. 2024. "Physical and Chemical Characteristics of Droppings as Sensitive Markers of Chicken Health Status" Animals 14, no. 9: 1389. https://doi.org/10.3390/ani14091389







