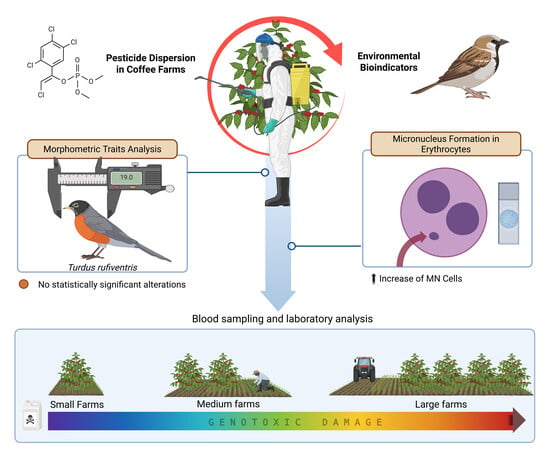
Birds as Environmental Bioindicators of Genotoxicity in Brazilian Cerrado Farmlands: An In Situ Approach
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
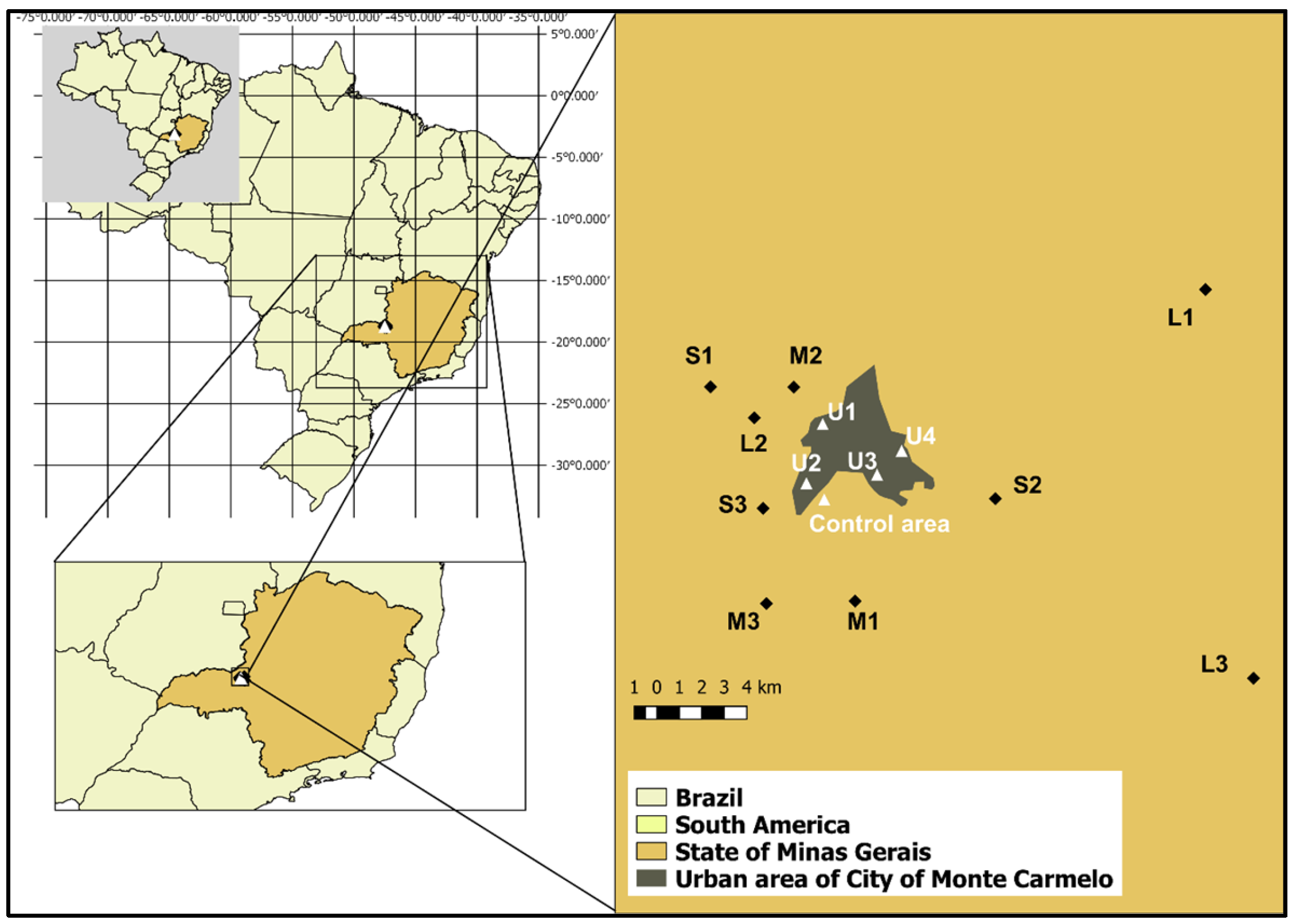
2.1. Study Areas
2.2. Data Collection (Birds)
2.3. Blood Samples, Micronuclei Tests, and Staining
2.4. Evaluation of Micronuclei
2.5. Statistical Analysis
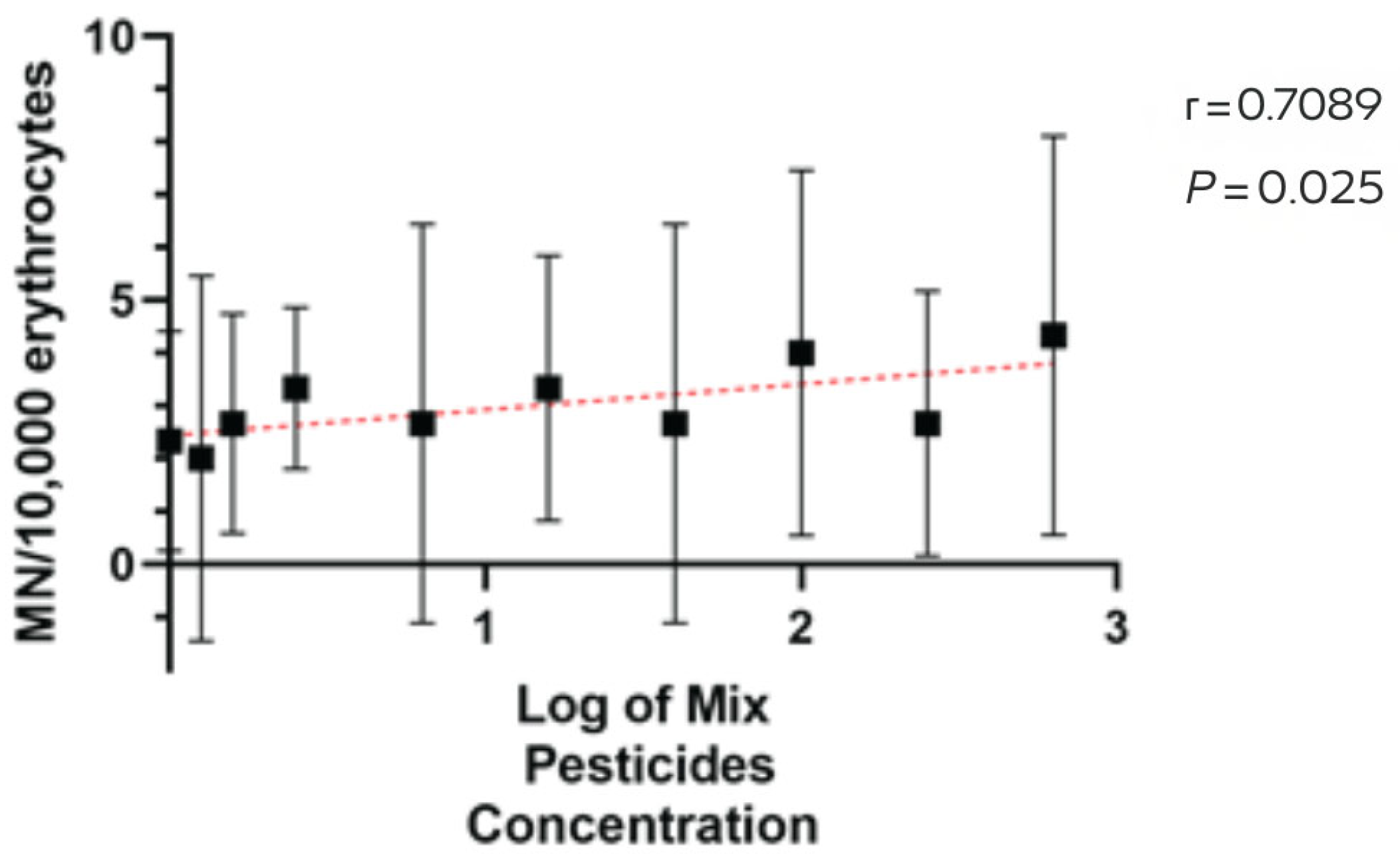
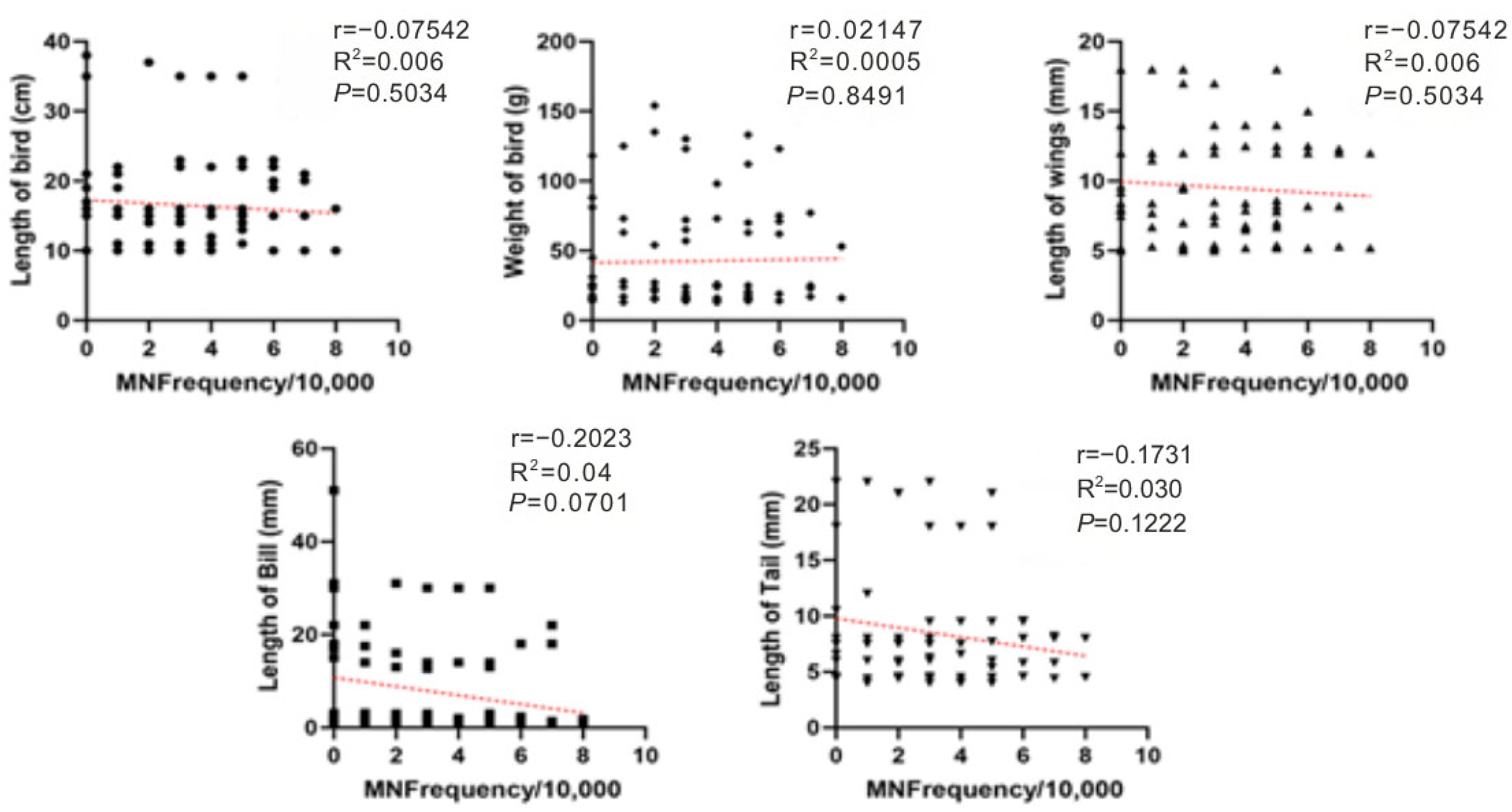
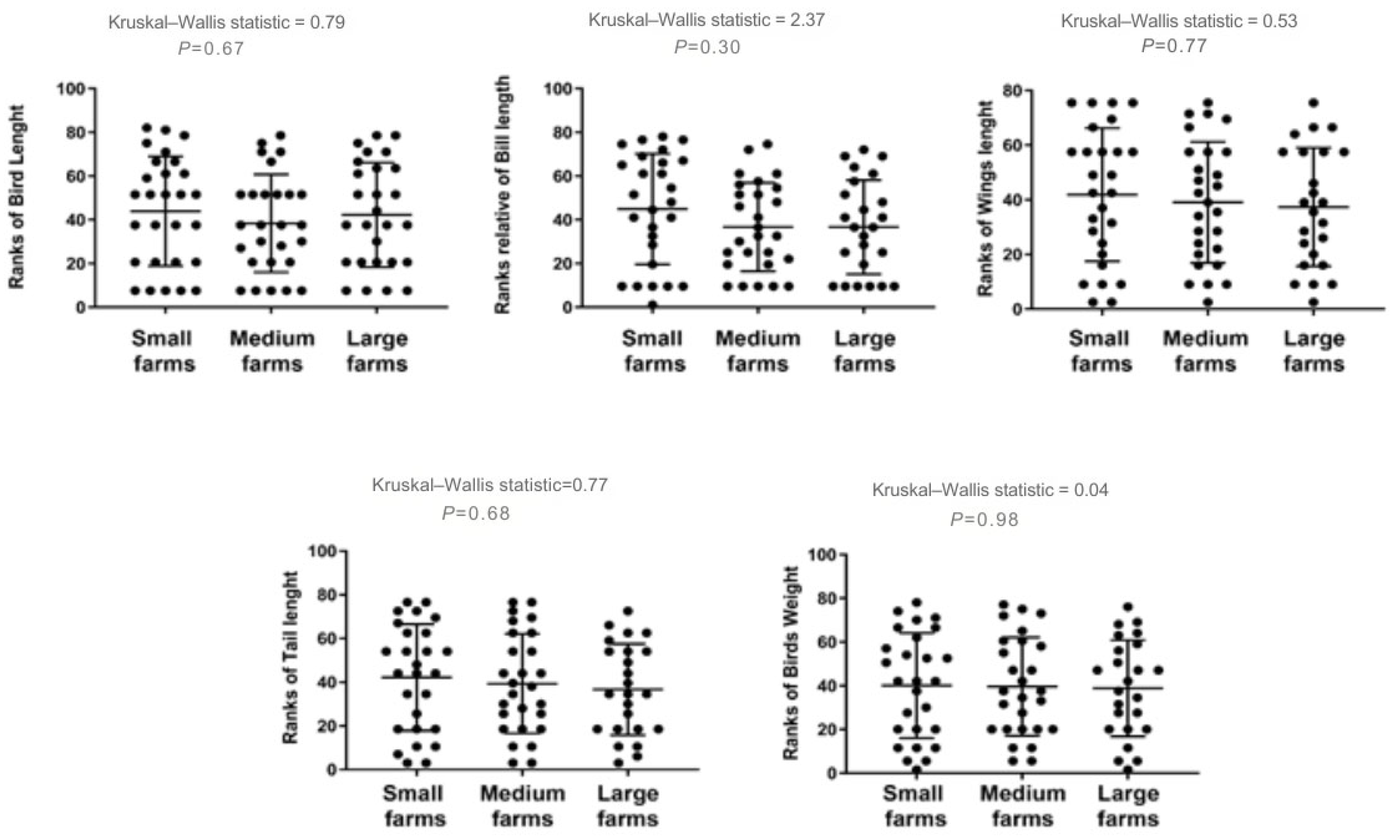
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rattner, B.A.; Bean, T.G.; Beasley, V.R.; Berny, P.; Eisenreich, K.M.; Elliott, J.E.; Eng, M.L.; Fuchsman, P.C.; King, M.D.; Mateo Soria, R.; et al. Wildlife ecological risk assessment in the 21st century: Promising technologies to assess toxicological effects. Integr. Environ. Assess. Manag. 2023, 20, 725–748. [Google Scholar] [CrossRef]
- Kessler, M.; Abrahamczyk, S.; Bos, M.; Buchori, D.; Putra, D.; Gradstein, S.; Höhn, P.; Kluge, J.; Orend, F.; Pitopang, R.; et al. Cost-effectiveness of plant and animal biodiversity indicators in tropical forest and agroforest habitats. J. Appl. Ecol. 2010, 48, 330–339. [Google Scholar] [CrossRef]
- Temple, S.A. Can Birds be indicators of Environmental Hazards? Passeng. Pigeon 1988, 50, 311–313. [Google Scholar]
- Koskimies, P. Birds as a tool in environmental monitoring. Ann. Zool. Fennici. 1989, 26, 153–166. [Google Scholar]
- Burger, J.; Gochfeld, M. Marine birds as sentinels of environmental pollution. Ecohealth 2004, 1, 263–294. [Google Scholar] [CrossRef]
- Fa, E.; Po, E.; Edet, D. Paramount roles of wild birds as bioindicators of contamination. Int. J. Avian Wildl. Biol. 2017, 2, 00041. [Google Scholar] [CrossRef]
- Mansfield, I.; Reynolds, S.J.; Lynch, I.; Matthews, T.J.; Sadler, J.P. Birds as bioindicators of plastic pollution in terrestrial and freshwater environments. Environ. Pollut. 2024, 330, 123790. [Google Scholar] [CrossRef]
- Saleh, K.; Sarhan, M. Clastogenic analysis of chicken farms using micronucleus test in peripheral blood. J. Appl. Sci. Res. 2007, 3, 1646–1649. [Google Scholar]
- Sheta, B.; El-Qenawy, I.; El-Gammal, H. Utilization of birds of different mating system behavior and feeding habits as a bioindicator for urbanization lead pollution. Catrina Int. J. Environ. Sci. 2022, 26, 67–73. [Google Scholar] [CrossRef]
- Baesse, C.Q.; Tolentino, V.C.M.; Silva-Marcos, A.; Silva-Andrade, A.; Ferreira, G.A.; Paniago, L.P.M.; Nepomuceno, J.C.; Melo, C. Micronucleus as biomarker of genotoxicity in birds from Brazilian Cerrado. Ecotoxicol. Environ. Saf. 2015, 115, 223–228. [Google Scholar] [CrossRef]
- Baesse, C.Q.; Tolentino, V.C.; Morelli, S.; Melo, C. Effect of urbanization on the micronucleus frequency in birds from forest fragments. Ecotoxicol. Environ. Saf. 2019, 171, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Souto, H.N.; Campos Júnior, E.O.; Campos, C.F.; Rodrigues, T.S.; Pereira, B.B.; Morelli, S. Bioindicatoring birds: The use of a micronuclei test as a tool to assess environmental pollutants on coffee farms in southeast Brazil. Environ. Sci. Pollut. Res. 2018, 25, 24084–24092. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Zheng, X.; Bai, F.; Wu, Y.; Lei, W.; Zhang, Z.; Chen, L.; Huang, W.; Li, X.; Wang, H.; et al. Pollutant exposure for Chinese wetland birds: Ecotoxicological endpoints and biovectors. ACS EST Water 2024, 4, 2076–2087. [Google Scholar] [CrossRef]
- Ribeiro, P.V.A.; Baesse, C.Q.; Cury, M.C.; Melo, C.D. Leukocyte profile of the helmeted manakin, antilophia galeata (passeriformes: Pipridae) in a cerrado forest fragment. Zoologia 2020, 37, e46441. [Google Scholar] [CrossRef]
- Tomazelli, J.; Rodrigues, G.Z.P.; Franco, D.; de Souza, M.S.; Burghausen, J.H.; Panizzon, J.; Kayser, J.M.; Loiko, M.R.; Schneider, A.; Linden, R.; et al. Potential use of distinct biomarkers (trace metals, micronuclei, and nuclear abnormalities) in a heterogeneous sample of birds in southern Brazil. Environ. Sci. Pollut. Res. Int. 2021, 29, 14791–14805. [Google Scholar] [CrossRef]
- Silveira, E.D.R.; Benvindo Souza, M.; Assis, R.A.; Santos, C.G.A.; Amorim, N.P.L.; Borges, R.E.; de Melo, C.; de Souza Santos, L.R. Micronucleus and different nuclear abnormalities in wild birds in the Cerrado, Brazil. Environ. Sci. Pollut. Res. Int. 2022, 29, 14279–14287. [Google Scholar] [CrossRef]
- Heddle, J.A. A rapid in vivo test for chromosomal damage. Mutat. Res. 1973, 18, 187–190. [Google Scholar] [CrossRef]
- Schmid, W. The micronucleus test. Mutat. Res. 1975, 31, 9–15. [Google Scholar] [CrossRef]
- Cid, F.D.; Gatica-Sosa, C.; Antón, R.; Caviedes-Vidal, E. Contamination of heavy metals in birds from embalse la florida (San Luis, Argentina). J. Environ. Monit. 2009, 11, 2044. [Google Scholar] [CrossRef]
- Markowski, M.; Kaliński, A.; Skwarska, J.; Wawrzyniak, J.; Bańbura, M.; Markowski, J.; Zieliński, P.; Bańbura, J. Avian feathers as bioindicators of the exposure to heavy metal contamination of food. Bull. Environ. Contam. Toxicol. 2013, 91, 302–305. [Google Scholar] [CrossRef]
- Olayemi, O.A.; Taiwo, A.A.; Jagun, J.A. Micronucleus as a biomarker of genotoxicity in village weaver bird (Ploceus cucullatus). World’s Vet. J. 2014, 4, 48–53. [Google Scholar] [CrossRef]
- Vargiya, D.; Jethva, B.; Pandya, D.J. Feather heavy metal contamination in various species of waterbirds from Asia: A review. Environ. Monit. Assess. 2021, 194, 26. [Google Scholar] [CrossRef] [PubMed]
- Peris, A.; Baos, R.; Martínez, A.; Sergio, F.; Hiraldo, F.; Eljarrat, E. Pesticide contamination of bird species from Doñana National Park (southwestern Spain): Temporal trends (1999–2021) and reproductive impacts. SSRN Electron. J. 2022, 323, 121240. [Google Scholar] [CrossRef]
- Zhou, W.; Li, M.; Achal, V. A comprehensive review on environmental and human health impacts of chemical pesticide usage. Emerg. Contam. 2025, 11, 100410. [Google Scholar] [CrossRef]
- Ghisari, M.; Long, M.; Tabbo, A.; Bonefeld-Jorgensen, E.C. Effects of currently used pesticides and their mixtures on the function of thyroid hormone and aryl hydrocarbon receptor in cell culture. Toxicol. Appl. Pharmacol. 2015, 284, 292–303. [Google Scholar] [CrossRef]
- Ogada, D.L. The power of poison: Pesticide poisoning of Africa’s wildlife. Ann. N. Y. Acad. Sci. 2014, 1322, 1–20. [Google Scholar] [CrossRef]
- Katagi, T.; Fujisawa, T. Acute toxicity and metabolism of pesticides in birds. J. Pestic. Sci. 2021, 46, 305–321. [Google Scholar] [CrossRef]
- Jayakumar, S.; Muralidharan, S.; Dhananjayan, V. Organochlorine Pesticide Residues Among Colonial Nesting Birds in Tamil Nadu, India: A Maiden Assessment from Their Breeding Grounds. Arch. Environ. Con. Tox. 2020, 78, 555–567. [Google Scholar] [CrossRef]
- Humann-Guilleminot, S.; Clément, S.; Desprat, J.; Binkowski, L.J.; Glauser, G.; Helfenstein, F. Large-scale survey of house sparrows feathers reveals ubiquitous presence of neonicotinoids in farmlands. Sci. Total Environ. 2019, 660, 1091–1097. [Google Scholar] [CrossRef]
- Pompeu, J.; Assis, T.O.; Ometto, J.P. Landscape changes in the Cerrado: Challenges of land clearing, fragmentation and land tenure for biological conservation. Sci. Total Environ. 2024, 906, 167581. [Google Scholar] [CrossRef]
- Borges-Almeida, K.; Benvindo-Souza, M.; Martello, F.; Mathias, L.; de Melo e Silva, D.; Collevatti, R.G. Habitat fragmentation mediates the frequency of cytogenetic endpoints in small non-flying mammals in agriculture landscapes in Cerrado. Environ. Sci. Pollut. Res. Int. 2025, 32, 16432–16445. [Google Scholar] [CrossRef] [PubMed]
- Zampiroli, J.A.; Ferraudo, A.S.; Oliveira, R.B.; Ferreira, M.C. Application technology for chemically controlling coffee leaf miner in the Cerrado of Minas Gerais State. Rev. Bras. Ciênc. Agrár. 2017, 12, 97–103. [Google Scholar] [CrossRef]
- Silva, F.C.; Pereira, A.A.; Reis, P.R.; Carvalho, G.R.; Silva, E.A.A.; Oliveira, A.C.B.; Souza, J.M. Genetic diversity and agronomic performance of Coffea arabica accessions in the Cerrado Mineiro. Agronomy 2025, 15, 222. [Google Scholar] [CrossRef]
- Rosa, R.; Lima, S.C.; Assunção, L.W. Abordagem preliminar das condições climáticas de Uberlândia (MG). Socied. Nat. 1991, 3, 91–108. [Google Scholar] [CrossRef]
- Sigrist, T. Guia de Campo Avis Brasilis: Avifauna Brasileira, 1st ed.; Avis Brasilis: São Paulo, Brazil, 2005. [Google Scholar]
- Rudkin, C.; Sewart, G.D. Behaviour of hens in cages: A pilot study using video tapes. A report for the Rural Industries Research and Development Corporation (RIRDC). Queensland 2003, 40, 102. [Google Scholar]
- CBRO–Comitê Brasileiro de Registros Ornitológicos. 2015. Available online: https://www.cbro.org.br/wp-content/uploads/2020/06/Piacentini-et-al-2015-RBO.pdf (accessed on 14 February 2021).
- Palhares, D.; Grisolia, C.K. Comparison between the micronuclei frequencies of kidney and gill erythrocytes in tilapia fish, following mitomycin C treatment. Gen. Mol. Biol. 2002, 25, 281–284. [Google Scholar] [CrossRef]
- Fenech, M. The in vitro micronucleus technique. Mutat. Res. 2000, 455, 81–95. [Google Scholar] [CrossRef]
- Ramírez-Muñoz, M.P.; Zuñiga, G.; Bugarín, O.T.; Portilla, E.; García-Martinez, D.; Ramos, A.; Cantú, J.M.; Sánchez-Corona, J. Evaluation of the Micronucleus Test in Peripheral Blood Erythrocytes by Use of the Splenectomized Model. Labor. Ann. Sci. 1999, 49, 418–420. [Google Scholar]
- Zúñiga-González, G.; Torres-Bugarín, O.; Zamora-Pérez, A.; Gomez-Meda, B.C.; Ramos-Ibarra, M.L.; Martínez-González, S.; González-Rodriguez, A.; Luna-Aguirre, J.; Ramos-Mora, A.; Ontiveros-Lira, D.; et al. Differences on the number of micronucleated erythrocytes among young and adult animals including humans. Spontaneous micronuclei in 43 species. Mutat. Res. 2001, 494, 161–167. [Google Scholar] [CrossRef]
- Polleta, G.L.; Larriera, A.; Kleinsorge, E.; Mudry, M.D. Caiman latirostris (broad-snouted caiman) as a sentinel organism for genotoxic monitoring: Basal values determination of micronucleus and comet assay. Mutat. Res. Mol. Mech. Mutagen. 2008, 650, 202–209. [Google Scholar] [CrossRef]
- Ismail, M.; Khan, Q.M.; Ali, R.; Ali, T.; Mobeen, A. Genotoxicity of chlorpyrifos in freshwater fish Labeo rohita using Alkaline Single-Cell Gel Electrophoresis (Comet) assay. Drug. Chem. Toxicol. 2014, 37, 466–471. [Google Scholar] [CrossRef]
- Cruz-Esquivel, A.; Viloria-Rivas, J.; Marrugo-Negrete, J. Genetic damage in Rhinella marina populations in habitats affected by agriculture in the middle region of the Sina River, Colombia. Environ. Sci. Pollut. Res. 2017, 24, 27392–27401. [Google Scholar] [CrossRef]
- Dutta, S.; Bahadur, M. Cytogenetic analysis of micronuclei and cell death parameters in epithelial cells of pesticide exposed tea garden workers. Toxicol. Mech. Methods 2016, 26, 627–634. [Google Scholar] [CrossRef]
- Jacobsen-Pereira, C.H.; Santos, C.R.; Maraslis, F.T.; Pimental, L.; Feijó, A.J.L.; Silva-Ioara, C.; Medeiros, G.S.; Zeferino, R.C.; Pedrosa, R.C.; Maluf, S.W. Markers of genotoxicity and oxidative stress in farmers exposed to pesticides. Ecotoxiol. Environ. Saf. 2018, 148, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Begon, M.C.R.; Townsend, E.; Harper, L. Ecologia de Indivíduos a Ecossistemas, 4th ed.; Artmed: Porto Alegre, Brazil, 2007. [Google Scholar]
- Stoncius, D. Spontaneus micronuclei in enbryos of the Black-headed Gull (Larus ridibundus, L.) populations. Ekologija 2003, 1, 63–66. [Google Scholar]
- Gómez-Meda, B.C.; Zamora-Perez, A.L.; Luna-Aguirre, J.; González-Rodríguez, A.; Luisa Ramos-Ibarra, M.; Torres-Bugarín, O.; Zúñiga-González, G.M. Nuclear abnormalities in erythrocytes of parrots (Aratinga canicularis) related to genotoxic damage. Avian Pathol. 2006, 35, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Kursa, M.; Bezrukov, V. Health status of an Antartic top predator: Micronuclei frequency and White blood cell differentials in the South Polar Skua (Catharacta maccorminck). Polarforschung 2007, 37, 1–5. [Google Scholar]
| Active Ingredient | Concentration (g/L) | Class of Pesticide | Pesticide Syrup Volume (L/ha) | ||
|---|---|---|---|---|---|
| Small Farms | Medium Farms | Large Farms | |||
| Copper hydroxide | 691 | Fungicide | 0.5 | 0.5 | 1 |
| Carbendazim | 250 | Fungicide | 5 | 5 | 5 |
| Pyraclostrobin | 133 | Fungicide | 500 | 500 | 500 |
| Imidacloprid | 700 | Insecticide | 50 | 100 | 100 |
| Spiromesifen | 240 | Insecticide/Acaricide | 400 | 600 | 1600 |
| Chlorantraniliprole | 350 | Insecticide | 4 | 4 | 4 |
| Total | 959.5 | 1209.5 | 2210 | ||
| Family | Scientific Name | Common Name | n | MN per 10,000 Erythrocytes (Mean ± SD) | ||
|---|---|---|---|---|---|---|
| Small Farms | Medium Farms | Large Farms | ||||
| Columbidae | Columbina talpacoti | Ruddy Ground Dove | 1 | 0 | - | - |
| Leptotila rufaxilla | Grey-fronted Dove | 9 | - | - | 6 ± 0 | |
| Cuculidae | Crotophaga ani | Smooth-billed Ani | 3 | 0 | 3 ± 0 | 4.5 ± 0.7 |
| Guira guira | Guira Cuckoo | 3 | 1.5 ± 0.7 | 1.67 ± 1.52 | 5 ± 0 | |
| Thraupidae | Dacnis cayana | Blue Dacnis | 2 | 1 ± 1.41 | 5 ± 0 | - |
| Tangara sayaca | Sayaca Tanager | 3 | 2 ± 0 | 0 | - | |
| Tersina viridis | Swallow Tanager | 1 | - | 2 ± 0 | - | |
| Volatinia jacarina | Blue-black Grassquit | 18 ** | 1.86 ± 1.07 | 2.43 ± 1.27 | 5.57 ± 1.62 | |
| Tyrannidae | Elaenia flavogaster | Yellow-bellied Elaenia | 1 | 0 | 0 | 4 ± 0 |
| Myiozetetes similis | Social Flycatcher | 4 | 2 ± 1 | 4 ± 0 | 4.5 ± 0.7 | |
| Tyrannus melancholicus | Suiriri Flycatcher | 3 | 1 ± 0 | 3.5 ± 0.7 | 4.5 ± 0.7 | |
| Galbulidae | Galbula ruficauda | Rufous-tailed Jacamar | 17 ** | 1 ± 0 | 0 | 7 ± 0 |
| Icteridae | Gnorimopsar chopi | Chopi Blackbird | 9 | 0.1 | - | 6.5 ± 0.7 |
| Molothrus bonariensis | Shiny Cowbird | 15 ** | 2 ± 0 | 3 ± 0 | 8 ± 0 | |
| Mimidae | Mimus saturninus | Chalk Mockingbird | 1 | - | 1 ± 0 | - |
| Passeridae | Passer domesticus | House Sparrow | 5 | - | 6 ± 0 | 6 ± 1.41 |
| Thamnophilidae | Thamnophilus doliatus | Barred Antshrike | 3 | 0 | - | 3 ± 0 |
| Thamnophilus torquatus | Rufous Antshrike | 1 | 1 ± 0 | - | - | |
| Turdidae | Turdus leucomelas | Pale-breasted Thrush | 4 | 3 ± 0 | 5 ± 0 | 6 ± 0 |
| Turdus rufiventris | Rufous-bellied Thrush | 16 ** | 4 ± 0 | 3 ± 0 | 4.5 ± 0.7 | |
| Passerellidae | Zonotrichia capensis | Rufous-collared Sparrow | 3 | 0 | 2.5 ± 0.7 | 5 ± 0 |
| TOTAL | 122 | 1.38 ± 1.20 | 2.59 ± 1.65 * | 5.31 ± 5.40 * | ||
| Reference site (multiple species) | 30 | 0.26 ± 0.52 | ||||
| Family | Scientific Name | Common Name | Guild | Bill Length (mm) | ||
|---|---|---|---|---|---|---|
| Small farms | Medium farms | Large farms | ||||
| Columbidae | Columbina talpacoti | Ruddy Ground Dove | Granivorous | 15.0 | - | |
| Leptotila rufaxilla | Grey-fronted Dove | Granivorous | - | - | 24.0 | |
| Cuculidae | Crotophaga ani | Smooth-billed Ani | Omnivorous | 35.0 | 34.0 | 35.0 |
| Guira guira | Guira Cuckoo | Omnivorous | 36.5 | 36.0 | 36.0 | |
| Thraupidae | Dacnis cayana | Blue Dacnis | Omnivorous | 31.0 | 13.0 | - |
| Tangara sayaca | Sayaca Tanager | Frugivorous | 21.0 | - | ||
| Tersina viridis | Swallow Tanager | Omnivorous | 15.0 | |||
| Volatinia jacarina | Blue-black Grassquit | Granivorous | 10.3 | 10.3 | 10.1 | |
| Tyrannidae | Elaenia flavogaster | Yellow-bellied Elaenia | Omnivorous | 18.0 | 17.0 | 16.0 |
| Myiozetetes similis | Social Flycatcher | Insectivorous | 17.3 | 16.5 | ||
| Tyrannus melancholicus | Suiriri Flycatcher | Omnivorous | 21.5 | 21.0 | 19.5 | |
| Galbulidae | Galbula ruficauda | Rufous-tailed Jacamar | Insectivorous | 22.0 | 20.0 | 19.0 |
| Icteridae | Gnorimopsar chopi | Chopi Blackbird | Omnivorous | 17.8 | 23.5 | |
| Molothrus bonariensis | Shiny Cowbird | Omnivorous | 18.0 | 16.0 | 16.0 | |
| Mimidae | Mimus saturninus | Chalk-browed Mockingbird | Omnivorous | - | 26.0 | - |
| Passeridae | Passer domesticus | House Sparrow | Omnivorous | - | 14.0 | 14.5 |
| Thamnophilidae | Thamnophilus doliatus | Barred Antshrike | Insectivorous | 20.0 | - | 17.0 |
| Thamnophilus torquatus | Rufous-winged Antshrike | Insectivorous | - | 21.0 | - | |
| Turdidae | Turdus leucomelas | Pale-breasted Thrush | Omnivorous | 23.0 | 22.0 | 22.0 |
| Turdus rufiventris | Rufous-bellied Thrush | Omnivorous | 25.0 | 21.0 | 21.0 | |
| Passerellidae | Zonotrichia capensis | Rufous-collared Sparrow | Granivorous | 17.0 | 14.0 | 13.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souto, H.N.; de Campos Júnior, E.O.; Siqueira, M.V.B.M.; Campos, C.F.; Morais, C.R.; Pereira, B.B.; Morelli, S. Birds as Environmental Bioindicators of Genotoxicity in Brazilian Cerrado Farmlands: An In Situ Approach. Animals 2025, 15, 3208. https://doi.org/10.3390/ani15213208
Souto HN, de Campos Júnior EO, Siqueira MVBM, Campos CF, Morais CR, Pereira BB, Morelli S. Birds as Environmental Bioindicators of Genotoxicity in Brazilian Cerrado Farmlands: An In Situ Approach. Animals. 2025; 15(21):3208. https://doi.org/10.3390/ani15213208
Chicago/Turabian StyleSouto, Henrique Nazareth, Edimar Olegário de Campos Júnior, Marcos Vinicius Bohrer Monteiro Siqueira, Carlos Fernando Campos, Cassio Resende Morais, Boscolli Barbosa Pereira, and Sandra Morelli. 2025. "Birds as Environmental Bioindicators of Genotoxicity in Brazilian Cerrado Farmlands: An In Situ Approach" Animals 15, no. 21: 3208. https://doi.org/10.3390/ani15213208
APA StyleSouto, H. N., de Campos Júnior, E. O., Siqueira, M. V. B. M., Campos, C. F., Morais, C. R., Pereira, B. B., & Morelli, S. (2025). Birds as Environmental Bioindicators of Genotoxicity in Brazilian Cerrado Farmlands: An In Situ Approach. Animals, 15(21), 3208. https://doi.org/10.3390/ani15213208